B. Arterial hypertension. ICH may be a complication of either acute or chronic arterial hypertension. Sustained chronic hypertension leads to degenerative changes in small penetrating arteries in the deep structures of the brain. Sudden, severe hypertension may overwhelm the autoregulatory responses of the cerebral vasculature and an arteriole may rupture. Acute, severe arterial hypertension may be secondary to acute glomerulonephritis, eclampsia, severe emotional stress, or the use of a sympathomimetic agent. The most common sites for hypertensive hemorrhage are the basal ganglia (putamen in particular), thalamus, pons, cerebellum, or deep lobar white matter. Hypertension should be considered as the likely cause of a hematoma located in deep gray matter structures of the cerebral hemisphere if the patient has a history of hypertension. Other features of chronic hypertension, such as retinopathy, left ventricular hypertrophy, or renal dysfunction, are supportive of the diagnosis. The magnetic resonance imaging (MRI) finding of asymptomatic microhemorrhages located in deep structures also points to chronic hypertension. Hemorrhagic stroke may be attributed to hypertension because of the presence of an elevated blood pressure measured upon arrival to an emergency department. However, arterial hypertension is common among acutely ill patients with intracranial hemorrhage of many causes and the finding of an elevated blood pressure should not automatically lead to the diagnosis of hypertensive hemorrhage. Acute hypertensive crises (hypertensive encephalopathy) may be associated with SAH that is most commonly located over the convexity of the cerebral hemisphere.
C. Saccular aneurysm. Rupture of a saccular aneurysm is the most common cause of nontraumatic SAH and it is an important cause of ICH. Annually, approximately 30,000 Americans will have aneurysmal SAH. Approximately 1% to 5% of adults harbor intracranial aneurysms but a minority of these lesions actually rupture. In general, the risk for rupture is correlated with the size of the aneurysm, with the highest risk found with aneurysms larger than 6 mm in diameter. Aneurysms in the posterior circulation are associated with a higher risk of bleeding than similarly sized aneurysms in the carotid circulation. Approximately 85% of saccular aneurysms are in the carotid circulation with the most common sites being the junction of the internal carotid artery–posterior communicating artery, the bifurcation of the middle cerebral artery, and the anterior communicating artery. The most common sites in the posterior circulation are the bifurcation of the basilar artery and the origin of the posterior inferior communicating artery. Patients with autosomal-dominant polycystic kidney disease have a high prevalence of intracranial aneurysms. Approximately 10% of patients have a family history of cerebral aneurysms.
D. Other aneurysms. Infective, neoplastic, and traumatic aneurysms are rare causes of intracranial hemorrhage. These lesions are usually located in peripheral branch pial arteries on the cortical surface of the cerebral hemispheres. They are smaller than saccular aneurysms, but they have a relatively high risk of hemorrhage. Dolichoectatic (fusiform) aneurysms are tortuous, elongated arterial enlargements most commonly found in the basilar arteries of patients with extensive atherosclerosis or Fabry’s disease. Hemorrhage is an uncommon complication. Spontaneous or traumatic dissecting aneurysms of intracranial arteries, particularly of the basilar or distal vertebral arteries, are potential causes of atypical SAH.
E. Vascular malformations. Vascular malformations are classified as arteriovenous malformation (AVM), developmental venous anomaly, cavernous malformation, and telangiectasia. They may be located in any part of the brain. Although familial cases, as with hereditary hemorrhagic telangiectasia (HHT) or familial cavernous malformations may occur, most are sporadic. The rate of bleeding is especially high among persons with HHT. The prevalence of vascular malformations is less than that of saccular aneurysms and most affected persons never have a hemorrhage. ICH is the presenting symptom in approximately 50% of cases. Nonhemorrhagic symptoms include recurrent and stereotypic headache, seizures, or progressive neurologic impairments. Patients with a large AVM that causes turbulent flow may have pulsatile tinnitus and a cranial bruit may be auscultated. A dural arteriovenous fistula also may cause a hemorrhage and may mimic a vascular malformation.
F. Cerebral amyloid angiopathy. Cerebral amyloid angiopathy (congophilic angiopathy) is a leading cause of lobar hemorrhage in older persons. With aging, amyloid is deposited in the walls of cortical and leptomeningeal arterioles. Presumably, the protein accumulation leads to vascular fragility and bleeding. The hemorrhages, which are most commonly located in the frontal and parietal lobes, usually arise at the junction of the cerebral cortex and adjacent white matter. Multiple or recurrent hemorrhages are common and cerebellar hemorrhages may develop. Cerebral amyloid angiopathy should be considered as the likely cause of lobar ICH in persons older than 75. Because approximately 70% of affected patients also have a history of Alzheimer’s disease, a past history of dementia or cognitive decline increases the likelihood that an ICH in an elderly patient is due to amyloid angiopathy. The presence of microhemorrhages at the cortical/white matter junction seen by MRI is also supportive of the diagnosis.
G. Vasculitis. Multisystem or primary angiitis of the CNS (PACNS) vasculitis is a rare cause of intracranial hemorrhage. Bleeding is most commonly associated with a necrotizing vasculitis, such as periarteritis nodosa. Vasculitis may be the cause of bleeding among some young patients who have hemorrhagic stroke following the use of a sympathomimetic agent. An association between ICH and vasculitis secondary to varicella-zoster virus has been reported and the risk of ICH also is increased among persons who have human immunodeficiency virus.
H. Bleeding disorders. Intracranial hemorrhage is a potential complication of several inherited or acquired bleeding diatheses including hemophilia, sickle cell disease, thrombocytopenia, and leukemia. Intracranial bleeding also may complicate the use of thrombolytic or antithrombotic agents. The medications may not be the sole cause of bleeding in some cases; rather, the agents may exacerbate hemorrhage from another cause. Still, the severity of bleeding is worse and the prognosis is poorer among patients with hemorrhage secondary to a coagulation disorder than among other causes of intracranial hemorrhage. Intracranial hemorrhage is a potential complication of use of warfarin; it is associated with a higher likelihood of mortality than with bleeding of other causes. This complication should be considered whenever a patient has acute neurologic symptoms while taking oral anticoagulants, even if there is no other evidence of bleeding. The risk of ICH is increased among the elderly and those who have leukoaraiosis present of brain imaging studies. Persons with a past history of stroke or poorly controlled hypertension also have a high risk of hemorrhage. The risk of intracranial bleeding increases when the international normalized ratio (INR) exceeds 3 to 4. One of the advantages of the new oral anticoagulants (dabigatran, rivaroxaban, apixaban, and edoxaban) for treatment of patients with nonvalvular atrial fibrillation is a lower risk of intracranial hemorrhage than that associated with warfarin. The risk of hemorrhagic stroke is lower with the use of antiplatelet agents. The combination of aspirin and clopidogrel does have a higher risk of serious bleeding than the use of either medication alone. The combination of warfarin and aspirin also is associated with an increased risk of bleeding.
I. Drug abuse. The abuse of drugs such as cocaine or methamphetamine has been associated with intracranial hemorrhage. These drugs may lead to bleeding because of sudden surges in blood pressure or because of the development of a vasculitis. In addition, a secondary increase in blood pressure may lead to rupture of a vascular malformation or aneurysm. Intracranial hemorrhage also has been associated with heavy alcohol use.
J. Moyamoya. Moyamoya is an uncommon cause of hemorrhagic stroke in children and young adults (![]() Video 41.1). The arteriographic hallmarks of moyamoya are occlusions of the major arteries of the anterior circulation and the appearance of a network of fine blood vessels at the base of the brain. Moyamoya disease appears to be inherited on an autosomal-dominant basis and is most common among persons of northeastern Asian ancestry. Moyamoya syndrome is diagnosed when similar arteriographic findings occur among patients with a number of acquired disorders. Hemorrhage may be secondary to rupture of an aneurysm, most commonly in the posterior circulation, or rupture of a small collateral vessel.
Video 41.1). The arteriographic hallmarks of moyamoya are occlusions of the major arteries of the anterior circulation and the appearance of a network of fine blood vessels at the base of the brain. Moyamoya disease appears to be inherited on an autosomal-dominant basis and is most common among persons of northeastern Asian ancestry. Moyamoya syndrome is diagnosed when similar arteriographic findings occur among patients with a number of acquired disorders. Hemorrhage may be secondary to rupture of an aneurysm, most commonly in the posterior circulation, or rupture of a small collateral vessel.
K. Venous thrombosis. Occlusion of a cortical vein (cortical vein thrombosis) or cerebral venous sinus (sinus thrombosis) is an uncommon etiology of hemorrhagic stroke. Bleeding is most common among patients with thrombosis of the superior sagittal sinus. In this situation, the areas of bilateral bleeding are parasagittal in location and in a thumbprint pattern. The clinical course of cerebral venous sinus thrombosis differs from that of most other hemorrhagic strokes. Most patients have worsening headache, seizures, altered consciousness, and focal neurologic signs that evolve over a few days. Cerebral venous sinus thrombosis is more common among women and is often correlated with the peripartum state or the use of oral contraceptives. It may also occur among persons who are dehydrated, have malignancies, have undergone a recent cranial operation, or have an otolaryngologic infection.
L. Brain tumors. Hemorrhage may be the initial symptom of a highly vascular metastatic brain tumor including choriocarcinoma, melanoma, or carcinoma of the kidney, thyroid, breast, or lung. The most common primary tumors associated with bleeding are glioblastoma and pilocytic astrocytoma. Patients may have a history of evolving neurologic symptoms such as headache or personality change prior to the bleeding event. The presence of extensive brain edema detected by brain imaging during the first hours after the hemorrhage or multiple hemorrhagic lesions should prompt consideration of an underlying brain tumor.
M. Hemorrhagic transformation of an ischemic stroke. Modern brain imaging allows for discovery of hemorrhagic changes in the ischemic lesions in a sizable proportion of patients with a recent stroke. A smaller percentage has neurologic worsening secondary to hemorrhagic transformation of the infarction. The risk of this complication increases with the use of thrombolytic agents, anticoagulants, or endovascular interventions within the first hours after stroke.
N. Reperfusion hemorrhage. Hemorrhage is a potential subacute complication of surgical interventions (carotid endarterectomy or angioplasty/stenting) used to treat severe stenoses of extracranial arteries. The hemorrhage is attributed to an increase in perfusion to an area of the brain in which autoregulation has been disturbed by the severe stenosis of the parent vessel. The bleeding, which is in the hemisphere ipsilateral to the treated artery, usually occurs 2 to 3 days after the surgical procedure and presents with headache, seizures, and new focal neurologic signs.
MANIFESTATIONS OF HEMORRHAGIC STROKE
The clinical features of hemorrhagic stroke are generally the same in children and adults. The symptoms and signs of primary IVH and SAH may differ from those of ICH in that focal neurologic signs are usually absent or subtle. Because of the absence of prominent focal neurologic signs, errors in diagnosis are more likely to occur among patients with SAH than among patients with bleeding primarily in the brain.
A. Clinical presentation. Hemorrhagic stroke is usually a sudden and dramatic event. The patient or observers often relate the circumstances surrounding the onset of symptoms. A headache, of any quality and location, usually described as intense and often is pronounced as the “worst headache of my life.” The term thunderclap is often used to describe these headaches. A severe headache accompanied by a transient loss of consciousness or one that is cataclysmic in onset is a premier symptom of aneurysmal SAH. Approximately 40% of patients with ICH will have severe headache. Other symptoms include nausea, vomiting, prostration, photophobia, phonophobia, and nuchal rigidity. The presence of nausea and vomiting and focal signs suggestive of a lesion in a cerebral hemisphere is predictive of a hemorrhage event. The exception to the rule of sudden onset of headache is the course of venous sinus thrombosis, in which the headache may slowly worsen over hours to days.
Disturbances in consciousness are common. Prolonged unresponsiveness, including coma, occurs among patients with major hemorrhages. Transient alteration in alertness, which may mimic syncope, may be present at the time of the hemorrhage. Disorientation, confusion, or delirium also may occur. Although focal or generalized seizures may develop, recurrent seizures or status epilepticus are uncommon.
Focal neurologic symptoms/signs reflect the location of the hematoma. The most common pattern is a contralateral hemiparesis and sensory loss secondary to a hematoma in the basal ganglia. Patients with cerebellar hemorrhages may have a subacute course. They report headache, vertigo, disturbed balance, nausea, and vomiting. Signs of increased intracranial pressure (ICP) or brainstem compression subsequently appear, including declines in consciousness, cranial nerve palsies, and motor impairments. Although most patients with SAH or primary IVH do not have focal neurologic signs, some patients with aneurysms will have focal impairments. The most common is a third nerve palsy secondary to a ruptured aneurysm located on the posterior communicating artery.
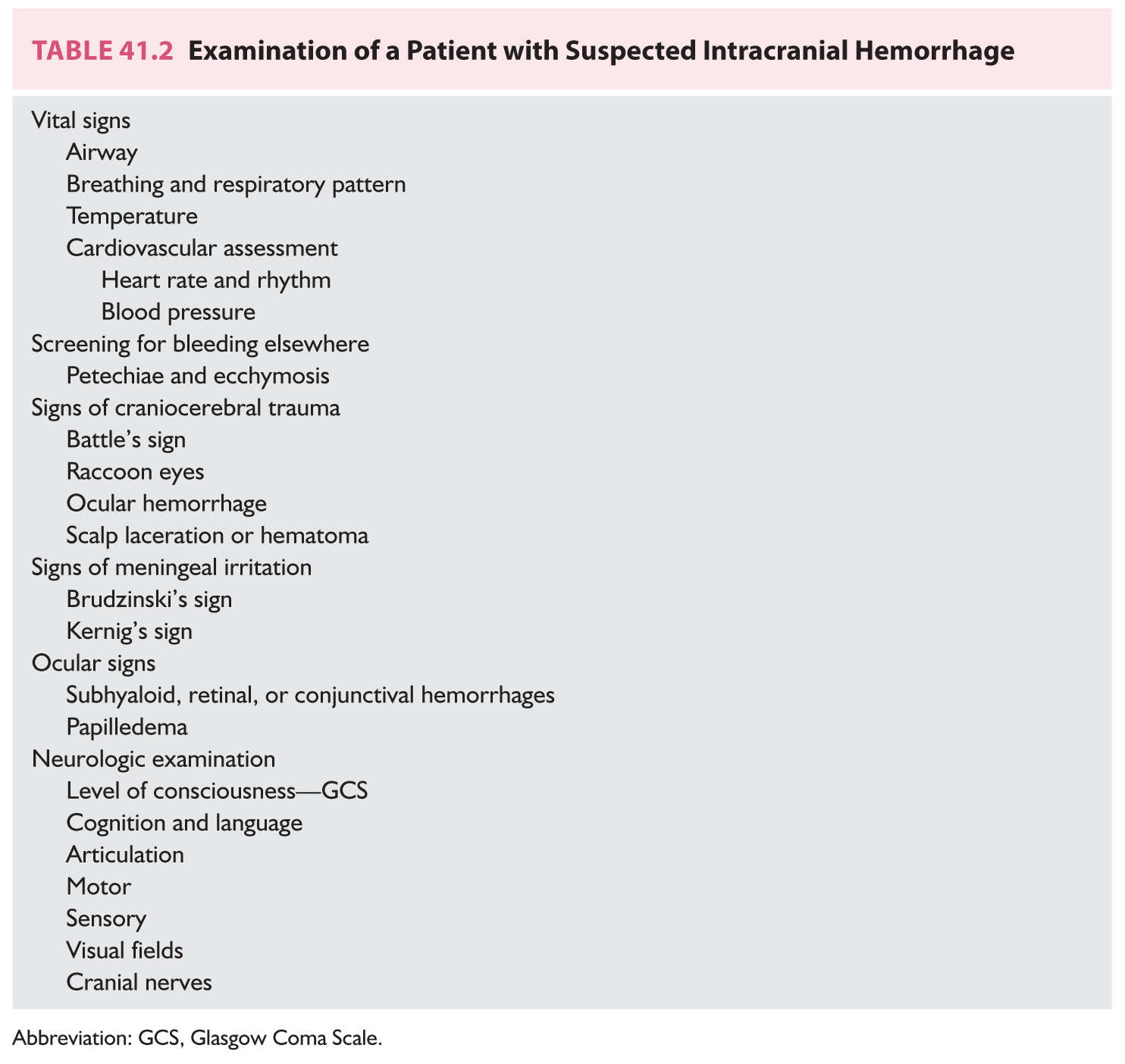
B. General examination. Assessment of the vital signs, airway, breathing, and circulation (ABCs) of emergency care is the first step in the examination of the critically ill person with a suspected intracranial hemorrhage (Table 41.2). Vital signs are measured closely and neurologic assessments are done frequently to monitor for evidence of neurologic worsening or improvement. Securing the airway, most commonly with intubation, should be used for treatment of those patients with impaired consciousness, seizures, vomiting, or evidence of brainstem dysfunction. Patients with severe bleeding may have respiratory abnormalities that lead to hypoxia, hypercapnia, or acidosis. Fever is relatively common especially among patients with IVH and SAH. Electrocardiographic abnormalities and cardiac arrhythmias may also be detected. Some of the cardiac arrhythmias may be life-threatening and require emergency treatment. Most patients will have an elevated blood pressure. Neurogenic pulmonary edema also is a potential complication of severe hemorrhages.
Detection of a bruit over the head may point to an AVM. Multiple areas of ecchymosis or petechiae may suggest infective endocarditis, recent trauma, or an underlying coagulation disorder. Evidence of cervical spine, facial, or cranial injury should be sought. Neck pain or tenderness may represent an associated cervical spine injury in a patient with cranial trauma that may be secondary to the hemorrhage. The neck should not be flexed to screen for signs of meningeal irritation until a cervical spine fracture has been excluded. Meningeal irritation may result from blood in the subarachnoid space. Nuchal rigidity (Brudzinski’s sign) may not be present in patients with a hematoma restricted to the brain or in those who are comatose. A stiff neck is prominent among patients with SAH but this sign may take several hours to appear. Ocular hemorrhages (subhyaloid, conjunctival, or retinal) may be detected in approximately 20% of patients, especially among those with impaired consciousness. Because the course of the illness is short, papilledema is not commonly found in the first hours of the illness. The exception to this rule is patients who have bleeding secondary to venous sinus thrombosis or in some patients with brain tumors.
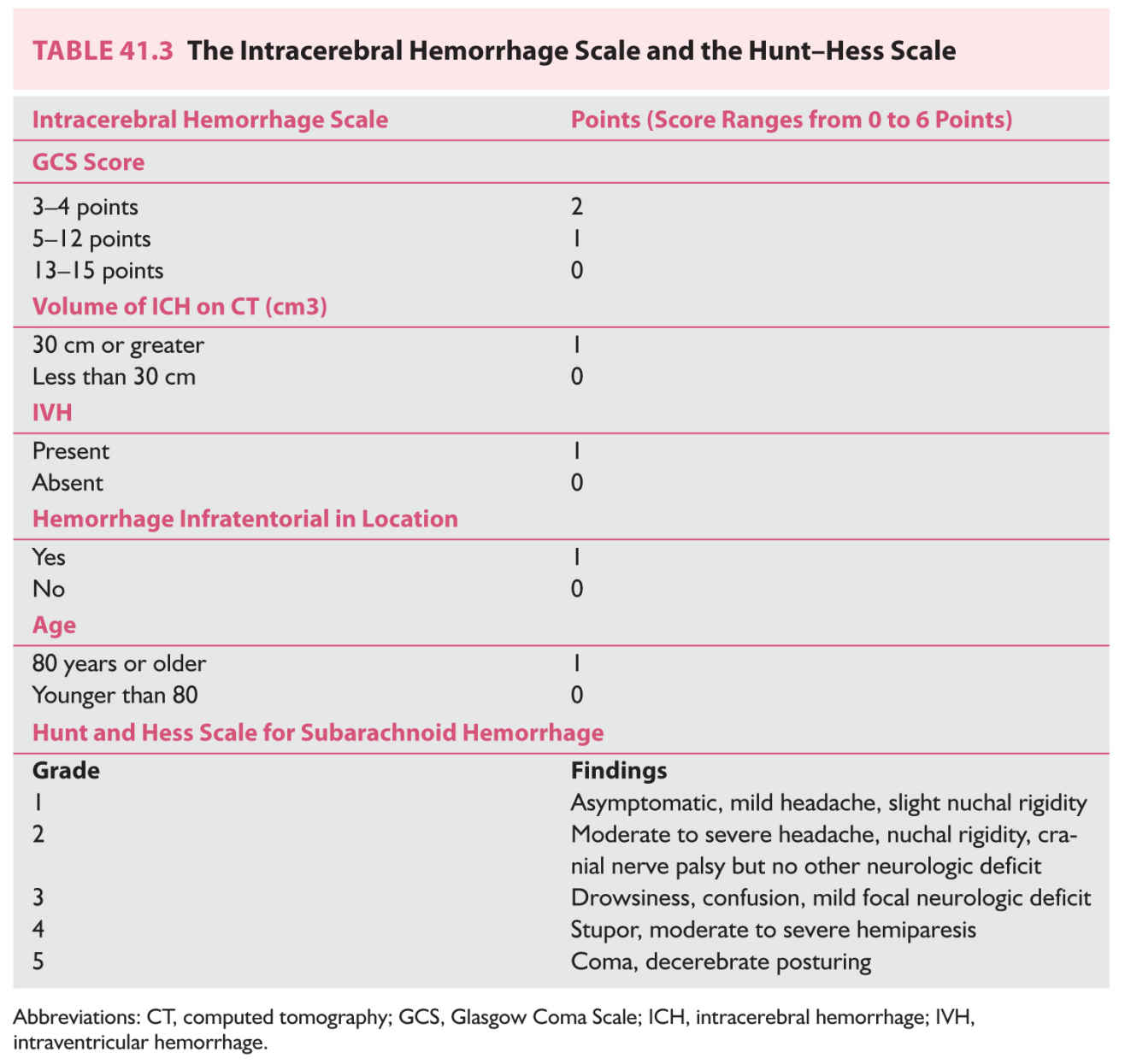
C. Assessment of consciousness. The most important component of the neurologic examination is the examination of the patient’s level of consciousness because it strongly correlates with the severity of the hemorrhage and prognosis. While the easily calculated Glasgow Coma Scale (GCS) was originally developed to assess patients with head injuries, it is directly applicable to persons with nontraumatic brain hemorrhages. In general, score of 8 or less on the GCS correlates with a very poor prognosis. The score on the GCS is also one of the major components of the Intracerebral Hemorrhage Score (Table 41.3). The level of consciousness is also a major contributor to scoring the Hunt–Hess scale to determine the severity of SAH.
D. Neurologic examination. The remainder of the neurologic examination is aimed at detecting abnormalities that reflect the location of the hemorrhage within the brain. Depending upon the site of the bleeding, motor, sensory, language, or cranial nerve impairments are found.
DIFFERENTIAL DIAGNOSIS OF HEMORRHAGIC STROKE
The differential diagnosis of an intracranial hemorrhage is not extensive. The brief course, clinical severity, and the prominent focal neurologic signs are relatively specific.
A. Ischemic stroke. The leading alternative diagnosis is acute ischemic stroke. Although there are no unique features, patients with hemorrhagic stroke are generally more seriously ill than those with ischemic events. Headaches, an early decline in consciousness, nausea, vomiting, photophobia, and phonophobia are more prominent with hemorrhagic lesions.
B. Craniocerebral trauma. Differentiation of traumatic from spontaneous intracranial hemorrhage may be difficult when a patient is comatose and no history is available. In general, deep hemorrhages within the brain are not secondary to trauma.
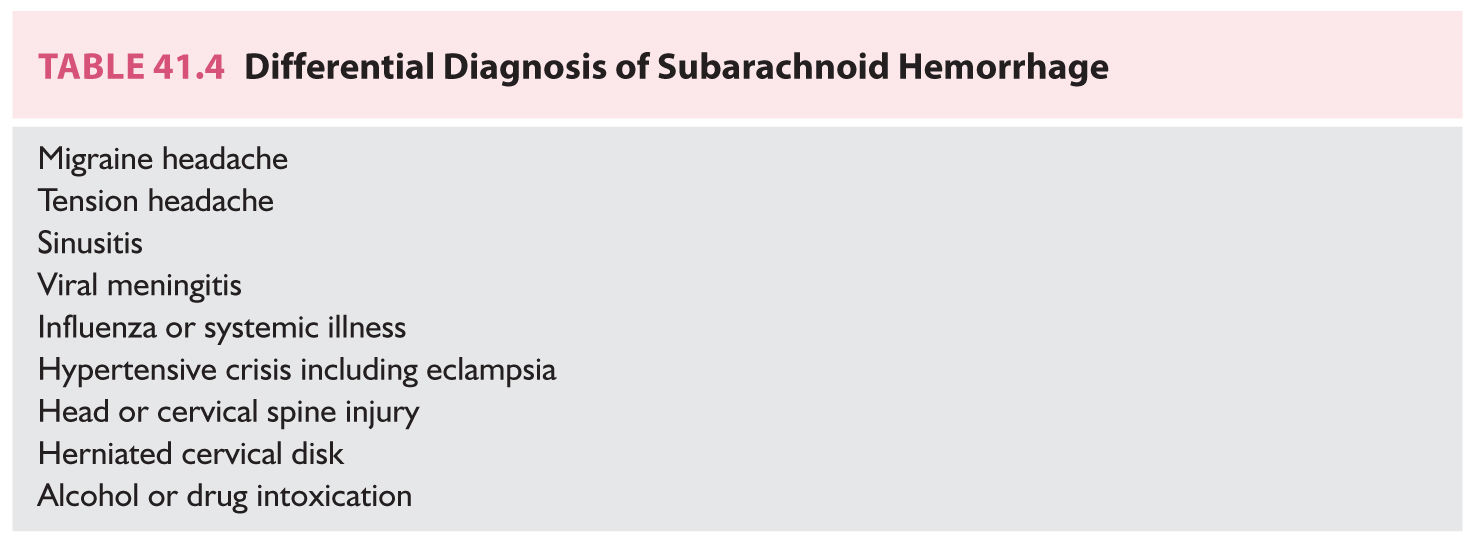
C. Subarachnoid and primary intraventricular hemorrhage. In contradistinction to ICH, the differential diagnosis of SAH and primary IVH is broad (Table 41.4). Although these patients usually seek medical attention because of the severity of their symptoms, physicians may be misled because of the absence of focal neurologic signs or disturbances in consciousness. The diagnosis of SAH is missed in approximately 15% of cases, a scenario that most commonly occurs in the least seriously ill patients. Failure to recognize a ruptured aneurysm has serious implications because of the high risk of a potentially fatal recurrent hemorrhage and because of the availability of effective therapies that can be administered. The only way to avoid missing an SAH or primary IVH is to maintain a high index of suspicion. Patients who have a sudden onset of an exceptionally severe headache or a headache associated with loss of consciousness need to be screened. The absence of focal neurologic signs or meningeal irritation does not preclude the diagnosis. Atypical symptoms of SAH include severe neck, face, shoulder, eye, or ear pain. A ruptured aneurysm in the posterior fossa may produce neck or back pain as a primary symptom.
DIAGNOSTIC STUDIES
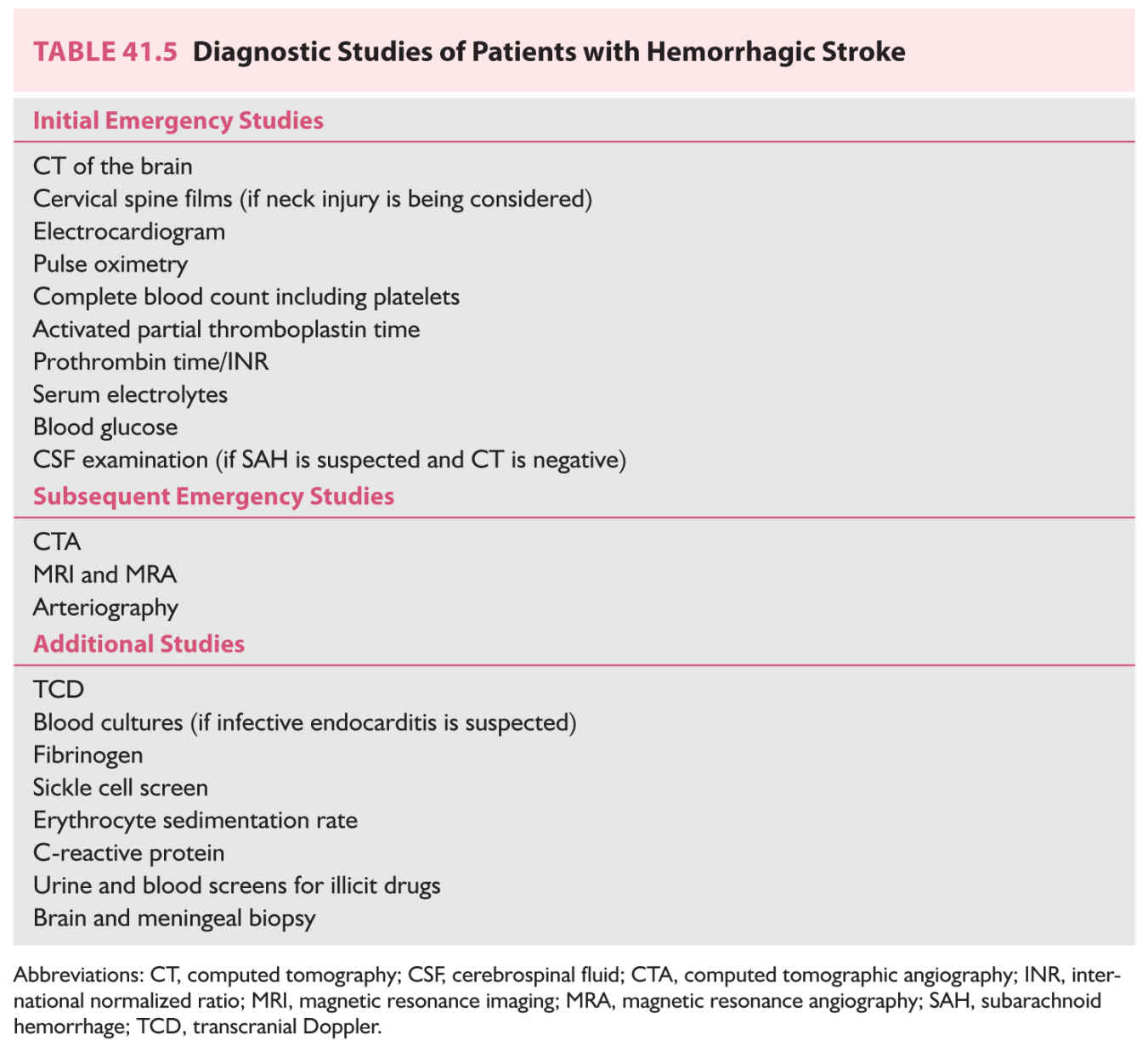
The goals of the emergency evaluation are (1) to confirm hemorrhage as the cause of the neurologic symptoms, (2) to screen for acute neurologic or medical complications, and (3) to determine the most likely explanation for the bleeding event (Table 41.5). These persons are at high risk for a variety of serious neurologic complications including brain edema, increased ICP, hydrocephalus, and seizures. Medical complications include myocardial ischemia, cardiac arrhythmias, neurogenic pulmonary edema, respiratory abnormalities, gastrointestinal bleeding, and fluid and electrolyte disturbances. Before they are moved, patients with possible craniocerebral trauma should have imaging of the cervical spine.
A. Computed tomography (CT) of the brain (Fig. 41.1). The single most important diagnostic study is CT of the brain. It is relatively inexpensive, noninvasive, quick, easy to perform, and available at most hospitals. The yield of CT is extraordinarily high. When it is performed within 24 hours of onset, blood density can be detected in almost 100% of patients with ICH and approximately 95% of patients with SAH. The presence of a “spot sign” on an early, contrast-enhanced CT study/computed tomographic angiography (CTA) predicts those patients who are likely to have enlargement of their hematoma. Sequential CT studies obtained during the first hours of the illness often show enlargement of a hematoma presumably as a result of continued bleeding. Besides detecting the blood in the subarachnoid space in most cases of SAH, the pattern and severity of the bleeding seen on CT help predict subsequent complications including vasospasm. With aneurysmal SAH, CT will demonstrate subarachnoid blood primarily in the basal cisterns near the Circle of Willis. The location of the bleeding also points to the likely site of the ruptured aneurysm. Isolated subarachnoid blood found over the convexity may represent a hypertensive crisis associated with cerebral vasoconstriction syndrome or hypertensive encephalopathy. Blood located around the brainstem is a feature of perimesencephalic SAH. CT may miss a small collection of subarachnoid blood in a patient with a mild hemorrhage or if the bleeding is in the posterior fossa. If the CT is performed several days after the event, the yield of the test in detecting the bleeding decreases considerably because the blood may have been reabsorbed. CT also detects early complications of hemorrhage including hydrocephalus, mass effect, brain edema, and herniation. Contrast-enhanced CT may detect an underlying vascular malformation or aneurysm. CTA is a valuable method to image the vasculature at the base of the brain, and, in particular, to examine the anatomic relationships of saccular aneurysms. CTA also may be used to monitor for the development of vasospasm that complicates SAH.
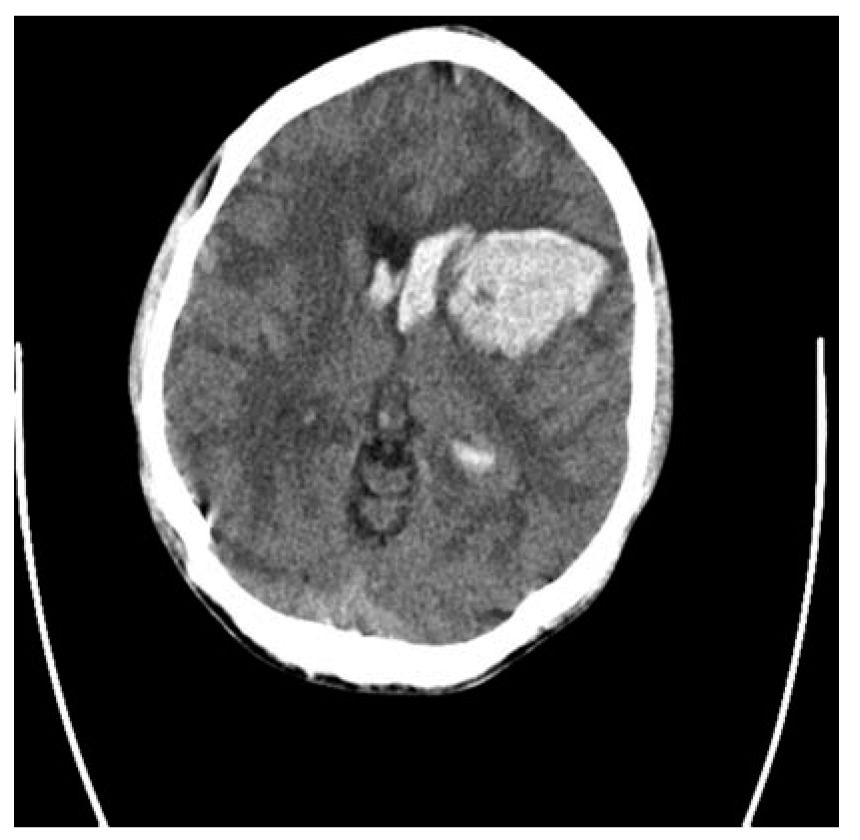
FIGURE 41.1 CT of the brain demonstrates a large putaminal hemorrhage with secondary IVH and shift of midline structures. In addition, the sulci are obliterated, which provides indirect evidence of mass effect and increased ICP. CT, computed tomography; ICP, intracranial pressure; IVH, intraventricular hemorrhage.
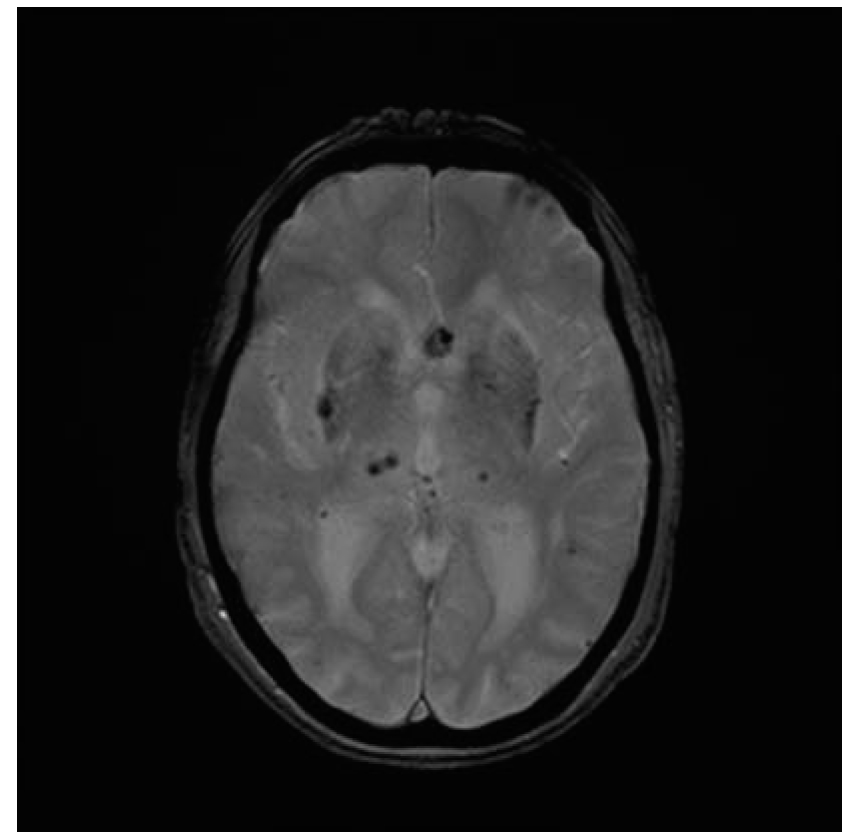
FIGURE 41.2 Gradient echo sequence MRI of the brain reveals several small microhemorrhages in the basal ganglia and the thalamus bilaterally. In addition, an aneurysm of the anterior cerebral artery is also visualized. MRI, magnetic resonance imaging.
B. MRI. Multisequence MRI is as sensitive as CT in the detection of intracranial bleeding (Fig. 41.2). It also provides additional data about the most likely cause of bleeding. Although more expensive and not as widely available as CT, MRI does have advantages. Because of time-linked changes in the responses of iron in the hematomas of varying ages, MRI provides information about the age of the hemorrhagic lesion. A gradient echo sequence is useful in detecting microhemorrhages, which are often found among patients with amyloid angiopathy or long-standing hypertension. Abnormal flow voids may be found with vascular malformations. MRI is also useful in screening for tumors or cerebral venous sinus thrombosis. Magnetic resonance venography is used to screen for venous sinus thrombosis and magnetic resonance angiography (MRA) also may be performed to detect aneurysms or vascular malformations.
C. Examination of the cerebrospinal fluid. The role of the examination of the cerebrospinal fluid (CSF) has declined with the advent of modern brain imaging. If CT or MRI shows a hemorrhage, there is little reason to evaluate the CSF for the presence of blood. Conversely, CSF examination remains important if SAH is suspected and CT does not show blood. The most common situation is among alert patients with mild signs. The risk of neurologic complications, including herniation, following lumbar puncture (LP) is low in an alert patient who does not have focal signs and has no mass found on CT. Determining whether the source of blood in the CSF is from an intracranial hemorrhage or is secondary to the procedure itself (blood tap) may be difficult. Bloody CSF from SAH usually does not clear in sequentially collected tubes. Xanthochromia (yellowing) of the CSF supernatant after centrifugation is the most reliable sign that the blood truly represents SAH but it can take up to 12 hours to appear. A physician should immediately centrifuge a blood CSF specimen to check for xanthochromia because a delay of several hours may give a false-positive result. The CSF findings evolve over subsequent days and if the LP is delayed, only slightly yellow fluid, an elevated CSF protein, or an inflammatory response that mimics viral meningitis may be found.
D. Arteriography. The role of catheter cerebral arteriography has changed with the increased use of CTA and MRA to detect vascular malformations or aneurysms (Fig. 41.3). It is usually not needed for evaluation of older patients with a history of hypertension and a hemorrhage located in the thalamus or basal ganglia. It may be useful in detecting small aneurysms or vasculitis. It may be used to screen for vasospasm after SAH. Currently, the most common scenario for arteriography is its performance as a preliminary step for endovascular treatment of an aneurysm or vascular malformation. It also may be combined with interventions to treat vasospasm.
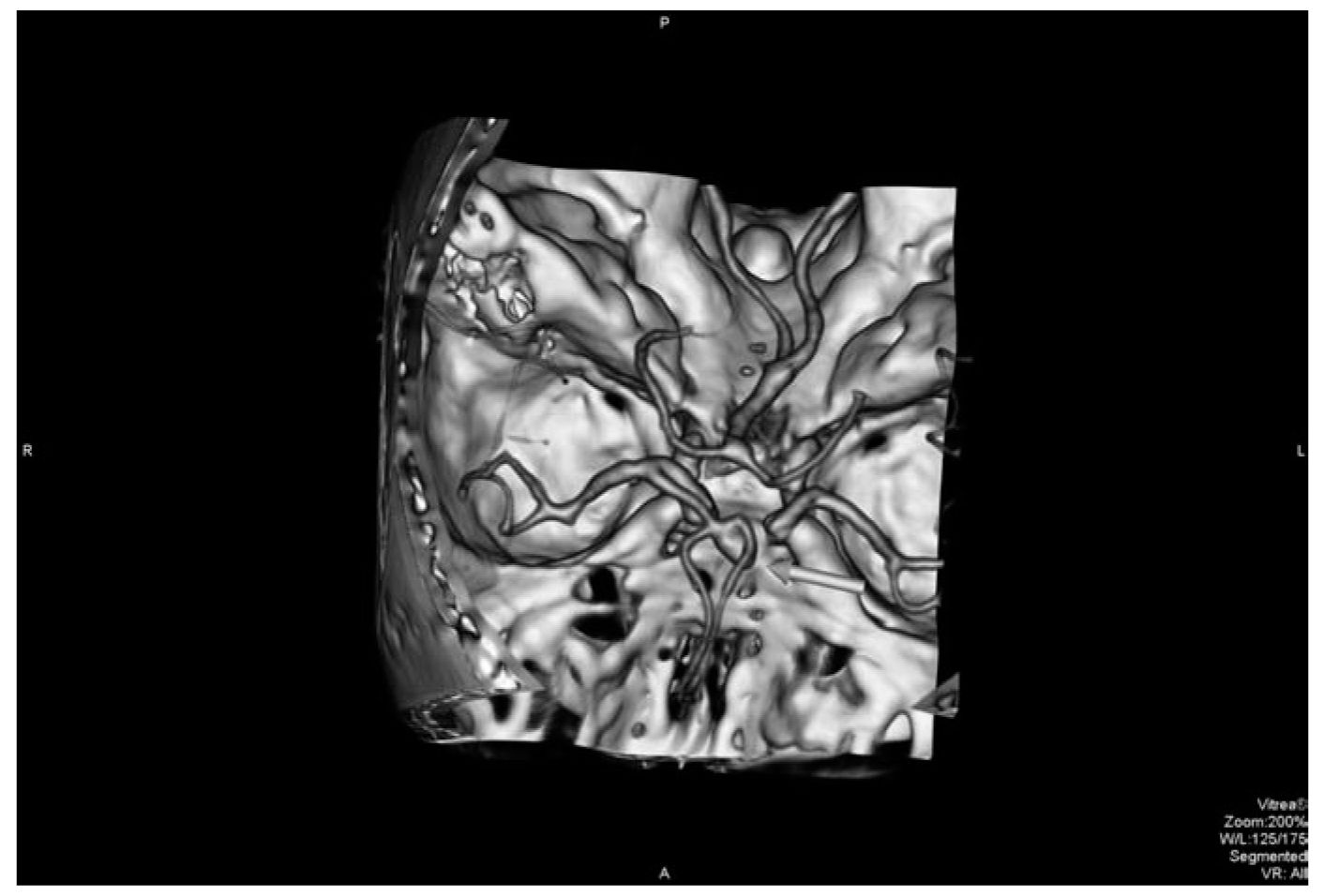
FIGURE 41.3 CTA visualizes an anterior communicating artery aneurysm (arrow) in a patient with an SAH. CTA, computed tomographic angiography; SAH, subarachnoid hemorrhage. (See color plates.)
E. Other diagnostic studies. Patients should have coagulation studies to screen for a possible explanation for the hemorrhage. In addition, the results of these tests will influence acute surgical management decisions. Electrocardiography and hematologic and biochemistry studies are part of the evaluation to screen for medical complications or comorbid illnesses. Transcranial Doppler (TCD) has been used to monitor for the development of cerebral vasospasm following SAH.
TREATMENT
A. Prevention. Prevention is the most cost-effective strategy for treatment of patients at high risk for hemorrhagic stroke. Administration of antihypertensive agents to patients with acute or chronic elevations of blood pressure may lower the risk of an ICH. Abstinence from use of vasoconstricting drugs of abuse also lessens the risk of hemorrhage. Careful administration of thrombolytic, anticoagulant, and antiplatelet agents also decreases the risk of an intracranial hemorrhage. Management of inherited or acquired disorders of coagulation, including, when appropriate, the administration of clotting factors, is also effective in lowering the risk of a hemorrhagic stroke. Management of an unruptured vascular malformation or aneurysm is often recommended. Choices include endovascular or direct surgical occlusion of larger aneurysms. Surgical resection, focused irradiation, or endovascular therapies alone or in combination are potential therapies for treatment of an AVM. However, recent evidence suggests that in some cases the risks of the interventions, which include stroke, may be greater than the likelihood of a hemorrhage. Unfortunately, in many cases, the initial presentation of the underlying disease is the hemorrhagic stroke and no specific preventive therapy could have been done.
B. Referral and admission. Patients with hemorrhagic stroke are critically ill. Inpatient care is warranted because of the life-threatening nature of the illness and because of the high risk of serious neurologic and medical complications. The facilities and personnel required for successful management of these patients are not usually available in most community hospitals. Admission to a specialized treatment facility that has monitoring capabilities and intensive unit level care is needed. The high-risk nature of hemorrhagic stroke means that most patients are referred to comprehensive stroke centers that have critical care, vascular neurology, and neurosurgical expertise.
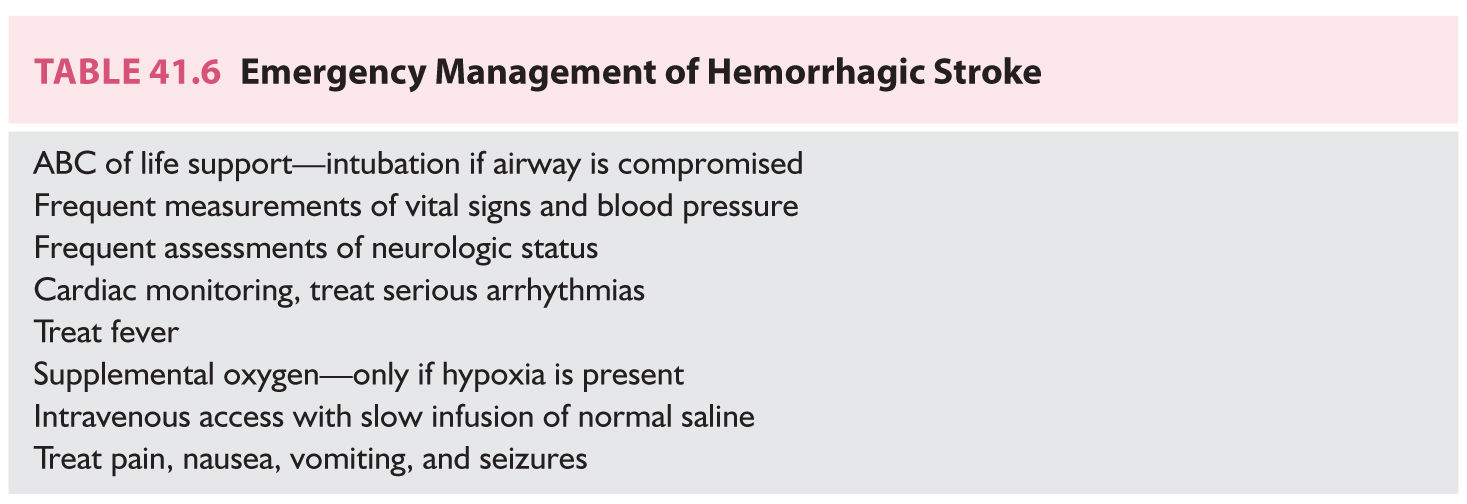
C. General management. Measures to control or prevent acute medical and neurologic complications are part of emergency general management (Table 41.6). Endotracheal intubation and ventilatory assistance may be needed. Cardiac monitoring to detect arrhythmias and frequent measurements of vital signs and the neurologic status should be performed. Hypoxic patients receive supplemental oxygen. Fever should be addressed. Access for intravenous administration of medications and fluids is needed and normal saline is infused slowly. Hypotonic solutions are generally avoided because of their potential effects on the formation of brain edema and because patients may have hyponatremia. Hypoglycemia and hyperglycemia should be promptly managed. Symptoms such as headache, nausea, vomiting, or agitation warrant treatment. Patients who have had seizures receive antiepileptic drugs but the prophylactic use of these agents to patients who have not had seizures is controversial. Because of concerns that the stress of a seizure may induce rebleeding, many physicians prophylactically prescribe anticonvulsants to patients with ruptured aneurysms.
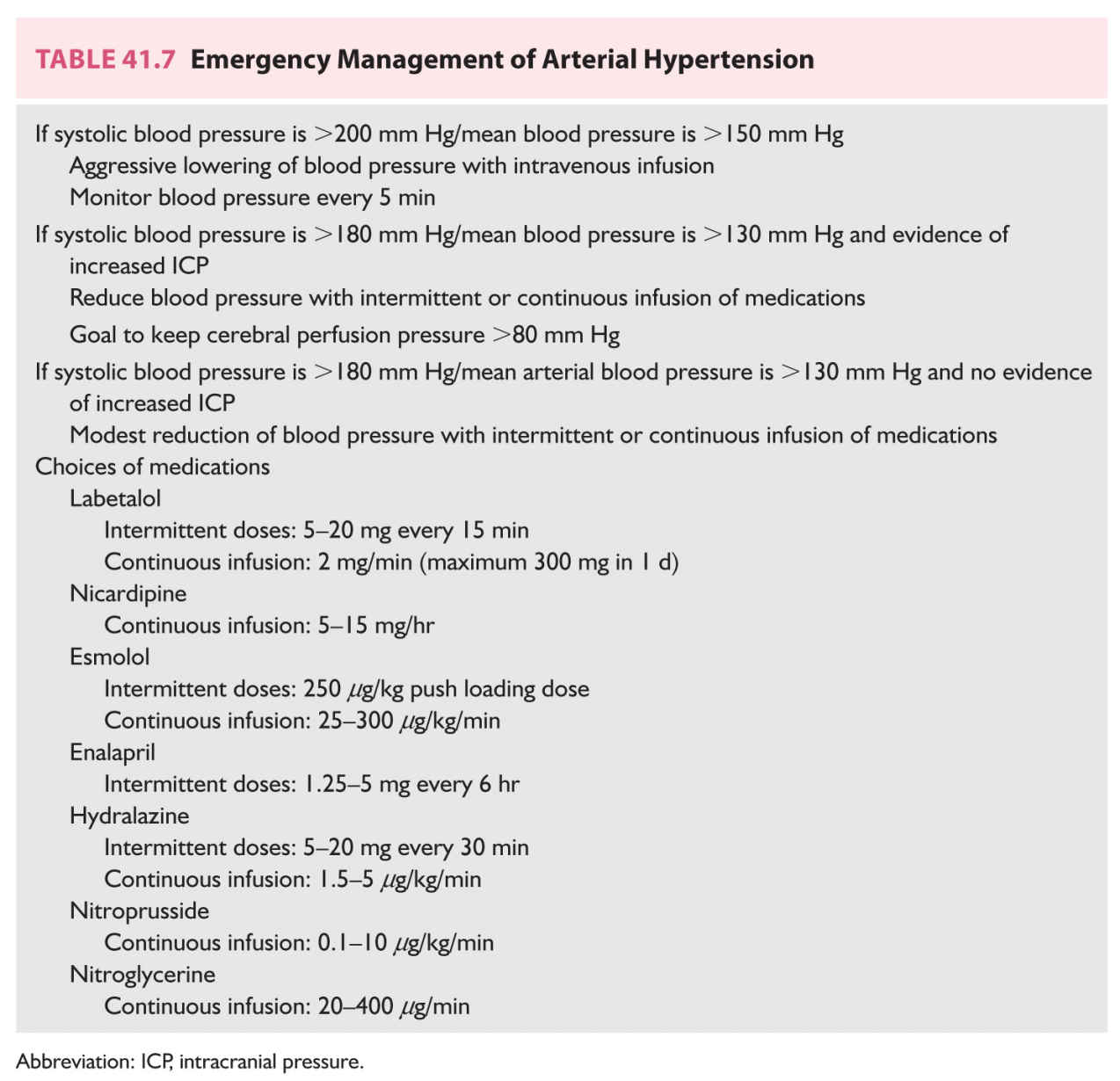
D. Treatment of arterial hypertension. Elevations in blood pressure may worsen intracranial bleeding, promote brain edema formation, or induce recurrent rupture of an aneurysm. Most patients’ blood pressures decline as pain, agitation, seizures, and vomiting are controlled. The level of blood pressure that mandates medical management is not known but the usual goal is a systolic blood pressure <160 mm Hg. Because elevations in ICP may induce compensatory increases in blood pressure, special caution is exercised when lowering the blood pressure among patients with intracranial hypertension. Responses to antihypertensive agents may be exaggerated. Short-acting parenteral medications are preferred because the dosages may be titrated in response to the patient’s blood pressure and neurologic status (Table 41.7). In addition, caution should be exercised when using sodium nitroprusside because its secondary cerebral vasodilatory actions may worsen ICP.
E. Halting continued bleeding. Many patients have growth of the hematoma during the first few hours of the illness. This is particularly true for patients who have hemorrhages complicating the use of oral antithrombotic agents. Recombinant factor VIIa did not improve outcomes among patients with spontaneous hemorrhages. Patients with bleeding secondary to warfarin are being treated with four-factor prothrombin complex concentrates; the INR can be reversed in a manner of minutes. These patients also receive vitamin K. There is no specific agent to reverse the anticoagulant effects of the new oral factor-X inhibitors although antidotes are being developed. A recent study demonstrated the utility of the monoclonal antibody, idarucizumab, in reversing the effects of dabigatran in patients with serious bleeding or who needed emergency surgical interventions.
F. Increased ICP and brain edema. Increased ICP is an important complication of major intracranial hemorrhages; it results from the mass effect of the hematoma, brain edema, or acute hydrocephalus. Monitoring for ICP may be used to guide treatment; most commonly, this involves placement of an intraventricular catheter. Impaired venous return, fever, agitation, hypoxia, hypercapnia, and hypoventilation may exacerbate ICP and should be managed. Early measures include elevation of the head of the bed, modest fluid restriction, and avoidance of potentially hypo-osmolar fluids. Corticosteroids are not useful. Intubation and hyperventilation are instituted if a patient’s condition is deteriorating. Hyperosmolar therapies, most commonly hypertonic saline or mannitol, are prescribed to treat seriously elevated ICP.
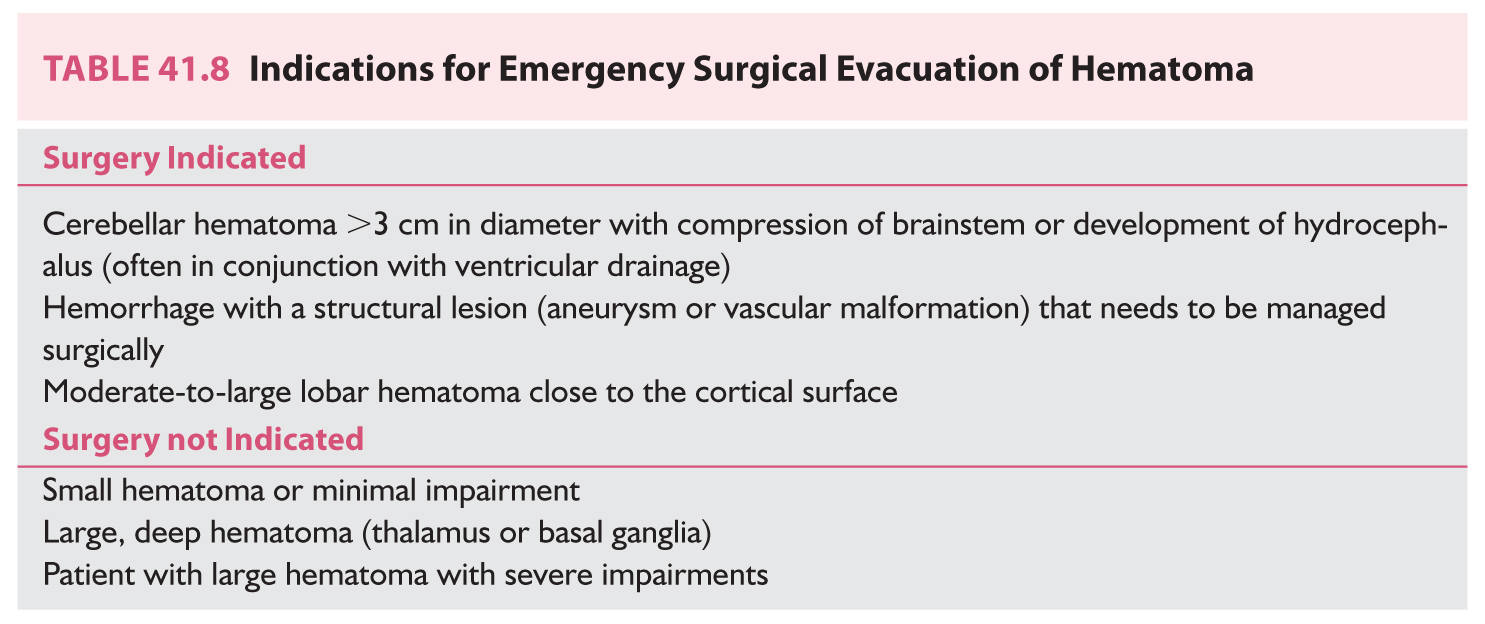
G. Emergency surgical management. An early decision involves the need for surgical evacuation of a hematoma that is causing mass effects or increased ICP. A ventricular catheter may be used to drain CSF if the patient has secondary hydrocephalus; it can be used to lower ICP and may forestall the need for a craniectomy. Removal of a large, mass-producing hematoma may be a life-saving procedure. Choices include an open craniotomy, minimally invasive surgery, or endoscopic aspiration. Administration of thrombolytic agents to aspiration of an IVH or ICH appears promising. The location and size of the hematoma and the patient’s neurologic status, course, and general health affect a decision about surgery (Table 41.8). In general, surgery is recommended for the treatment of a large cerebellar hematoma that is compressing the brainstem or obstructing CSF outflow. Patients with lobar hematomas within 1 cm of the cortical surface may be considered for surgery. However, patients with small-to-moderate-sized hematomas of the cerebral hemisphere do not need surgery. Patients with hemorrhages arising in the thalamus or basal ganglia are not usually surgically treated. There is no evidence that surgical evacuation of such hematomas improves outcomes. There are no data about the utility of decompressive craniectomy in improving outcomes after intracranial hemorrhage. Administration of rtPA into an intracerebral hematoma or IVH is being studied. The rationale for the use of the thrombolytic agent is that it may promote resolution of the thrombus, which in turn augments aspiration of the hematoma. The doses of recombinant tissue plasminogen activator (rtPA) used in this situation are very low.
H. General inpatient care. The components of emergency management are continued after admission to the hospital. Given the seriousness of the illness, most patients are admitted to an intensive care unit setting for monitoring and treatment. Careful nursing care and regular assessments of the patient’s condition and vital signs are continued. To decrease the risk of aspiration and pneumonia, liquids and food are not given by mouth until the patient’s ability to swallow safely has been confirmed. If the patient cannot take fluids by mouth, a nasogastric tube should be placed. Care in avoiding pulmonary complications is part of general management. Modest fluid restriction is continued for patients who have a large hematoma. Management of intravenous fluids should emphasize maintaining normal electrolyte levels. Some patients will have hyponatremia secondary to cerebral salt wasting and these patients will need hypertonic fluids. Incontinence often mandates placement of an indwelling bladder catheter. Because of the risk of urinary tract infection, the catheter should be removed as quickly as possible. Because bedridden patients have a high risk of deep venous thrombosis that may lead to pulmonary embolism, they may be given enoxaparin or heparin after the risk of continued bleeding has been abated. If a risk for recurrent bleeding is high and the patient has deep vein thrombosis, a filter can be placed in the inferior vena cava. When the patient’s condition has stabilized, increased activity, mobilization, and rehabilitation may begin.
CAUSE-SPECIFIC TREATMENT
Management of the cause of bleeding is a key component of the overall treatment for patients with intracranial hemorrhage. Treatment of bleeding diatheses and arterial hypertension already has been discussed.
A. Vascular malformations. Patients with a ruptured vascular malformation, most commonly an AVM, may be treated to prevent a second hemorrhage. Because the risk of early recurrent bleeding is relatively low, treatment is usually delayed until the hematoma has reabsorbed. Options for treatment include surgical resection, endovascular administration of vascular occlusive materials, and focused irradiation; these interventions may be done alone or in combination. Decisions for treatment are influenced by the size and location of the malformation and the number and caliber of feeding arteries. Lesions in neurologically eloquent areas and those that are deep in the brain may not be surgically approachable. A high-flow malformation is also a problem because a postoperative state of hyperperfusion leading to hemorrhage or severe brain edema that may follow resection. A staged series including both endovascular and surgical procedures may be performed. Small and deep vascular malformations may be treated with focused, high-intensity radiation that leads to secondary fibrosis and gradual occlusion of the vessels. Some patients with very large vascular malformations may not be treatable with any of the currently available modalities.
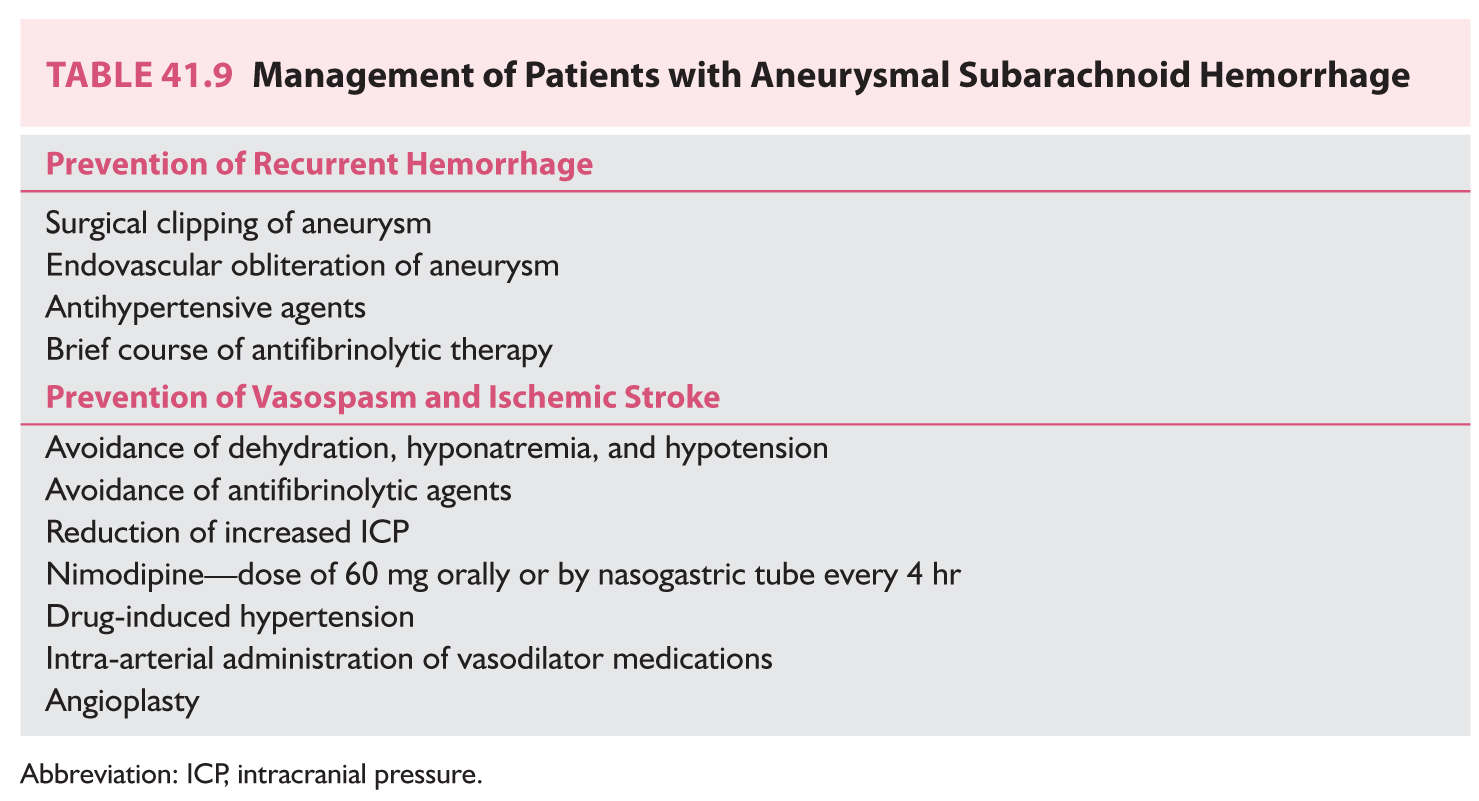
B. Saccular aneurysms. Patients with ruptured saccular aneurysms are vulnerable to both recurrent hemorrhage and ischemic stroke (Table 41.9). Rebleeding is a largely fatal event that peaks during the first 24 hours, when the risk is approximately 4%. The overall risk of recurrent hemorrhage during the first 10 days approaches 20%. The symptoms of rebleeding are similar to those of the initial hemorrhage; another CT will show increased intracranial hemorrhage. The most effective measures to forestall rebleeding are direct surgical obliteration of the aneurysm by clipping or endovascular occlusion of the aneurysm by inserting coils. In general, the goal is to treat the patient as quickly as possible. The selection of endovascular or direct surgical treatment is influenced by the patient’s condition, the location of the aneurysm, the presence of serious comorbid diseases, and the presence of vasospasm. Because the morbidity of endovascular treatment is less than that accompanying surgical clipping, the use of endovascular procedures has expanded. Lowering blood pressure or the administration of antifibrinolytic agents to prevent recurrent bleeding can be prescribed but they may be associated with an increased risk of ischemia. Vasospasm is an arterial process that occurs almost exclusively in association with aneurysmal SAH. It is most likely to occur among patients with extensive subarachnoid blood found on CT. The progressive arterial narrowing peaks at 7 to 10 days after SAH and thereafter gradually abates. The arterial narrowing leads to hypoperfusion, which causes brain ischemia. The symptoms of vasospasm are worsening headache, altered consciousness, and focal neurologic signs that may wax and wane. TCD ultrasonography may detect alterations in flow velocities in major arteries before the clinical signs appear. The arterial narrowing also may be detected on sequential CTA studies. Nimodipine is initiated after the diagnosis of SAH because it does improve outcomes. The medication presumably lessens the ischemic effects of the vasospasm; it is unclear whether the medication has any effect on the arterial process. Drug-induced hypertension is prescribed to patients in whom ischemic symptoms develop. Although no controlled trials have shown efficacy of this regimen, several studies report success. The regimen is rigorous and requires monitoring. Besides inducing myocardial ischemia, the regimen could promote recurrent aneurysmal rupture if the aneurysm has not been treated. Hypervolemic hemodilution, which was combined with the hypertension, is no longer used. Arteriography, which may be used to confirm the vasospasm, may be complemented by angioplasty or endovascular administration of medications to treat the arteriopathy.
• ICH accounts for approximately 20% of all strokes. It is the primary alternative diagnosis to ischemic stroke.
• ICH remains a highly fatal disease with death commonly because of brain injury itself or secondary to increased ICP.
• A broad spectrum of diseases cause intracranial hemorrhage. The most common are hypertension, saccular aneurysms, vascular malformations, and bleeding disorders. Determination of the cause of the hemorrhage is crucial in both prognosis and treatment.
• With the use of modern brain imaging, the diagnosis of ICH is becoming easier. In addition, vascular imaging is performed to look for the underlying blood vessel disease.
• Initial management involves general emergency care, treatment of the increased ICP, and measures aimed at the hematoma, including surgery. Other surgical procedures are done to treat the causes of hemorrhage such as intracranial aneurysms or vascular malformations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree