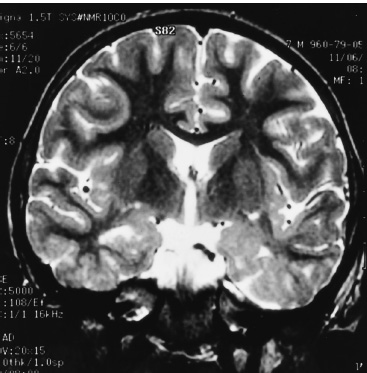
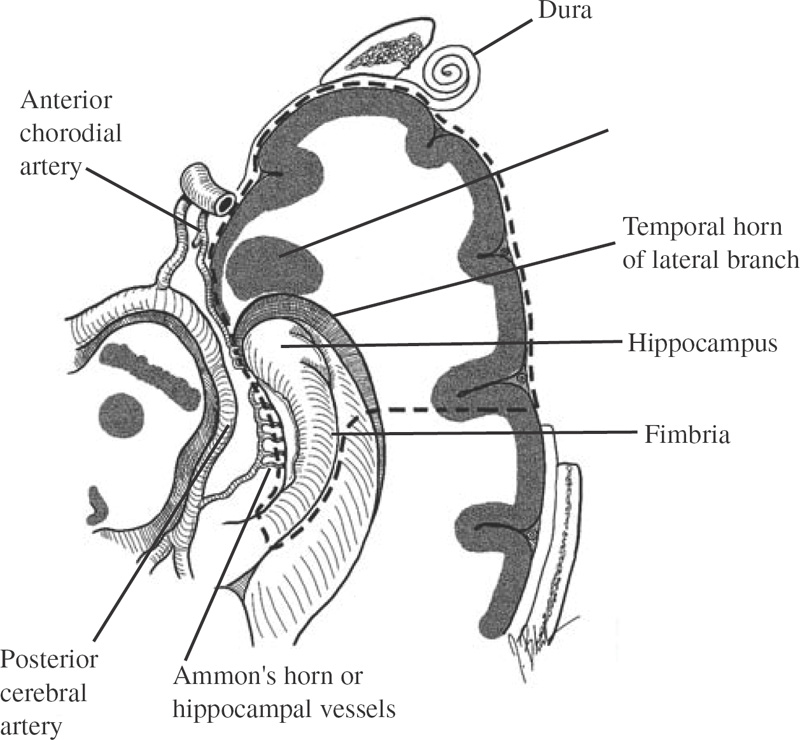
Functional Disorders Epilepsy affects approximately 0.5 to 1.0% of the population, and its onset most frequently occurs during childhood. In young children, uncontrolled seizures adversely affect intellectual maturation and the development of normal social behaviors, which may be a result of prolonged and often toxic levels of anticonvulsant medication or the constant abnormal electric activity during development. Because intractable seizures in children have a “malignant” natural history with eventual declines in both intellectual and behavioral functions, surgery has become an acceptable early treatment modality for these patients. The temporal lobe and its medial structures, the amygdalohippocampal complex, are the most common source of medically intractable complex partial seizures in adults and are the origin for approximately 30% of such seizure disorders in children. Historically, temporal-lobe epilepsy has been the most common surgically treated form of epilepsy because its distinct pathology and accessible location, low morbidity of surgery, and excellent outcomes. The percentage of temporal-lobe resections in most pediatric series exceeds 56%. The determination of intractability and the evaluation of surgical candidacy in children can be done in a systematic and effective way to identify which patients would benefit from surgical intervention when they have failed medical management. Several techniques have been used for temporal lobectomy for epilepsy. The original description by Penfield, later modified by Falconer, consisted of an en block resection of the temporal lobe and the medial structures. Falconer initially described the successful use of his technique for temporal lobectomy, specifically for the treatment of epilepsy in children, almost 30 years ago. This author prefers a two-stage resection similar to that described by Spencer, which involves an anterolateral cortical resection followed by a microscopic removal of the medial structures. This method allows limited resection of the anterior temporal lobe and yet provides adequate access to the medial basal structures. Often the best method is that which the surgeon is comfortable with and performs most commonly. This description is meant as a guide to the anatomy and one approach that provides a low incidence of morbidity with excellent outcomes. The goal of the surgical evaluation is to characterize the seizures, define the epileptogenic zone by lateralizing and localizing them to a particular region, and, finally, determine the relationship of the epileptic focus to the eloquent cortex. This is achieved through the formalized evaluation process, which attempts to obtain concordant data as to the origin of the abnormal region. The initial phase is noninvasive and includes a battery of tests to define the seizures, their semiology, and their electrophysiologic dysfunction. Besides electroencephalography (EEG), a neuropsychologic battery, imaging with magnetic resonance (MR), and some type of functional imaging are used to obtain a better understanding of the preoperative anatomical and functional anomalies. These imaging studies, in conjunction with the EEG, often are able to define the seizure syndrome and its etiology. If necessary, further evaluation may include EEG and videotelemetry to characterize the seizure semiology or amobarbital (Wada) testing, where needed, for language and memory localization. Concordance between the EEG and imaging as indispensible for defining the epileptogenic zone and for making the decision to proceed with a surgical intervention. With the further refinement and improvement in neu-roimaging capability and resolution, particularly with the development of new sequencing modalities (e.g., fluid attenuated inversion recovery (FLAIR) for MR, lesions and subtle structural differences between normal and abnormal cortical areas have been increasingly defined that have been correlative with the epileptogenic zone with increased sensitivity (Fig. 22–1). Because epilepsy is a functional disorder of the brain, defining the anatomic abnormalities may not be sufficient to define the epileptic focus fully. In these instances, functional neuroimaging may detect the generalized and focal functional abnormalities and their relationship to the EEG and anatomic imaging. These imaging modalities have been useful in temporal-lobe evaluation and are believed to have a significant role in the evaluation of patients with extratemporal epilepsy as well. The MR can be used for MR spectroscopy (MRS) and functional MR (fMR); the efficacy of positron emission tomography (PET) and single-photon emission computerized tomography (SPECT) also have had a major impact on the evaluation of patients with temporal lobe epilepsy. FIGURE 22–1. Coronal MR (T2-weighted) scan of a 3-year-old boy with a 2-year history of complex partial seizures intractable to medication. Previous scans were reportedly normal. A cortical signal abnormality is noted in the left inferior temporal, parahippocampal gyri and the medial structures. With the improved evaluation techniques now available, many pathologic and physiologic changes that were previously undetectable can be identified, which can further support the decision for surgical intervention when medical therapy has failed. Many young children would have previously had a prolonged observation period prior to surgical resection with little medical efficacy. With modern neuroimaging techniques, a child with a clear cytoarchitectural, neoplastic, or metabolic abnormality that correlates with the seizure syndrome can be identified and a focused surgical resection performed without unnecessary prolongation. These new technologies also have decreased the need for invasive monitoring with intracranial electrodes in some adult series by 50% and in children in up to 90% by identifying those patients who would have had a previously undefined epileptogenic zone, with the necessary concordant data defining their epileptic focus before implantation. These patients were able to benefit directly from a focal surgical resection without a second operation. There are two sources for a temporal-lobe seizure focus: the medial basal portion and the lateral neocortex. The medial basal portion includes the fusiform and the parahippocampal gyri as well as the amygdalohippocampal complex. Anteromedially, the parahippocampal gyrus curves in front of the midbrain to form the uncus; immediately posterolateral to the uncus is the entorhinal cortex. Medial to these structures lies the hippocampal formation. When approaching from lateral to medial along the parahippocampal gyrus, the subiculum forms arising from the parahippocampal gyrus. Directly medial to the subiculum are the fields of the cornu ammonis (CA1–CA4) and the dentate gyrus. In medial temporal sclerosis, the greatest damage occurs in Sommer’s sector (CA1 and prosubiculum) and the end-folium (CA4 and hilus). The other areas, such as granule cell layer are CA2, also are severely damaged. The only region not as badly affected is the subiculum, although there is some damage. Inside the temporal horn of the lateral ventricle, the body of the hippocampus is covered by the alveus, which posteriorly, in the tail of the hippocampus, forms into the fimbria of the fornix. Within the ventricle and superomedial are the choroidal fissure and the choroid plexus, which lie in the roof of the temporal horn and have a vascular supply from the anterior choroidal artery. The lateral cortex includes the superior and medial gyri and the anterior part of the inferior temporal gyrus. Any of these cortical regions also may support an epileptic focus either due to a lesion or architectural abnormality but may also include eloquent or functionally important tissue, especially in the dominant hemisphere. The vascular supply to the temporal lobe consists of multiple perfusing vessels. There is a lateral and supero-lateral supply from the middle cerebral artery as it arises from the sylvian fissure. The inferior surface of the temporal lobe is supplied by the posterior cerebral artery with the P2 and P3 segments traversing the posterior portion of the ambient cistern to reach the inferior part of the temporal lobe. The P2 segment also supplies part of the hippocampus and parahippocampal gyrus via the small Ammon’s horn arteries medially. The anterior choroidal artery also contributes to the medial vascular supply as it extends posteriorly from the internal carotid artery through the choroid plexus within the temporal horn and supplies part of the cascade of microvasculature to the hippocampus and parahippocampal gyrus (Fig. 22–2). Cranial nerves III and IV extend from the brainstem within the ambient cistern anteriorly along the edge of the tentorium. Pupillary dilatation is a harbinger of un-cal herniation because of third-nerve compression by the uncus anterior to the hippocampus. Injury to these nerves during a temporal lobectomy can be minimized by maintaining the integrity of the pia–arachnoid border of the superior compartment. FIGURE 22–2. The P-2 segment of the posterior cerebral artery provides part of the arterial supply to the hippocampus via the small Ammon’s horn arteries medially. The anterior choroidal artery also contributes to the medial vascular supply as it extends posteriorly to the choroid plexus within the temporal horn. The planned resection is mapped in the dashed lines. The anesthetic issues for patients undergoing a temporal lobectomy depend on whether intraoperative electrophysiologic studies will be performed. In general, the anesthetic technique includes variable combinations of inhalation agents, narcotics, and paralytics. After induction, anesthesia often is maintained using nitrous oxide along with a continuous fentanyl infusion. There is usually no contraindication to paralysis for the standard procedure, and vecuronium is usually the drug of choice. Other inhalation agents, such as isoflurane, may be used at minimal dosages if necessary but may impact on the intraoperative EEG. Benzodiazepines and barbiturates also are usually avoided because of their long-acting effect on the EEG. “Awake” craniotomies are rarely done in children except in the older, mature child. In these instances, a combination of local injections of a long-acting anesthetic, such as marcaine hydrochloride, and a general, short-acting amnestic medication, such as propofol, is continued throughout the initial intervention until the eloquent area is exposed. Once the patient’s cooperation is needed, the infusion can be discontinued and the intraoperative functional testing can proceed. Overall, we prefer to map these functional areas extra-operatively with grid and strip electrodes in a planned two-stage procedure with the presumed craniotomy adequately placed to expose these areas. Mapping of the epileptogenic focus then can be performed and cortical stimulation algorithms used to define the areas of eloquent cortex prior to resection in a more relaxed setting. Routine monitoring is used in all patients unless special circumstances or concerns exist (e.g., excessive blood loss from an arteriovenous malformation). The routine for the surgical preparation includes adequate venous access for medications and fluids; an arterial line for constant blood pressure monitoring; a urinary drainage catheter; and, in cases in which blood loss may be significant, a central venous catheter. Surface EEG leads also may be placed before positioning for intraoperative monitoring. Anticonvulsant medications are continued in full dosages throughout the intraoperative and postoperative periods. Preoperative medications are dependent on the preferences of the surgeon and operative team. Antibiotics are given “preincision” and sometimes are continued at specific intervals during the procedure. The use of vancomycin and tobramycin or cefazolin has been described and probably has the same efficacy and outcome. Infection, although the most commonly reported complication, occurs only rarely. The use of preoperative steroids is also variable and often depends on the etiology of the seizures. As in standard tumor surgery, if there is a “lesional” component to the seizures and there is associated edema or mass effect, dexamethasone is often used at a dose of 1 to 10 mg every 6 hours. Some surgeons prefer to use steroids in all cases as a prophylactic measure for the swelling associated with surgical manipulation. Alternatively, a steroid regimen could be instituted postoperatively if there were any obvious neurologic deficits. Osmotic diuresis with mannitol also has been described to facilitate surgical manipulation of the cerebral structures; however, brain relaxation often can be achieved simply by drainage of cerebrospinal fluid and the use of mild hyperventilation (PaCO2 of 30–35) without creating major fluid shifts. Patients are placed supine on the operating table with a roll under the ipsilateral shoulder and the head turned away, exposing the operative side. The head is positioned so that it is parallel to the floor with the vertex pointed downward 15 degrees, and the patient is placed in three-point fixation (Figs. 22–3A and B). The hair and scalp are scrubbed with an antibacterial soap, and a small strip is shaved along the planned incision. In this way, the need to shave this exposed area of the head is minimized. Following skin preparation, a question mark incision is marked out; inferiorly, it extends from 1 cm below the zygoma, just anterior to the tragus of the ear, and curves around the ear to the apex of the ear, which is its posterior extent. The incision then curves superiorly and anteriorly above the superior temporal ridge, staying within the hairline, and as far anteriorly as possible to optimize the anterior exposure. Infiltration of the skin with lidocaine and epinephrine can be used to assist hemostasis. The skin incision is carried out with a no. 15 blade and carried through the subcutaneous tissues and the galea. Skin clips then are applied for hemostasis. Alternatively, the skin can be scored to the subcutaneous tissue and further incised using a needle-tip cautery to avoid the skin clips and to cause minimal blood loss. The scalp flap then is sharply dissected through the subgaleal tissue using the no. 15 blade and reflected anteriorly using fishhooks (Fig. 22–4). The periosteum is left intact on the bone, as is the temporalis muscle and its fascia. The temporalis muscle then is incised in a T-shaped manner with the vertical arm placed anteriorly so that approximately one third of the muscle is reflected anteriorly and two thirds reflected posterinferiorly using the fishhooks or suture. The superior muscle incision should leave a cuff of tissue attached to the superior temporal line for later reattachement (Fig. 22–5).

SURGICAL INDICATIONS AND PREOPERATIVE EVALUATION
Imaging
SURGICAL ANATOMY OF THE TEMPORAL LOBE
INTRAOPERATIVE TECHNIQUE
Anesthetic Techniques and Preoperative Medications
Positioning and Initial Exposure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


