Fig. 10.1
(a) Preoperative T2-weighted MRI of a patient with Chiari I malformation. The image demonstrates a small posterior fossa, a slight caudal displacement of the tentorial insertion (arrow) and a syringomyelia at C2–C3. (b) The postoperative scan shows the decompression at the foramen magnum (arrow) with resolution of the syrinx
Apart from a small posterior fossa volume, additional bony anomalies are common in Chiari I malformation and may involve the articulations at the craniocervical junction. Assimilations of the atlas to the occiput, basilar invaginations or Klippel-Feil syndromes may also be encountered (Kagawa et al. 2006; Tubbs et al. 2011; Smith et al. 2010). It is important to recognise that the compression of neural structures and CSF flow obstruction are localised at the foramen magnum in all variants of Chiari I malformation although these may not be the only mechanisms responsible for the patients’ symptoms. Instabilities of the craniocervical junction or upper cervical spine are important features to recognise in a significant proportion of Chiari I patients.
10.1.2 Basilar Invagination
Basilar invagination is defined as a protrusion of the odontoid peg into the foramen magnum (Fig. 10.2). A line between the posterior rim of the foramen and the hard palate constitutes Chamberlain’s line . If the odontoid crosses this line for more than 2.5 mm, this is considered pathological. Basilar invagination may be associated with osteogenesis imperfecta , Hajdu-Cheney syndrome , Paget’s disease (Menezes 2008b), Marfan’s syndrome (Hobbs et al. 1997), Down’s syndrome (Menezes 2008a) or rheumatoid arthritis (Krauss et al. 2010). The congenital form is caused by bony anomalies of the clivus , occipital bone , atlas and upper cervical vertebrae. The result of this altered anatomy is a gradual upward shifting of the upper cervical vertebrae towards the foramen magnum. The C1/2 intervertebral joints appear to play a major role for this effect as distraction of these joints may reverse the ventral compression by the odontoid peg (Jian et al. 2010; Goel 2004).
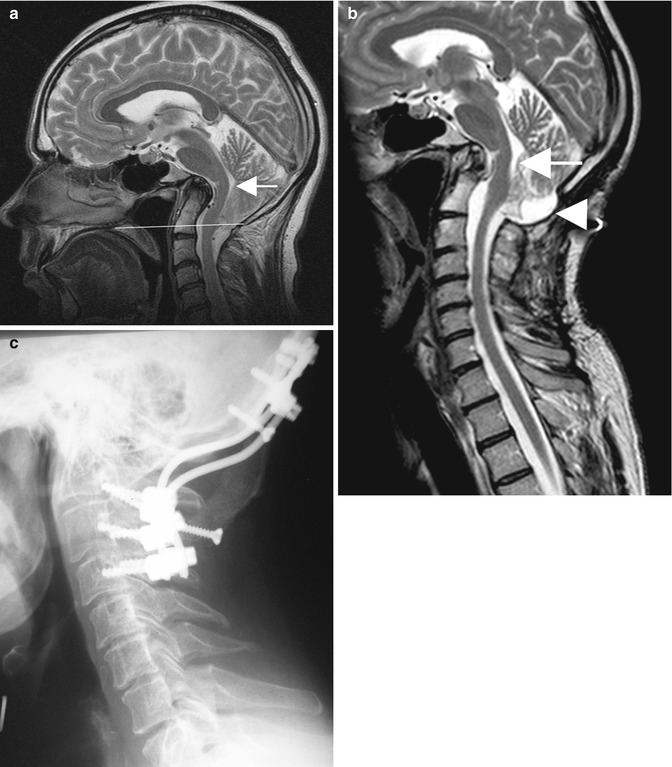

Fig. 10.2
(a) The preoperative T2-weighted MRI shows a Chiari I malformation associated with a profound basilar invagination and compression of the brainstem (arrow). The odontoid extends far above Chamberlain’s line (white horizontal line). (b) The postoperative MRI demonstrates the result of a combined decompression with transoral resection of the dens (arrow) and posterior decompression (arrowhead) and fusion. (c) The postoperative lateral radiograph demonstrates the position of all implants and a good sagittal profile of the cervical spine
10.1.3 Chiari II Malformation
In Chiari II malformation, the compression and cerebrospinal fluid flow obstruction occur in the upper spinal canal and not at the level of the foramen magnum. In contrast with Chiari I, the foramen magnum is enlarged in Chiari II, and the tonsils, the vermis and the brainstem are all herniated into the cervical canal (Fig. 10.3). Almost all patients with this malformation will also have a spinal myelomeningocoele . The pathophysiology of this malformation has been elegantly described by McLone and Knepper. Due to the spinal myelomeningocoele, CSF drains in utero into the amniotic fluid, resulting in a low intracranial pressure, which then inhibits the formation of a normally sized posterior fossa. The growth of the brain finally leads to herniation of cerebellar tonsils, vermis and brainstem into the spinal canal (McLone and Knepper 1989). As in the majority of Chiari I patients, the size of the skull forces the brain to grow towards the spinal canal in Chiari II. The major difference is the timing: in Chiari I, this effect takes place after birth, whereas in Chiari II the major pathological changes occur before birth with much graver consequences. Support for this hypothesis comes from results of intrauterine operations on myelomeningoceles before the 26th week of gestation. If the spinal dysraphism could be closed successfully, then no Chiari II malformation developed (Danzer et al. 2011; Tulipan et al. 1999).
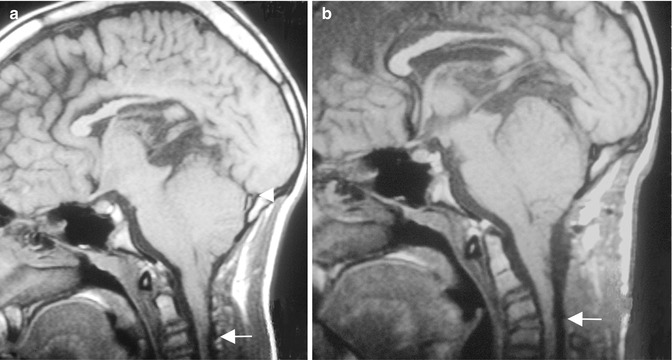

Fig. 10.3
(a) Preoperative T1-weighted MRI of a 14-year-old boy with a Chiari II malformation, demonstrating the enlarged foramen magnum with cerebellar tonsillar herniation to C3 (arrow). The tentorium inserts close to the posterior margin of the foramen magnum (arrowhead). (b) The postoperative image demonstrates the decompression of the upper cervical spine and discloses a kyphotic angulation at C3/4 (arrow)
10.1.4 Foramen Magnum Arachnoiditis
Foramen magnum arachnoiditis is the only pathology at the craniocervical junction associated with syringomyelia without there being additional compression of brainstem or spinal cord (Fig. 10.4). Arachnoiditis at this level may be related to a previous episode of meningitis or trauma or other causes of haemorrhage (Klekamp et al. 2002; Appleby et al. 1969).
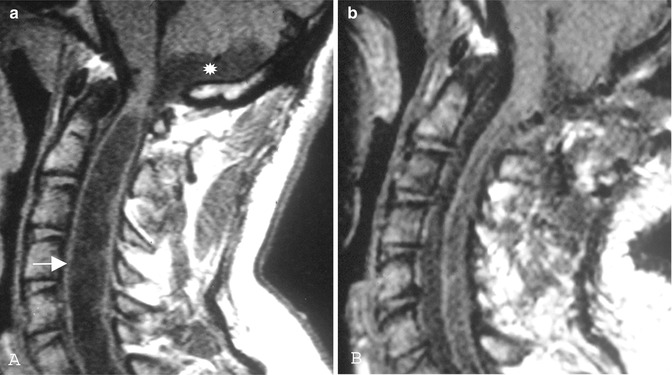

Fig. 10.4
(a) Preoperative T1-weighted MRI indicates foramen magnum arachnoiditis related to birth injury in a 29-year-old man with progressive tetraparesis. There is not a cerebellar tonsillar herniation, but there is an arachnoid pouch close to the foramen magnum and a significant syrinx (arrow). (b) The postoperative MRI shows a collapse of the arachnoid pouch and the syrinx
10.2 Neuroradiology
The diagnosis of Chiari I malformation should be straightforward these days, given the wide accessibility of magnetic resonance imaging (MRI) in western countries. To what extent tonsillar herniation may sometimes be seen as physiological and how neuroradiological criteria should be defined are, however, still a matter of controversy. A tonsillar herniation of more than 5 mm is widely considered pathological in adults (Aboulezz et al. 1985), but in young children, cerebellar growth causes a physiological herniation of the cerebellar tonsils . Conversely, in old age, atrophy of the brain may lead to tonsillar ascent (Mikulis et al. 1992). Tonsillar descent of less than 5 mm does not exclude the diagnosis of a Chiari I malformation (Milhorat et al. 1999), and in doubtful cases cardiac-gated cine MRI is very helpful to demonstrate a CSF flow obstruction and a clinically relevant herniation (Haughton et al. 2003; Panigrahi et al. 2004; Ellenbogen et al. 2000; Tubbs et al. 2007; Milhorat et al. 1999; Hofkes et al. 2007). Likewise neurophysiological examinations (Henriques Filho and Pratesi 2006) and neuro-otological evaluations (Kumar et al. 2002) have been proposed, as means of providing evidence of compression of the medulla oblongata or spinal cord.
In patients with Chiari I malformation, the radiological examination should include more than simply defining how far the tonsils are descended into the spinal canal. It is also important to consider the bony anatomy of the craniocervical junction. For example, is there evidence of anterior compression by the odontoid peg, i.e. basilar invagination (Fig. 10.2)? Is the atlas assimilated to the occiput? Are cervical segments fused, i.e. Klippel-Feil syndrome? If these anomalies are present, then instability of the craniocervical junction must be ruled out with flexion and extension studies , using either conventional radiography or CT imaging. The latter has the advantage of being able to visualise each of the different joints, in multiple planes and in both flexion and extension. In addition, sagittal and coronal reconstructions are particularly useful.
For all craniocervical pathologies, ventricular sizes should be evaluated. In Chiari I malformation, overt hydrocephalus is rare, but some degree of ventricular enlargement is not uncommon and was observed in 9 % of the author’s series. With Chiari II malformation, on the other hand, hydrocephalus is almost ubiquitous. In fact, it is uncommon for the neurosurgeon to encounter such a patient who has not already been managed with a ventricular shunt. If a Chiari II patient is evaluated because of new neurological symptoms, the first priority must be to assess the function of a previously implanted ventricular shunt. This requires a comparison of recent and old CT or MRI images, to look for changes of ventricular size that may indicate under- or over-drainage.
One important aspect for surgical planning in Chiari patients is the position of the tentorium , in relation to foramen magnum and the external occipital protuberance . Normally, the tentorium will insert at the level of this protuberance, and this indicates the position of the large intradural sinuses , such as the transverse sinus and the confluence of sinuses. In Chiari I patients, this tentorial insertion may be shifted by a centimetre or more towards the foramen magnum. When planning an occipital craniectomy, this must be taken into account and is even more important with regard to the dural incision. In Chiari II malformation, the foramen magnum is widened, and the tentorium usually inserts at the level of or very close to the foramen magnum (Fig. 10.3). For this reason, the suboccipital dura should not be incised in patients with a Chiari II malformation.
In patients with primary foramen magnum arachnoiditis, bony anomalies will not be apparent. The diagnosis is made by MRI and, in the absence of tonsillar herniation and brainstem or cervical cord compression, will require a cine MRI to demonstrate a CSF flow obstruction in the foramen magnum area. Such obstruction often involves the fourth ventricle exit foramina as well, and ventriculomegaly is common, being observed in half of the author’s series of foramen magnum arachnoiditis. Another common feature of primary foramen magnum arachnoiditis is the formation of arachnoid cysts or pouches in the posterior fossa (Fig. 10.4).
Finally, the presence or absence of an associated syringomyelia or syringobulbia should be demonstrated as well as the entire extension of the cavity, to rule out additional abnormalities such as a tethered cord.
10.3 Clinical Presentation
With all craniocervical pathologies, clinical symptoms may evolve from different pathophysiological components. These may include hydrocephalus , compression of the brainstem and spinal cord, craniocervical instability , disturbances of brainstem or spinal cord blood flow, tethering mechanisms related to chronic arachnoiditis and CSF flow obstruction leading to syringomyelia. Clinical and radiological examinations therefore need to be analysed carefully, to identify the appropriate targets for treatment.
Considering all Chiari patients, it is interesting to note that the presentation is very much dependent on the age of the patient. In early childhood below age 2 years, signs of brainstem compression predominate, with apnoeic spells, cyanosis attacks and swallowing problems, whereas in later childhood scoliosis becomes the most common presenting sign. What are regarded as the more typical clinical features of a Chiari I malformation – occipital headaches , gait ataxia , sensory disturbances and motor weakness – are uncommon in children and are observed predominantly in adults (Rauzzino and Oakes 1995; Menezes et al. 2005) (Table 10.1). This age-related clinical profile can be explained by the postnatal growth of the cerebellum. At birth, most parts of the brain have reached about a third of their adult volume, but the cerebellum is the smallest part of the central nervous system, with just 15 % of its adult volume at this time; presumably, this serves to protect the brainstem during delivery . The adult volume of the cerebellum is reached late in the second year of life, indicating that the cerebellar volume increases by a factor of seven in that period (Klekamp et al. 1989). Therefore, if a Chiari malformation does become symptomatic before 2 years of age, dramatic presentations with respiratory problems are likely to be observed, something unknown in adult patients. Once the cerebellum is fully grown, the clinical course tends to be less dramatic and is characterised by slow progression.
Table 10.1
Preoperative neurological symptoms (author’s series)
Group | Occipital pain (%) | Neuropathic pain (%) | Hyperaesthesia (%) | Gait (%) | Motor power (%) | Sphincter function (%) | Swallowing difficulties (%) |
|---|---|---|---|---|---|---|---|
Chiari I | 79 | 50 | 71 | 62 | 40 | 16 | 20 |
Chiari II | 25 | 19 | 64 | 100 | 67 | 92 | 22 |
FMA | 74 | 39 | 83 | 78 | 83 | 48 | 15 |
As with every rule, however, there are exceptions. Minor traumas may cause acute symptoms in formerly asymptomatic patients with Chiari I malformation (Yarbrough et al. 2011; Murano and Rella 2006). In extremely rare cases, even sudden deaths have been reported (Wolf et al. 1998; Stephany et al. 2008; Agrawal 2008; Yoshikawa 2003). This raises questions as to whether asymptomatic children with Chiari I malformations should be allowed to participate in sport activities and whether prophylactic surgery for such patients is warranted. Chiari decompressions certainly cannot be considered to be no-risk procedures and are associated with a mortality of about 1 %. Given the fact that severe neurological deficits after minor trauma are extremely rare – no such instance was reported by a single patient in the author’s series of more than 600 patients – it appears reasonable to leave decisions regarding timing of surgery and which sport activities can be pursued to the patients and their parents, without pressing them one way or the other.
In the author’s series, patients with Chiari I presented with an average age of 43 ± 16 years, while those with Chiari II tended to be younger, at 17 ± 13 years. Compared to patients with a Chiari I malformation, patients with foramen magnum arachnoiditis presented at a similar age (37 ± 10 years) but with more severe neurological deficits (Table 10.1). Interestingly, this appeared not to hold for swallowing dysfunctions, which were more common with Chiari malformations, despite the significant scarring at the medulla oblongata level, which was present in all patients with foramen magnum arachnoiditis. The length of history was significantly longer for patients with arachnoiditis compared to Chiari patients (101 ± 96 months, compared to 71 ± 106 months for Chiari I and 26 ± 39 months for Chiari II).
10.4 Management
10.4.1 Chiari I Malformation
For all Chiari I patients, treatment of symptomatic hydrocephalus should be prioritised. Endoscopic third ventriculostomy is now preferred to traditional ventriculoperitoneal shunting as the first option for CSF diversion whenever patients present with clinical signs of raised intracranial pressure (Massimi et al. 2011).
Management options for arachnoid cysts include resection or fenestration (Fig. 10.5). Insertion of cyst shunts is not considered by the author due to their high failure rate. Treatment for Chiari I malformations due to solid masses requires tumour resection with foramen magnum duraplasty (Fig. 10.6).
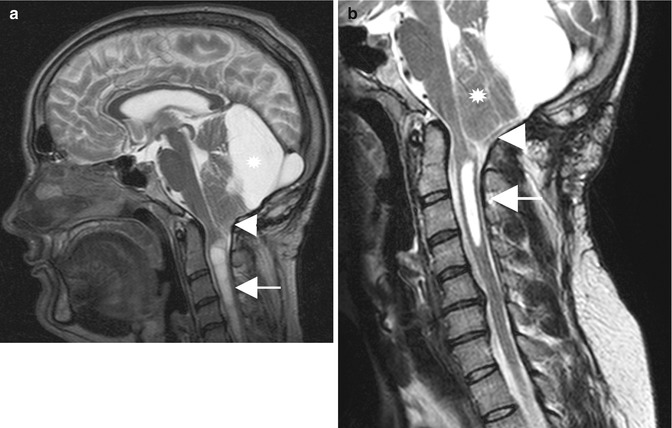
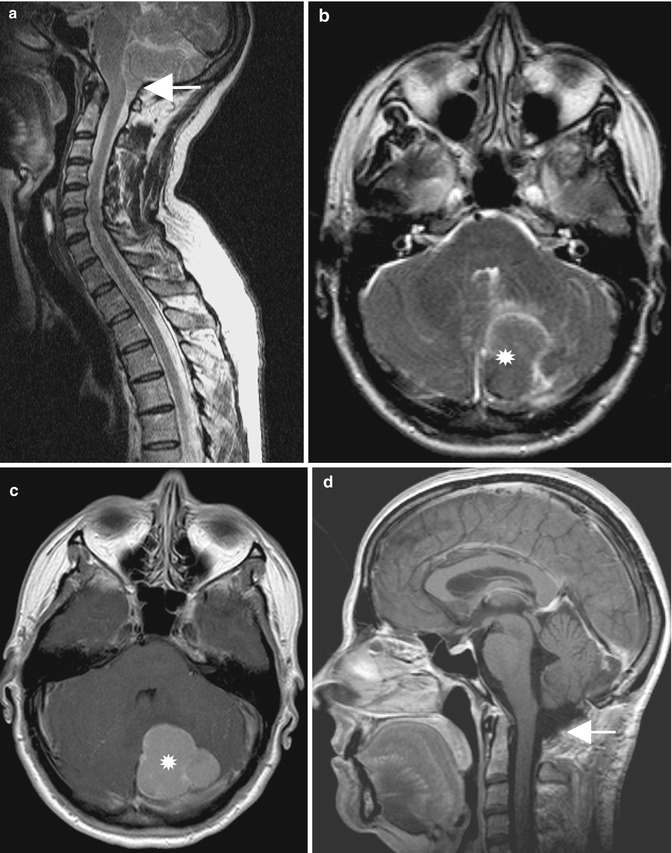

Fig. 10.5
(a) Preoperative T2-weighted MRI of a patient with syringomyelia (arrow) and a cerebellar tonsillar herniation (arrowhead) secondary to a large retrocerebellar arachnoid cyst . (b) The postoperative scan demonstrates a decrease in syrinx size (arrow) and a decompression at the foramen magnum (arrowhead). Despite large fenestration of the arachnoid cyst, the vermis has not changed its shape

Fig. 10.6
(a) This preoperative T2-weighted MRI seems to demonstrate a typical Chiari I malformation (arrow). (b) The axial T2-weighted image indicates a lesion underneath the tentorium on the left side . (c) This turned out to be a large meningioma in T1 after contrast . (d) The postoperative T1-weighted scan with gadolinium shows a complete tumour removal and a decompressed foramen magnum (arrow)
For Chiari I hindbrain hernias, there is general agreement that surgical treatment should be reserved for symptomatic patients. In children, however, it may be unclear which complaints are linked to the malformation. It can also be a challenge to differentiate between physiological and pathological tonsillar descent, as revealed by MRI. In the absence of neurological symptoms or progressive scoliosis , the author does not recommend decompression for children with a Chiari I malformation, unless they demonstrate the typical occipital headache provoked by Valsalva-like manoeuvres, such as sneezing or coughing.
With all patients, it should be borne in mind that not every headache is due to tonsillar herniation, even when such an abnormality is present. Nor does the presence of syringomyelia constitute an indication for surgery in its own right, in children or adults alike. When neurological symptoms are present, however, surgery should be recommended. As a general rule, progression of neurological symptoms occurs more rapidly than does enlargement of an underlying syrinx. The author has not observed enlargement of a syrinx in an asymptomatic patient with a Chiari I malformation.
Even though foramen magnum decompression is widely accepted as the treatment of choice for Chiari I malformation, there is no general agreement on how this operation should be performed (Schijman and Steinbok 2004; Haroun et al. 2000). Gardner’s original operation consisted of a wide craniectomy of the posterior fossa and opening of the 4th ventricle in order to plug the obex with a piece of muscle. The dura was left open. He reported 5 mortalities after 74 such procedures (Gardner 1965). Similar mortality rates, as well as significant morbidity caused by manipulations at the obex (Williams 1978), led other surgeons to modify this operation. Guided by modern imaging techniques such as MRI in the 1980s and modern CT scanners, the anatomy of patients with Chiari I malformation could be studied in much more detail than was previously possible. This led to less invasive procedures, such as leaving the arachnoid intact after dural opening (Logue and Edwards 1981), incising only the outer dural layer (Gambardella et al. 1998) or even restricting the operation to a purely bony decompression (James and Brant 2002). It should, of course, be the intention of every neurosurgeon to restrict any operation to its essential requirements in order to limit surgical morbidity, complications and discomfort for the patient but, at the same time, to do so without compromising the beneficial effects of the procedure.
The following account of a surgical technique describes the author’s preferred method, as do subsequent accounts of operative techniques in this chapter. It should be noted, however, that there are many variations, and it is true to say that the only manoeuvre that all methods have in common is removal of bone from the occipital squama.
Surgery is performed in prone position. The decompression is limited to a foramen magnum decompression of 3–4 cm, together with removal of the posterior arch of the atlas. The atlanto-occipital membrane is coagulated and dissected off the dura, which is then incised in a Y-shape, under the microscope, and held open by sutures. Care should be taken to leave the arachnoid intact in order not to pull on and tear underlying bridging veins or small blood vessels supplying the spinal cord, brainstem or cerebellum. Leaving the arachnoid intact at this stage also avoids contamination of the subarachnoid cisterns with blood. Venous sinuses may be encountered upon opening the dura, in the midline or at the foramen magnum, and these will require suturing. The arachnoid should be examined for evidence of scar formation or adhesions to the cerebellum, brainstem or spinal cord. The arachnoid is then incised, starting below the cerebellar tonsils and continuing to an extent that allows these structures to be spread apart for inspection of the foramen of Magendie. For this purpose, the cerebellar tonsils are coagulated at their tips and medially. Resection of tonsils is advised against as this may risk injury to important blood vessels such as the posterior inferior cerebellar artery (PICA). If Magendie is patent and no arachnoid adhesions are detectable elsewhere, then no further intradural dissections need be performed. If the foramen is obstructed, then it should be opened by sharp dissection. In patients with severe arachnoid scarring, dissection will need to create at least a communication between the cranial and the spinal subarachnoid channels. In such instances, it may not always be possible to open Magendie without risking injury to important structures, such as the PICA. The dissection should not, in these circumstances, be carried out laterally, to avoid injury to perforating vessels of brainstem or spinal cord. A duraplasty is then inserted using alloplastic material. To avoid formation of adhesions between nervous tissue and the duraplasty or suture line, the graft is lifted off the cord by tenting sutures, which are fixed to muscle attachments laterally. Finally, the wound is closed, paying particular attention to the muscular layer in order to avoid CSF fistulas . Postoperatively, all patients should be supervised on the intensive care unit for at least 24 h before returning to the normal ward.
10.4.2 Basilar Invagination
In patients with additional basilar invagination , a combination of ventral and dorsal compression may be associated with instability of the craniocervical junction. These pathophysiological components may not, however, be relevant in all affected patients. Of a group of 53 patients with basilar invagination in the author’s series, 35 were managed surgically. In 16 patients, there was neither a ventral compression by the odontoid nor craniocervical instability. These patients were managed with foramen magnum decompression for their Chiari malformation as the only procedure. In another 10 of the 35 patients, who demonstrated no clinical signs of ventral compression by the odontoid such as caudal cranial nerve deficits but either radiological evidence of craniocervical instability or assimilation of the atlas to the occiput, foramen magnum decompression was combined with craniocervical stabilisation. In the remaining 9 patients, ventral compression of the medulla oblongata had caused caudal cranial nerve dysfunctions. These patients underwent transoral resection of the odontoid , followed by posterior decompression and craniocervical fusion (Fig. 10.2) (Klekamp and Samii 2001).
Whenever the position of the odontoid leads to brainstem compression, the key elements of surgical treatment are distraction of the C1/2 intervertebral joints and C1/2 fusion. This distraction may reverse the ventral compression to a degree that no additional transoral resection of the odontoid is required. In the author’s series, the decision for a transoral decompression was based on clinical signs of caudal cranial nerve deficits in the presence of compression of the medulla by the odontoid. Whether a transoral resection of the odontoid is obsolete (Goel and Shah 2009) or still required for patients with substantial and irreducible ventral compression (Smith et al. 2010) remains a controversial issue.
For craniocervical stabilisations, the implants need to be adjusted carefully to the abnormal anatomy. Precise planning is required to allow their safe fixation at the occipital bone as well as the allocation of the bone graft despite of the craniectomy required for foramen magnum decompression. All implants need to be covered completely by the muscular layer during closure, without too much strain being placed on soft tissues; otherwise, local discomfort or even CSF fistulas may result.
10.4.3 Chiari II Malformation
If clinical signs of hydrocephalus are present in Chiari II patients, treatment of the increased intracranial pressure has always the first priority. Endoscopic third ventriculostomy is again an optional alternative to ventriculoperitoneal shunts (Elgamal et al. 2011). In the literature, it has been stated in two large series that if the hydrocephalus has been managed successfully, then only a small minority of Chiari II patients require a decompression (Talamonti and Zella 2011; Rauzzino and Oakes 1995).
If one considers the pathophysiological considerations put forward by McLone (McLone and Knepper 1989) and the positive results obtained following intrauterine operations on foetuses (Danzer et al. 2011; Tulipan et al. 1999), Chiari II malformations are potentially preventable or reversible, if the decompression is carried out early enough in symptomatic patients with a sufficiently treated hydrocephalus. A number of studies have reported a benefit if decompression for Chiari II is performed as soon as neurological symptoms begin (Rauzzino and Oakes 1995; Teo et al. 1997; Pollack et al. 1992, 1996). Yet, there appears to be very little scientific evidence to support routine use of this approach for symptomatic infants (Tubbs and Oakes 2004). This may reflect the fact that, once severe brainstem dysfunctions are present, a decompression may not reverse established neurological deficits (Kirsch et al. 1968).
In the author’s series, 42 patients presented with a Chiari II malformation. Surgical management was only recommended in 13 of these patients, when there was no evidence for ventricular shunt malfunction but a clear history of progressive brainstem or cervical cord dysfunction (Charney et al. 1987; Kirsch et al. 1968). Six were under 2 years of age, presenting with signs of central dysregulation. Three patients presented between 5 and 14 years of age (Fig. 10.3) and the remainder in adulthood, with progressive upper extremity dysfunction.
When operating for Chiari II malformation, the decompression must be undertaken at the spinal levels corresponding to the tonsillar descent, rather than at the foramen magnum, which is enlarged in this entity. Patients are operated in prone position. The exposure extends from the foramen magnum to the lowest lamina covering the herniated tonsils. After laminectomy of these segments, the atlanto-occipital membrane should be coagulated and dissected off the dura. The dural incision then starts at the level of the foramen magnum and extends over all levels involved. Then the arachnoid is opened. Dissection should be limited strictly to the midline in order to avoid injury to perforating vessels or caudal cranial nerves. It should be borne in mind that the brainstem is displaced caudally in these patients, taking with it important structures such as the PICA or caudal cranial nerves. As outlined for Chiari I operations, it is also desirable to open the foramen of Magendie in Chiari II patients, although this should be undertaken only if it can be performed safely. Coagulation of tonsils , which is a safe technique in Chiari I to gain access to Magendie , is not recommended in Chiari II as these are very often tightly adhered to the underlying brainstem and any such manoeuvre carries considerable risks . Arachnoid dissection should concentrate on creating a passage between the intracranial and spinal subarachnoid channels. Once that is achieved, a duraplasty should be inserted. As described for Chiari I surgery (above), the duraplasty is lifted off the cord by tenting sutures.
Laminectomies in this patient group carry a significant risk of producing postoperative kyphosis or swan-neck deformities (Lam et al. 2009) (Fig. 10.3). They should therefore be combined with posterior fusion , which can be achieved elegantly with lateral mass screws (Fig. 10.7).
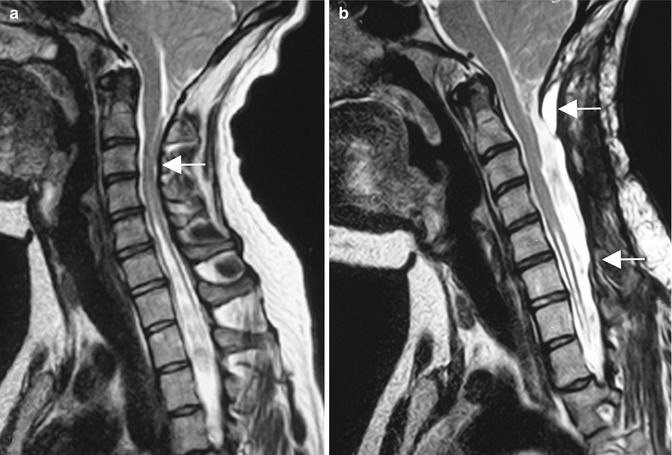

Fig. 10.7
(a) Preoperative T2-weighted MRI of an adult patient with Chiari II malformation presenting with progressive weakness of both hands. The image shows the tonsillar descent to C3 (arrow) with osteochondrosis and stenosis of the cervical spine at that level. (b) After bony decompression and duraplasty from C1 down to C5 (arrows), the enlarged subarachnoid space and decrease of the syrinx are apparent. To prevent a kyphotic deformity, a stabilisation was added with lateral mass screws. Postoperatively, she regained function in her hands
10.4.4 Foramen Magnum Arachnoiditis
In the author’s series, ventriculomegaly was more common in foramen magnum arachnoiditis compared to Chiari I malformations (52 % vs. 8.6 %). This implies that if a borderline tonsillar herniation is associated with ventricular dilatation, then arachnoiditis at the foramen magnum may well be present. Depending on the extent and severity of the arachnoiditis, surgical management may require CSF diversion, in addition to foramen magnum decompression.
When foramen magnum arachnoiditis is not diagnosed preoperatively, its presence, extent and severity must be determined after dural opening. Dural opening has to respect the arachnoid layer in such cases in order to avoid surgical morbidity related to vascular injuries in particular. Thereafter, the aim of surgery is to create a free CSF passage between the intracranial and spinal subarachnoid spaces. It is not advisable to dissect all arachnoid scarring off the spinal cord, medulla oblongata and cerebellar tonsils. On the contrary, such attempts are risky and simply lead to new adhesions and scar tissue formation. In foramen magnum arachnoiditis, the foramen of Magendie is always obstructed. A decision must therefore be made, intraoperatively, as to whether or not it can be opened safely. If important structures such as the PICAs are embedded in arachnoid scar tissue, then the risk may be too high. Some authors recommend placement of small catheters to provide an outflow for the 4th ventricle (Abe et al. 1995). However, even with ultrasound guidance, this remains a very risky manoeuvre, and the author has encountered patients with severe neurological deficits as a consequence of malpositioned catheters in this region. A safer strategy is to leave the foramen Magendie closed and place a supraventricular shunt. Indeed, because of such concerns, some surgeons have previously recommended limiting the management of foramen magnum arachnoiditis to ventricular shunting (Appleby et al. 1969).
10.5 Surgical Results and Complications
10.5.1 Chiari I Malformation
Two published analyses of foramen magnum decompressions gave extensive overviews on complications encountered during and immediately after surgery as well as delayed postoperative problems but did not provide any data (Menezes 1991; Mazzola and Fried 2003). When it comes to quantifying such complications, analysis of the literature shows enormous variations in reported figures. Not all studies seem to use the same standards when complications are analysed; how else can one explain figures of 2.4 % (Tubbs et al. 2011) and 37 % (Zerah 1999), both from series of more than 100 children, undergoing the same decompression procedure, carried out in respected institutions?
Tables 10.2 and 10.3 provide a literature overview, comparing complication rates, syrinx reduction rates and the frequency of surgical revisions , for different surgical decompression techniques for Chiari I. These include decompressions involving only bone removal, those with incision of the outer dural layer, those opening the dura completely and those where additional arachnoid dissection was performed.
Table 10.2
Literature review of the results of decompressions for Chiari I malformations, without dural opening
Authors | Group | N | Follow-up period | Peri- and postoperative complications | Syrinx size reduced | Recurrences/deaths |
|---|---|---|---|---|---|---|
Bony decompression only | ||||||
James and Brant (2002) | C | 4 | Not reported | None | Not reported | None |
Hayhurst et al. (2008) | A, C | 16 | 43 monthsa | 27 %a | 87 %a | 25 %/none |
McGirt et al. (2008b) | C | 116 | 25 months | 1 % | Not reported | 7.8 %/none |
Mutchnik et al. (2010) | A, C | 56 | Not reported | None | Not reported | 12.5 %/none |
Yilmaz et al. (2011) | A | 24 | Not reported | 8.3 % | 91.1 % | 9.5 %/not reported |
Bony decompression with outer dural decompression | ||||||
Gambardella et al. (1998) | A | 8 | Not reported | Not reported | 88 % | 12.5 %/none |
Munshi et al. (2000) | A | 11 | Not reported | 10 % | 50 % | 18.2 %/none |
Navarro et al. (2004) | C | 71 | 28 monthsa | 5.6 % | 65.7 %a | 10.8 %/none |
Limonadi and Selden (2004) | C | 12 | 15.7 months | Not reported | No syrinx cases | Not reported |
Caldarelli et al. (2007) | C | 30 | 55 months | Not reported | 50 % | 6.7 %/none |
Chauvet et al. (2009) | A | 11 | 18 months | 9.1 % | 80 % | None |
Table 10.3
Literature review of the results of decompressions for Chiari I malformations, with dural opening
Authors | Group | N | Follow-up period | Peri- and postoperative complications | Syrinx size reduced | Recurrences/deaths |
|---|---|---|---|---|---|---|
Bony decompression with arachnoid left intact and dura left open | ||||||
Di Lorenzo et al. (1995) | A | 20 | 29 months | Not reported | 100 % | 15 %/none |
Zerah (1999) | C | 79 | Not reported | 37 %a | 69 %a | 1.6 %a/none |
Bony decompression with arachnoid left intact and duraplasty | ||||||
Guyotat et al. (1998) | A, C | 42 | 39 months | Not reported | 58 % | 50 %/4.7 % |
Zerah (1999) | C | 79 | Not reported | 37 %a | 69 %a | 1.6 %a/none |
Munshi et al. (2000) | A, C | 34 | Not reported | 42 % | 100 % | None/none |
Limonadi and Selden (2004) | C | 12 | 14.8 months | 8.3 % | 100 % | Not reported |
Navarro et al. (2004) | C | 24 | 28 monthsa | 42.1 % | 65.7 %a | 4.2 %/none |
Galarza et al. (2007) | C | 20 | 21 monthsa | 8.3 %a | 64a | Not reported |
McGirt et al. (2008b) | C | 140 | 29 months | 3 % | Not reported | 7.1 %/None |
Wetjen et al. (2008) | A | 29 | 36 months | Not reported | 100 % | Not reported |
Hoffman and Souweidane (2008) | A, C | 40 | 11.4 months | CSF related 2.5 % | Not reported | 5 %/None |
Attenello et al. (2008) | C | 49 | 41 months | 10 %a | 55 %a | 10.2 %a/none |
A | 44 | 48 months | 20.5 % | 60 %
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||




