FIGURE 15-1 Hypertensive pregnancy disorders: classification and diagnostic criteria. (From Garovic VD. The role of angiogenic factors in the prediction and diagnosis of preeclampsia superimposed on chronic hypertension. Hypertension 2012;59:555–557.)
BLOOD PRESSURE MONITORING DURING PREGNANCY
Office Readings
The vagaries of office BP readings, noted in Chapter 2, obviously are in play during pregnancy. However, errors in BP measurement have even more immediate importance, possibly leading to overtreatment of some incorrectly diagnosed as hypertensive, but even more harm in those with elevated pressures that presage PE who are not recognized.
The various guidelines described in Chapter 2 should be followed in measuring the BP during pregnancy. Initially, BP should be taken in both arms since a difference of 10 mm Hg or more was found in 8.3% of pregnant women (Poon et al., 2008). The arm with the higher reading should be used.
In a meta-analysis of 34 studies involving 60,599 women, Cnossen et al. (2008) found that the most accurate predictor of PE in those considered to be at low risk was a mean BP of 90 mm Hg, or higher, during either the first or second trimester. For women considered to be at high risk, the best predictor was a diastolic BP of 75 mm Hg, or higher, during weeks 13 to 20 of gestation.
Home Readings
In their review of BP measurements during pregnancy, Chancellor and Thorp (2008) conclude
that pregnant women might benefit from bypassing clinic assessment and its inherent inaccuracies. Home blood pressure recording devices are inexpensive and overcome some of the problems in the clinic setting. In our experience, women are likely to take the time and energy to standardize the environment and follow protocols consistently. Armed with these data, they will be able to provide their clinicians with more accurate information about their trends in blood pressure across pregnancy.
Until home BP monitoring becomes more widely used, most women will be monitored by occasional readings in the office. The definitions given earlier in this chapter are based on office readings, with the caveat that unless the woman is in serious trouble, repeated readings be taken before diagnosing any form of hypertension.
Ambulatory Monitoring
In normal pregnancy, lower pressures are found in the midportion, with rises to nonpregnant levels near term (Macdonald-Wallis et al., 2012) (Fig. 15-2). The data in Figure 15-2 are from repeated (mean of 14) office readings on 13,016 women followed throughout pregnancy. A longitudinal, prospective study in 403 women who started with normal casual BP during the first trimester and who had repeated ABPM recordings made every 4 weeks found a highly significant higher level of both daytime and sleep BPs was noted during the first trimester in the 128 women who later developed GH and the 40 who later developed PE, in comparison to the 235 who remained normotensive (Hermida et al., 2004). These data suggest that ABPM may provide the best tool now available for the early identification of women who are predisposed to GH or PE. Moreover, those who developed PE had a greater blunting of the nighttime dipping of BP during the third trimester as compared to those who only had GH, so the procedure may provide additional warning of the impending development of PE (Ayala & Hermida, 2013).
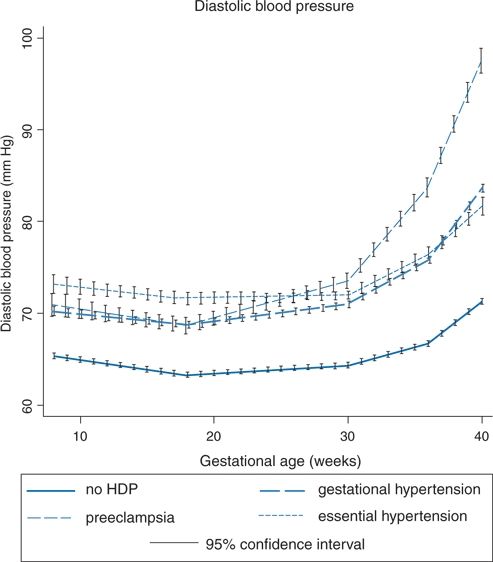
FIGURE 15-2 Average trajectories of diastolic blood pressures by hypertensive disorders of pregnancy in the unadjusted joint model (N = 13,016). (From Macdonald-Wallis C, Lawlor DA, Fraser A, et al. Blood pressure change in normotensive, gestational hypertensive, preeclamptic, and essential hypertensive pregnancies. Hypertension 2012;59:1241–1248.)
Pulse Wave Analysis
As described in Chapters 2 and 3, pulse wave analysis is being used increasingly as a noninvasive way to measure arterial compliance and central BP. Unfortunately, the procedure did not prognosticate the development of PE better than brachial blood pressure readings and a maternal risk factor profile (Carty et al., 2013).
CIRCULATORY CHANGES IN NORMAL PREGNANCY
Serial measurements begun before conception have portrayed the evolution of the profound changes of normal pregnancy that are apparent as early as 6 to 7 weeks (Mahendru et al., 2012). In 10 women, 9 nulliparous, who were studied before and repeatedly during pregnancy, significant decreases in systemic vascular resistance resulted in a fall in BP, despite an increase in cardiac output (CO), even before placentation (Chapman et al., 1998) (Fig. 15-3). As the authors note, “Therefore, it is likely that maternal factors, possibly related to changes in ovarian function or extended function of the corpora lutea, are responsible for the initial peripheral vasodilation found in human pregnancy” (Chapman et al., 1998).
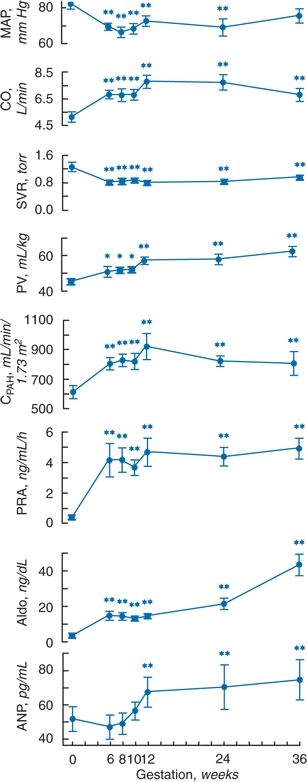
FIGURE 15-3 Changes in mean arterial pressure (MAP), cardiac output (CO), systemic vascular resistance (SVR), plasma volume (PV), effective renal plasma flow measured by para-aminohippurate clearance (CPAH), plasma renin activity (PRA), plasma aldosterone (Aldo), and atrial natriuretic peptide (ANP) in 10 women studied in the midfollicular phase of the menstrual cycles and at weeks 6, 8, 10, 12, 24, and 36 of gestation. p < 0.05; **p < 0.01. (Adapted from Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 1998;54:2056–2063.)
The progressive rises in plasma and blood volume are likely adaptations, via renal sodium retention, to the vasodilation and fall in BP. The low pressure and underfilled circulation provoke an increase in renin secretion (Watanabe et al., 2012) and, secondarily, a rise in aldosterone levels aided by stimulation from vascular endothelial growth factor (VEGF) (Gennari-Moser et al., 2013). The somewhat later rise in plasma atrial natriuretic peptide is evidence that, despite the increased blood volume, the central circulation is not overexpanded. As a consequence of renal vasodilation, renal plasma flow and glomerular filtration increase and renal vascular resistance decreases.
At the same time as various forces raise levels of renin–angiotensin–aldosterone, normal pregnancy brings forth numerous mechanisms including nitric oxide, carbon monoxide, and hydrogen sulfide (Holwerda et al., 2013) to protect the circulations of both mother and fetus from the intense vasoconstriction, volume retention, and potassium wastage that high angiotensin II and aldosterone levels would ordinarily engender (Gennari-Moser et al., 2014).
The large amounts of potent mineralocorticoids would be expected to increase sodium reabsorption at the cost of progressive renal wastage of potassium, yet pregnant women are normokalemic, likely the result of the high level of progesterone, which acts as an aldosterone antagonist (Brown et al., 1986).
Rang et al. (2008) showed that normal pregnancy is a low BP state associated with marked vasodilation that reduces peripheral resistance, along with an expanded fluid volume that increases CO. Renal blood flow is markedly increased, and the renin–aldosterone system is activated but with blunted effects. C-type natriuretic peptide levels remain low in normal pregnancies (Reid et al., 2014).
PREECLAMPSIA
Most PE becomes manifest near the end of pregnancy with few severe fetal or maternal complications. In a smaller percentage, 10% to 30%, PE becomes manifest earlier, before the 34th week, with frequent intrauterine growth restriction (IUGR) and more maternal complications (Sibai, 2008b). Valensise et al. (2008) characterized the maternal hemodynamics of 75 women with early PE and 32 with late PE, all initially studied by uterine artery Doppler ultrasonography at 24 weeks’ gestation. Figure 15-4 summarizes their findings that have also been observed by other investigators (Khaw et al., 2008; Mei et al., 2008; Rang et al., 2008).
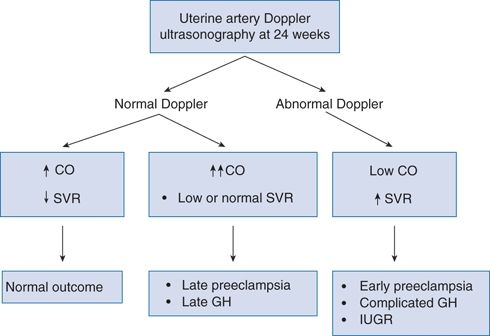
FIGURE 15-4 Uteroplacental and maternal hemodynamics at 24 weeks and subsequent pregnancy outcome. CO, cardiac output; SVR, systemic vascular resistance; GH, gestational hypertension; IUGR, intrauterine growth restriction. (Modified from Valensise H, Vasapollo B, Gagliardi G, et al. Early and late preeclampsia: Two different maternal hemodynamic states in the latent phase of the disease. Hypertension 2008;52:873–880.)
From these studies, Valensise et al. (2008) conclude that “early PE is placental mediated, linked to defective trophoblast invasion with a high percentage of altered uterine artery Doppler.”
Problems in Diagnosing Preeclampsia
There are problems inherent in diagnosing a syndrome of unknown cause on the basis of only highly nonspecific signs. For example, the BP in normal pregnancy usually falls during the first and middle trimesters, only to return toward the prepregnant level during the third trimester. Because women with chronic hypertension have an even greater fall early on, their subsequent rise in later pregnancy may give the appearance of the onset of PE. In addition, those with chronic hypertension may have previously unrecognized proteinuria: If seen only after midterm, the diagnosis of PE looks even more certain.
The distinction between chronic hypertension and PE is of more than academic interest: In the former, hypertension is the major problem, whereas “preeclampsia is more than hypertension; it is a systemic syndrome and several of its ‘nonhypertensive’ complications can be life threatening even when blood pressure elevations are quite mild” (National HBPEP Working Group, 2000). The management of the hypertension and the pregnancy, as well as the prognosis for future pregnancies, varies with the diagnosis. The bottom line, however, is clear: When in doubt, diagnose PE and institute its treatment, because even mild PE may rapidly progress. If PE is correctly diagnosed and managed, the risks to both mother and baby can be largely overcome (Lindheimer et al., 2009).
Obviously, women should be evaluated before conception. If hypertensive, therapy should be revised to exclude ACEIs, ARBs, or direct renin inhibitors. If renal disease is present, more careful observation is needed since there is an increased risk of adverse outcomes (Vikse, 2013). Foreknowledge of BP and renal function is essential.
Epidemiology
The causes of PE must explain the following features, as delineated by Chesley (1985):
- It occurs almost exclusively during the first pregnancy; nulliparas are six to eight times more susceptible than are multiparas. Older primigravidas are more susceptible than are younger.
- It occurs more frequently in those with multiple fetuses, hydatidiform mole, or diabetes.
- The incidence increases as term approaches; it is unusual before the end of the second trimester.
- The features of the syndrome are hypertension, edema, proteinuria, and, when advanced, convulsions and coma.
- There is characteristic hepatic and renal pathology.
- The syndrome has a hereditary tendency; in the families of women who had PE, the syndrome developed in 25% of their daughters and granddaughters but in only 6% of their daughters-in-law.
- It rapidly disappears when the pregnancy is terminated.
As listed in Table 15-1, multiple risk factors for PE have been identified (Dekker & Sibai, 2001). What remains elusive is the initiating mechanism, the trigger that sets off the oftentimes explosive course of this strange malady that disturbs up to 1 in 10 first pregnancies and is rarely seen again. The difficulty in identifying a specific cause is related to the likely presence of multiple mechanisms and, until recently, the lack of an experimental model for PE. Another difficulty is the inability to identify the early pathogenetic mechanisms, which remain invisible to current technology. Most of what is recognized are relatively late manifestations of a process that is initiated much earlier. As will be noted, no clinically useful screening test to predict the development of PE has been available until now, despite the recognition of circulating angiogenic (Levine et al., 2004) and antiangiogenic factors (Levine et al., 2006).
TABLE 15-1 Risk Factors for PE

Modified from Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet 2001;357:209–215.
Pathophysiology
As stated by Delles and Freel (2013):
The pathogenesis of preeclampsia is thought to be triggered by excessive maternal immune response to the developing trophoblast leading to placental oxidative stress, hypoperfusion, and hypoxia, and the subsequent release of placental factors causing widespread endothelial dysfunction in the maternal circulation. In turn, the resulting placental hypoperfusion is probably further aggravated by reduced activity of growth factors, including vascular endothelial growth factor (VEGF), placental growth factor, and transforming growth factor β 1. Antiangiogenic factors, such as soluble fms-like tyrosine kinase-1 (sFlt-1), a soluble form of the VEGF-1 receptor, and soluble endoglin, a part of the transforming growth factor β receptor, are released from apoptotic trophoblast cells and interact with and reduce the systemic and local levels of VEGF, placental growth factor, and transforming growth factor β 1.
Dechend and Staff (2012) have used the three-stage model proposed by Redman and Sargent (2010) thusly:
Dysregulated immunologic factors (stage 1) underlying defective placentation with reduced invasion of fetal extravillous trophoblast cells and reduced remodeling of maternal uteroplacental spiral arteries (stage 2) are initial pathophysiological events (Fig. 15-5). An unfavorable uteroplacental circulation ensues, with enhanced oxidative and endoplasmic reticulum stress and increased release of trophoblast-derived factors to the maternal circulation, which are thought to contribute to an excessive maternal inflammatory response and endothelial dysfunction (Buurma et al., 2013; Rajakumar et al., 2012). This induces the maternal clinical signs of preeclampsia with hypertension and proteinuria (stage 3).
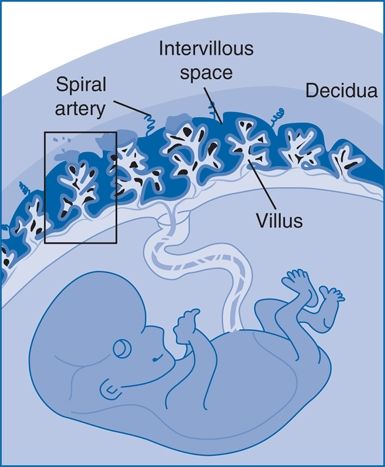
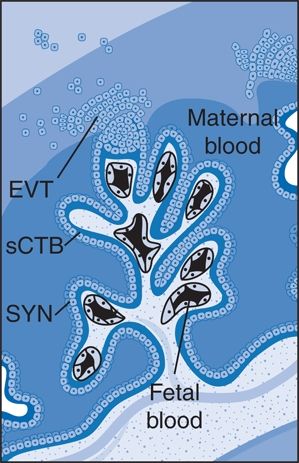
FIGURE 15-5 The intervillous space of the placenta. Maternal blood bathes the villous trees, which are covered by syncytiotrophoblast (SYN), that are underlaid by a population of progenitor cells called cytotrophoblast (sCTB). The syncytial knots are formed on these villi, and trophoblast-derived material is transferred into the maternal circulation. EVT, extravillous trophoblasts. (Adapted from Dechend R, Staff AC. Placenta messages to the mother: Not just debris. Hypertension 2012;59:191–193.)
Subsequently, Staff et al. (2013) recommended a redefinition of preeclampsia using placenta trophoblast-derived biomarkers (Fig. 15-6). Moreover, they emphasize that the current definition based on “thresholds of blood pressure and proteinuria does not correlate well with more severe maternal and perinatal outcome.” The rational for a redefinition has become more logical since the findings of Rajakumar et al. (2012) and Buurma et al. (2013). As Dechend and Staff (2012) note:
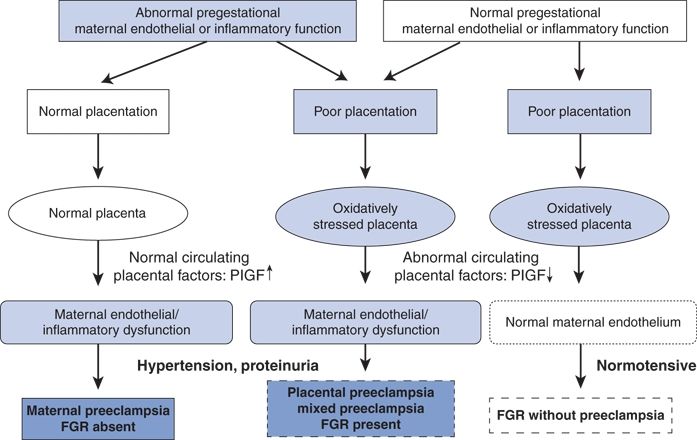
FIGURE 15-6 A model of extended definition of PE on the basis of placenta-derived biomarkers. The figure illustrates the main alternative pathways during pregnancy leading to same diagnosis of PE, with or without fetal growth restriction (FGR), or to development of FGR without PE. The pathways are differentiated by maternal circulating PlGF as a biomarker of placental dysfunction. At present, it is not clear whether the addition of other placenta-derived biomarkers (such as soluble fms-like tyrosine kinase-1 and soluble endoglin), or even maternally derived biomarkers (such as inflammatory cytokines), should be added to this flow chart to improve the definition and subclassification of PE types. (From Staff AC, Benton SJ, von Dadelszen P, et al. Redefining preeclampsia using placenta-derived biomarkers. Hypertension 2013;61:932–942.)
“The interface between the fetally derived placenta and maternal blood is formed by syncytium of multinucleated syncytiotrophoblasts, which is a result from the fusion of an underlying mononucleate cytotrophoblast. The syncytiotrophoblasts come into direct contact with maternal blood in the placental intervillous space (see Fig. 15-5)…. these deported trophoblast-derived structures are one part of a spectrum of traffic of material derived from the syncytiotrophoblast. This material includes trophoblast-derived, anucleate microvesicles and the much smaller trophoblast-derived nanovesicles, which together have been called placental debris. Apoptosis may be a mechanism regulating the shedding of subcellular debris. The words “debris” and “garbage” are, however, misleading, because all subcellular vesicles may not be waste from a tired placenta but instead be important bioactive messengers from the fetally derived placenta to the mother…. In the maternal circulation, they are believed to contribute to a generalized systemic inflammation, endothelial dysfunction, followed by hypertension and proteinuria in the pregnant woman. Although the precise mechanisms are unknown, there is evidence that the vesicles can modify the sequence of several cellular responses that contribute to the proinflammatory phenotype and impair maternal vascular dilation. … Rajakumar et al. (2012) report novel mechanistic data on one route by which the “antiangiogenic” protein soluble fms-like tyrosine kinase 1 (sFlt1), which is generated in the placenta, enters the systemic maternal circulation.” The authors show that some placental structures easily detach from the placenta and result in free, multinucleated fragments of 50 to 150 μm diameter that are loaded with sFlt1 protein and mRNA.
Buurma et al. (2013) extend their findings with the proof of the particular entrapment of these particles in the maternal lung, concluding
that detachment of syncytial knots from the placenta results in free transcriptionally active syncytial aggregates that represent an autonomous source of the anti-angiogenic sFlt1 delivery into the maternal circulation. The process of syncytial knot formation, shedding of syncytial aggregates, and appearance of placental microparticles in the maternal circulation appears to be greatly accelerated in preeclampsia and may contribute to the maternal vascular injury that characterizes this disorder. More recently, studies by Lyall et al (2014) have been interpreted by Burke and Karamanchi (2014) that “the lack of intramural trophoblasts in the myometrial vessels rather than defective interstitial troph -oblast invasion may be the primary abnormality in PE.
In a clinically oriented text as this, a great deal of the pathophysiology of preeclumpsia cannot be provided. Publications by Staff et al. (2013), Wang et al. (2014), and Warrington et al. (2013) provide additional details. Warrington et al. (2013) focus primarily on the development of hypertension (Fig. 15-7).
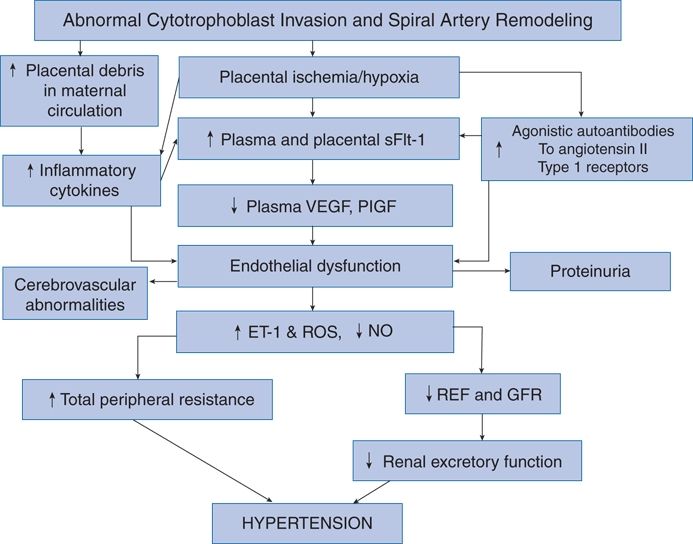
FIGURE 15-7 Schematic representation of the proposed initiating events and factors in the pathophysiology of preeclampsia. ET-1, endothelin-1; GFR, glomerular filtration rate; PlGF, placental growth factor; RBF, renal blood flow; ROS, reactive oxygen species; sFlt-1, soluble fms-like tyrosine kinase-1; VEGF, vascular endothelial growth factor. (From Warrington JP, George EM, Palei AC, et al. Recent advances in the understanding of the pathophysiology of preeclampsia. Hypertension 2013;62:666–673.)
Diagnosis
Early Diagnosis
Despite the impressive number of published series on the use of various angiogenic and antiangiogenic markers, uncertainty remains as to the most specific and sensitive to correctly identify those who are destined to develop PE early enough that mitigating therapies such as low-dose aspirin can be used.
Recently published data suggest that prediction may have improved to the level of clinical usefulness. Myatt et al. (2013) assayed biomarkers in low-risk nulliparous women, 153 who would develop PE and 468 who remained normotensive. They concluded that “changes in angiogenic biomarkers between first and early second trimester combined with clinical characteristics have strong utility for predicting early-onset preeclampsia.”
Similar evidence has been presented by Ohkuchi et al. (2013) and Verlohren et al. (2014). Both groups used different cutoffs at two gestational phases to improve the diagnostic accuracy of levels of antiangiogenic factor soluble fms-like tyrosine kinase-1 (sFlt-1) and the pro-angiogenic placental growth factor (PlGF) and vascular endothelial growth factor (VEGF). Therefore, the question of whether the cost-effectiveness of early diagnostic testing for early PE is worthwhile that was posed by Hyde and Thornton (2013) may soon be answered “Yes” according to Schnettler et al. (2013).
Until these or other markers of early PE become clinically useful, the diagnosis of PE still depends on maternal characteristics.
Current Diagnosis
Hypertension developing after the 20th week of gestation with proteinuria in a young nullipara is probably PE, particularly if she has a positive family history for the syndrome. Because patients usually have no symptoms, prenatal care is crucial to detect the signs early and thereby prevent the dangerous sequelae of the fully developed syndrome.
In keeping with the list of known risk factors (Table 15-1), women with the following features should be more closely evaluated and monitored (Ananth and Cleary, 2013)
- First pregnancy
- Previous PE
- ≥10 years since last baby
- Body mass index ≥35
- Family history of PE (mother or sister)
- Patient had low birth weight
- Diastolic BP ≥ 80 mm Hg
- Proteinuria (≥ + on more than one occasion and ≥300 mg per 24 hours)
- Multiple pregnancy
- Underlying medical condition
- Preexisting hypertension
- Preexisting renal disease
- Preexisting diabetes
- Presence of antihypertensive phospholipid antibodies
- Preexisting hypertension
Hypertension
The BP criterion is based on readings of 140/90 mm Hg or higher recorded on at least two occasions, 6 hours or more apart. Obviously, it is not possible to reconfirm the pressure levels over many weeks, as is recommended in nonpregnant patients.
Overdiagnosis
Despite the greater overall perinatal mortality with even transient elevations in pressure, for the individual patient, there is a significant chance of overdiagnosing PE on the basis of these values, which have been found to have only a 23% to 33% positive predictive value and an 81% to 85% negative predictive value (Dekker & Sibai, 2001). Higher ambulatory BP and heart rate are present at 18 weeks’ gestation in those who later developed PE; but those signs, too, have low predictive value (Hermida et al., 2004). Therefore, multiple readings and careful follow-up over at least a few days or weeks are needed for women who display such findings in the absence of any other suggestive features before the clinician should make the diagnosis or institute therapy.
Consequences
On the other hand, the level of pressure may not be inordinately high for it to have serious consequences: Women may convulse because of hypertensive encephalopathy with pressures of only 160/110 mm Hg. As noted in the report of the National HBPEP Working Group (2000):
The clinical spectrum of preeclampsia ranges from mild-to-severe forms. In most women, progression through this spectrum is slow, and the disorder may never proceed beyond mild preeclampsia. In others, the disease progresses more rapidly, changing from mild to severe in days or weeks. In the most serious cases, progression may be fulminant, with mild preeclampsia evolving to severe preeclampsia or eclampsia within days or even hours. Thus, for clinical management, preeclampsia should be overdiagnosed, because a major goal in managing preeclampsia is the prevention of maternal or perinatal morbidity and mortality, primarily through timing of delivery.
Proteinuria
Proteinuria is defined as more than 300 mg of protein in a 24-hour urine collection or 300 mg/L in two random, cleanly voided specimens collected at least 4 hours apart. The protein–creatinine ratio in a random urine sample has been found to be a poor predictor if levels are below 2,000 mg/d (Kayatas et al., 2013).
Hyperuricemia
Roberts et al. (2005) found that hyperuricemia was as important as proteinuria in identifying the fetal risk in women with GH. Bellomo et al. (2011) found that in a group of 206 primigravidas with a recent onset of hypertension, a serum uric acid above 5.2 mg/dL (309 μmol/L) predicted the development of PE with 88% sensitivity and 93% specificity.
Differential Diagnosis
Most women with typical features of de novo hypertension in pregnancy with no other obvious disorders turn out to have PE (Maynard et al., 2008). The recognition of PE superimposed on chronic hypertension may be more difficult. As described in the report of the National HBPEP Working Group (2000):
Preeclampsia may occur in [15–25% of] women already hypertensive (i.e., who have chronic hypertension).… [T]he diagnosis of superimposed preeclampsia is highly likely with the following findings:
- New onset or sudden increase of proteinuria
- In women with hypertension and no proteinuria early in pregnancy (<20 weeks)
- In women with hypertension and proteinuria before 20 weeks’ gestation
- A sudden increase in BP in a woman whose hypertension has previously been well controlled
- Thrombocytopenia (platelet count <100,000 cells per mm3)
- An increase in ALT [alanine aminotransferase] or AST [aspartate aminotransferase] to abnormal levels
The presence of hypertensive retinopathy, described in Chapter 4, or left ventricular hypertrophy would favor chronic hypertension.
Treatment
Nonpharmacologic Management
Smoking Cessation: Through 2007, 26% of mothers smoked during pregnancy and their children had more hypertension, but this was mainly ascribed to their obesity (de Jonge et al., 2013).
Bed Rest: In women who were hospitalized for various preterm indications, strict bed rest was said to reduce the incidence of PE and IUGR (Abenhaim et al., 2008). But, on the basis of a complete Cochrane review, McCall et al. (2013) call it “unsupported by data and unethical.”
Exercise: Most studies find a protection against PE by moderate exercise (Genest et al., 2012).
Sodium: Maintenance of usual sodium intake has been recommended to avoid further reducing placental perfusion (Knuist et al., 1998).
Calcium Supplements: Although once claimed to be effective for prevention of PE in high-risk populations, they are not useful for therapy (Hofmeyr et al., 2008). However, in an in vitro study, calcium protected endothelial activation by trophoblastic debris (Chen et al., 2013).
Caffeine: Caffeine may increase the risk of miscarriage, so it seems prudent to restrict its intake even more in women with PE (Weng et al., 2008).
Alcohol: Most observational data report no adverse effects on the children of mothers who have consumed small amounts of alcohol during pregnancy (Kelly et al., 2013; McCarthy et al., 2013), but Lewis et al. (2012) performed a genetic study of 4,117 eight-year-old children of mothers who consumed various amounts of alcohol during pregnancy and found that four genetic variants in alcohol-metabolizing genes in these children were strongly related to lower IQ. These data prompted Gray (2013) to recommend avoidance of alcohol during pregnancy.
Pharmacologic Therapy
The indications for drug therapy for hypertension during pregnancy remain uncertain since there is no evidence that such therapy improves neonatal outcomes. As stated by the 2013 ESH/ESC guidelines (Mancia et al., 2013) (Table 15-2):
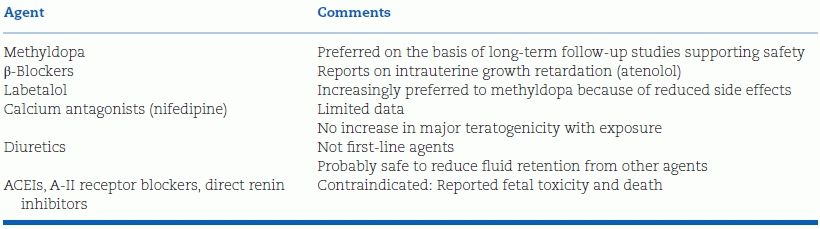
TABLE 15-2 Oral Drugs for Treatment of Chronic Hypertension in Pregnancy
In the absence of RCTs, recommendations can only be guided by expert opinion. While there is consensus that drug treatment of severe hypertension in pregnancy (>160 for SBP or >110 mm Hg for DBP) is required and beneficial, the benefits of antihypertensive therapy are uncertain for mildly or moderately elevated BP in pregnancy (<160/110 mm Hg), either preexisting or pregnancy-induced, except for a lower risk of developing severe hypertension. International and national guidelines vary with respect to thresholds for starting treatment and BP targets in pregnancy.
… Despite lack of evidence, the 2013 Task Force reconfirms that “physicians should consider early initiation of antihypertensive treatment at values >140/90 in women with (i) gestational hypertension (with or without proteinuria), (ii) preexisting hypertension with the superimposition of gestational hypertension, or (iii) hypertension with asymptomatic organ damage or symptoms at any time during pregnancy….
No additional information has been provided on the antihypertensive drugs to be used in hypertensive women: therefore the recommendations to use methyldopa, labetalol, or nifedipine (as the only calcium antagonist) really tested in pregnancy can be confirmed. Beta-blockers (possibly causing foetal growth retardation if given early in pregnancy) and diuretics (in preexisting reduction of plasma volume) should be used with caution. As mentioned, all agents interfering with the renin-angiotensin system (ACE inhibitors, ARBs, renin inhibitors) should absolutely be avoided. In emergency (preeclampsia), intravenous labetalol is the drug of choice with sodium nitroprusside or nitroglycerin in intravenous infusion being the other option.
These generally accepted preferences (Al Khaja et al., 2014) may reflect the hypertension per se and not the medications. In a population-based retrospective cohort study of over 100,000 deliveries including 1,964 with chronic hypertension, Orbach et al. (2013) found a similar rate of adverse perinatal outcomes in those women given no antihypertensive drugs as seen among those given either methyldopa or atenolol.
The previous preference given to hydralazine is not warranted. As noted in a meta-analysis of all 21 randomized controlled trials published between 1966 and 2002 involving 893 women given short-acting antihypertensives for severe hypertension in pregnancy, hydralazine was associated with more maternal and fetal side effects than nifedipine, isradipine, or labetalol (Magee et al., 2003).
Magnesium sulfate has been conclusively documented to be needed to prevent eclamptic convulsions, both when compared to placebo (Magpie Trial Collaborative Group, 2002) or a calcium channel blocker (Belfort et al., 2003). In addition, its use provides neuroprotection to infants delivered before 30 weeks’ gestation, as may be needed in women with severe PE (Crowther et al., 2003).
Long-Term Consequences
Maternal
Postpartum, women who have suffered PE, particularly if early onset, continue to be at greater risk for hypertension, diabetes, and obesity (Ahmed et al., 2014; Collen et al., 2013). Part of this continued risk reflects their prepregnancy state, in particular obesity (Gademan et al., 2013). As a consequence, these women suffer more cardiovascular (Hermes et al., 2013) and renal (Vikse, 2013) diseases later in life. Their immediate risk is low for serious complications, and, if given proper advice and follow-up, they may alter lifestyles better than most because of their prior experience during pregnancy (Hertig et al., 2008). As the consequences of maternal obesity have been recognized, prenatal weight reduction and little if any weight gain during pregnancy have been emphasized (Kominiarek et al., 2013).
Even without overt PE, women who have small for gestational age babies have more long-term cardiovascular (Melchiorre et al., 2012) and renal (Vikse, 2013) diseases. Similarly, preterm birth is a risk for subsequent maternal hypertension (Catov et al., 2013).
About 15% to 20% of women who have had PE will suffer it again during subsequent pregnancies. They should be more closely monitored during subsequent pregnancies as early as in the 12th week (Spaan et al., 2012).
Fetal
Children of women who are obese before their pregnancy have a significantly greater risk of being hypertensive (Gademan et al., 2013) and having early cardiovascular mortality (Reynolds et al., 2013) even more so if their mother had hypertension during the pregnancy.
Infants born small for gestational age, often from maternal undernutrition, suffer more hypertension and cardiovascular–renal diseases (Ingelfinger and Nuyt, 2012). Similar risks are seen with infants born preterm (de Jong et al., 2012). The consequences of maternal hypertension on their children extend to greater cognitive impairment 70 years later (Tuovinen et al., 2013).
Prevention
Dekker and Sibai (2001) have divided prevention into three stages:
1.Primary prevention will obviously be difficult without knowledge of the cause. However, avoidance of the known risk factors (Table 15-1) should help. In particular, avoiding teenage pregnancy, reducing obesity and insulin resistance, providing adequate nutrition, and avoiding multiple births during assisted pregnancies (Thomopoulos et al., 2013) should be protective.
2.Secondary prevention involves identifying the syndrome as early as possible and using strategies that are thought to influence pathogenic mechanisms. These include low-dose aspirin if given by the 16th week of gestation (Roberge et al., 2012) but not calcium supplementation (Levine et al., 1997). In addition, reduction of oxidative stress by antioxidants may work in animal models (Hoffmann et al., 2008), but neither vitamin C nor vitamin E have been preventative (Rossi & Mullin, 2011).
3.Tertiary prevention involves the various lifestyle changes and therapies described under management.
As noted in the ESH/ESC guidelines (Mancia et al., 2013):
There is considerable controversy regarding the efficacy of low-dose aspirin …. Two recent analyses came to opposing conclusions. Rossi and Mullin (2011) used pooled data from approximately 5,000 women at high risk and 5,000 at low risk for preeclampsia and reported no effect of low-dose aspirin in the prevention of the disease. Bujold et al. (2010), however, pooled data from over 11,000 women enrolled in RCTs of low-dose aspirin in pregnant women and concluded that women who initiated therapy at <16 weeks of gestation had a significant and marked reduction of the relative risk for developing preeclampsia (relative risk: 0.47) and severe preeclampsia (relative risk: 0.09) compared with control. [In yet another meta-analysis published before those used in the ESH/ECH guidelines, Roberge et al. (2012) concluded that “low-dose aspirin initiated at or before 16 weeks significantly reduces the risk of severe preeclampsia (relative risk: 0.22) but not mild preeclampsia (relative risk: 0.81)”]…. Faced with these discrepant data, only prudent advice can be offered: women at high risk of preeclampsia…. may be advised to take 75 mg of aspirin daily from 12 weeks until the birth of the baby, provided that they are at low risk of gastrointestinal haemorrhage.
ECLAMPSIA
Eclampsia is defined by the occurrence of seizures due to hypertensive encephalopathy on the background of PE (Fong et al., 2013). This serious complication is becoming less common as better prenatal care is given, but is still seen in about 1% of all pregnancies in developing societies (Miguil & Chekairi, 2008).
Manifestations of More Severe Disease
Intravascular Coagulation
As seen in Figure 15-8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








