One other change is the substitution of “Hypertension with retinal hemorrhages or papilledema” for “Accelerated-malignant hypertension with Grade 3 or 4 retinopathy,” as suggested by van den Born et al. (2011).
HYPERTENSION WITH RETINAL HEMORRHAGES AND/OR PAPILLEDEMA
Mechanisms
When BP reaches some critical level—in experimental animals at a mean arterial pressure (MAP) of 150 mm Hg—fibrinoid necrosis appears in arterial walls, likely to be a nonspecific consequence of very high BP (Beilin & Goldby, 1977). In humans, fibrinoid necrosis is relatively rare, perhaps because those who die from an acute episode have not had time to develop the lesion and those who live with therapy are able to repair it. The typical lesions, best seen in the kidney, are hyperplastic arteriosclerosis and accelerated glomerular obsolescence (Kitiyakara & Guzman, 1998).
Clinical Features
Hypertension with retinal hemorrhages or papilledema may be accompanied by various symptoms and complications, the most characteristic being microangiopathic hemolysis (Caro et al, 2013) or renal dysfunction (Table 8-2).
TABLE 8-2 Presenting Symptoms and Associated Complications in Patients with Hypertensive Crisis and Advanced Retinopathy
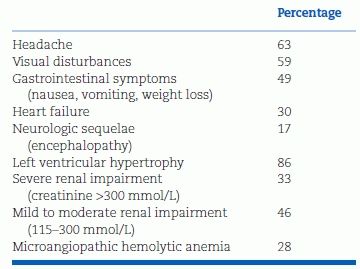
Adapted from van den Born BJ, Beutler JJ, Gaillard CA, et al. Dutch guideline for the management of hypertensive crisis—2010 revision. Neth J Med 2011;69:248–255.
Less common clinical presentations include
- Aortic dissection with giant cell arteritis (Smulders & Verhagen, 2008)
- Intramural hematoma of the aorta (Marfatia et al., 2012)
- Fibrinoid necrosis within abdominal arteries producing major gastrointestinal tract infarction with an acute abdomen (Padfield, 1975)
- Necrotizing vasculitis as a feature of lupus (Mitchell, 1994), polyarteritis nodosa (Blaustein et al., 2004), or Takayasu arteritis (Kettritz & Luft, 2012)
- Hematospermia or hematuria (Fleming et al., 2008)
Funduscopic Findings
The effects of the markedly elevated BP are displayed in the optic fundi (Fig. 8-1). Acute changes may include arteriolar spasm, either segmental or diffuse; retinal edema, with a sheen or ripples; retinal hemorrhages, either superficial and flame shaped or deep and dot shaped; retinal exudates, either hard and waxy from resorption of edema or with a raw cotton appearance from ischemia; and papilledema and engorged retinal veins (Bruce et al., 2012; Foguet et al., 2008).
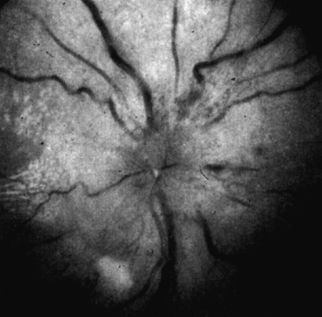
FIGURE 8-1 Funduscopic photography showing typical features of accelerated-malignant hypertension.
Similar retinopathy with hemorrhages and even papilledema rarely occurs with severe anemia or subacute bacterial endocarditis. Some patients have pseudopapilledema associated with congenital anomalies, hyaline bodies (drusen) in the disc, or severe farsightedness. Fluorescein fundus photography will distinguish between the true and the pseudo states. In addition, benign intracranial hypertension may produce real papilledema but is usually a minimally symptomatic and self-limited process (Jain & Rosner, 1992).
Evaluation
In addition to an adequate history and physical examination, a few laboratory tests should be done immediately to assess the patient’s status (Table 8-3).
TABLE 8-3 Initial Evaluation of Patients with a Hypertensive Emergency

Laboratory Findings
In 28% of patients with hypertension and retinal hemorrhages and/or papilledema, van den Born et al. (2011) found thrombotic microangiopathy, characterized by thromboses of small vessels, intravascular hemolysis with fragmented red blood cells, elevated lactic dehydrogenase, and consumption of platelets.
The urine contains protein and red cells. In a few patients, acute oliguric renal failure may be the presenting manifestation (Lip et al., 1997).
Various features of renal dysfunction including proteinuria may be present. Approximately half of patients have hypokalemia, reflecting secondary aldosteronism from increased renin secretion induced by intrarenal ischemia (Kawazoe et al., 1987). Hyponatremia is usual and can be extreme (Trivelli et al., 2005).
Multiple markers of inflammation, coagulation, platelet activation, and fibrinolysis were found in the blood from 20 patients with various types of hypertensive emergencies compared to the levels seen in hypertensive patients without target organ damage and normotensive subjects (Derhaschnig et al., 2012).
Cardiac troponin I levels were elevated in one-third of patients with a hypertensive emergency, in one series predictive of future cardiovascular events (Pattanshetty et al., 2012) and in another, not predictive (Afonso et al., 2011).
The electrocardiogram usually displays evidence of left ventricular hypertrophy, strain, and lateral ischemia. Echocardiography may show incoordinate contractions with impaired systolic and diastolic function and delayed mitral valve opening. Regression of these abnormalities usually occurs after lowering of BP by antihypertensive therapy (Gosse et al, 2011).
Evaluation for Identifiable Causes
Once causes for the presenting picture other than severe hypertension are excluded and necessary immediate therapy is provided, an appropriate evaluation for identifiable causes of the hypertension should be performed as quickly as possible. It is preferable to obtain necessary blood and urine samples for required laboratory studies before institution of therapies that may markedly complicate subsequent evaluation. None of these procedures should delay effective therapy.
Renovascular hypertension is the most likely secondary cause and, unfortunately, the one that may be least obvious by history, physical examination, and routine laboratory tests. It should be particularly looked for in older patients with extensive atherosclerosis (see Chapter 10).
If there are suggestive symptoms of pheochromocytoma, blood for a plasma metanephrine assay should be collected (see Chapter 12).
Primary aldosteronism should be considered, particularly if significant hypokalemia is noted in the initial blood sample. Plasma renin activity and aldosterone levels should be obtained. In most cases of primary aldosteronism presenting with a hypertension emergency, plasma renin activity levels have been very low despite the intrarenal necrotizing process (Prejbisz et al., 2013).
Prognosis
If untreated, most patients with hypertension and retinal hemorrhages and/or papilledema will die within 6 months. The 1-year survival rate was only 10% to 20% without therapy (Dustan et al., 1958). With current therapy, 5-year survival rates as high as 91% have been reported (Lane et al, 2009) showing the major protection provided by antihypertensive therapy.
Many patients when first seen have significant renal damage, which markedly worsens their prognosis (Szczech et al., 2010). In one series of 100 consecutive patients, the 5-year survival rate of those without renal impairment (serum creatinine <1.5 mg/dL) was 96%, no different from that of the general population (Bing et al., 2004). However, among those with renal impairment, 5-year survival fell to 65%. In another series of 120 patients followed for a mean of 5.6 years, end-stage renal disease developed in 31%, with the major predictors being an initial serum creatinine >1.9 mg/dL and uncontrolled hypertension (Amraoui et al., 2012).
When vigorous antihypertensive therapy is begun, renal function often worsens transiently, but in nearly half of those with initial renal insufficiency, function remains invariant or improves (Lip et al., 1997). In one series of 54 patients requiring dialysis, 12 recovered sufficient renal function to allow withdrawal of dialysis (James et al., 1995).
HYPERTENSIVE ENCEPHALOPATHY
With or without the structural defects of hypertension with retinal hemorrhages and/or papilledema, progressively higher BP can lead to hypertensive encephalopathy, reported in 10% to 15% of patients with the retinopathy. Conversely, one-third of patients with hypertensive encephalopathy do not have the funduscopic findings (van den Born et al., 2011).
Pathophysiology
Breakthrough Vasodilation
With changes in BP, cerebral vessels dilate or constrict to maintain a relatively constant level of cerebral blood flow (CBF), the process of autoregulation. Figure 8-2 shows direct measurements taken in cats, with progressive vasodilation as pressures are lowered and progressive vasoconstriction as pressures rise (MacKenzie et al., 1976). Note, however, that when MAPs reach a critical level, approximately 180 mm Hg, the previously constricted vessels, unable to withstand such high pressures, are stretched and dilated—first in areas with less muscular tone, producing irregular sausage-string patterns, and later diffusely, producing generalized vasodilation. This vasodilation allows a breakthrough of CBF, hyperperfusing the brain under high pressure, causing leakage of fluid into the perivascular tissue, leading to cerebral edema and the clinical syndrome of hypertensive encephalopathy (Strandgaard & Paulson, 1989).
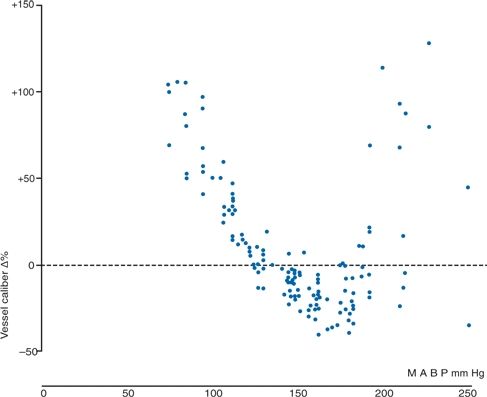
FIGURE 8-2 Observed change in the caliber of pial arterioles with a caliber of less than 50 mm in eight cats, calculated as a percentage of change from the caliber at a mean arterial blood pressure (MABP) of 135 mm Hg. The BP was raised by intravenous infusion of angiotensin II. (Reprinted from MacKenzie ET, Strandgaard S, Graham DI, et al. Effects of acutely induced hypertension in cats on pial arteriolar caliber, local cerebral blood flow, and the blood–brain barrier. Circ Res 1976;39:33, with permission.)
Breakthrough vasodilation has also been demonstrated in humans (Strandgaard et al., 1973). Figure 8-3 shows curves of autoregulation constructed by measuring CBF repetitively, while arterial BP was lowered by vasodilators or raised by vasoconstrictors. CBF is constant between MAPs of 60 and 120 mm Hg in normotensive subjects. However, when pressure was raised beyond the limit of autoregulation, breakthrough hyperperfusion occurred.
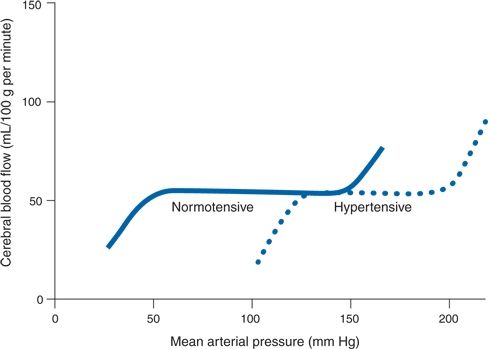
FIGURE 8-3 Idealized curves of CBF at varying levels of systemic BP in normotensive and hypertensive subjects. Rightward shift in autoregulation is shown with chronic hypertension. (Adapted from Strandgaard S, Olesen J, Skinhøj E, et al. Autoregulation of brain circulation in severe arterial hypertension. Br Med J 1973;1:507–510.)
Pressures such as these are handled without obvious trouble in chronic hypertensives, whose blood vessels adapt to the chronically elevated BP with structural thickening and stiffness (Iadecola & Davisson, 2008). Thereby, the entire curve of autoregulation is shifted to the right (see Fig. 8-3). Even with this shift, breakthrough will occur if MAPs are markedly raised to levels beyond 180 mm Hg.
These findings explain a number of clinical observations. Previously normotensive people who suddenly become hypertensive may develop encephalopathy at relatively low levels of hypertension, which are nonetheless beyond their upper limit of autoregulation. These include children with acute glomerulonephritis and young women with eclampsia. On the other hand, chronically hypertensive patients less commonly develop encephalopathy and only at much higher pressures.
In regard to the lower portion of the curve, when the BP is lowered by antihypertensive drugs too quickly, chronic hypertensives often are unable to tolerate the reduction without experiencing cerebral hypoperfusion, manifested by weakness and dizziness. These symptoms may appear at levels of BP that are still well within the normal range of autoregulation and well tolerated by normotensives. The reason is that the entire curve of autoregulation shifts, so that the lower end also is moved, with a falloff of CBF at levels of 100 to 120 mm Hg MAP (see Fig. 8-3). Moreover, patients with severe hypertension may lose their ability to autoregulate, increasing their risk of cerebral ischemia when BP is lowered acutely (Immink et al., 2004).
As detailed in Chapter 7, if the BP is lowered gradually, the curve can shift back toward normal so that greater reductions in pressure can eventually be tolerated. However, maneuvers that increase CBF further and thereby increase intracranial pressure, such as drugs that induce cerebral vasodilation, e.g., hydralazine, nitroglycerin, and nitroprusside, may be harmful in patients with encephalopathy (Sheta et al., 2011).
Central Nervous System Changes
Symptoms in encephalopathic patients include severe headaches, vomiting, confusion, seizures, visual changes, and, if untreated, coma (Table 8-2). The cerebrospinal fluid rarely shows pleocytosis (McDonald et al., 1993) but is usually under increased pressure. Computed tomography or magnetic resonance imaging usually shows a characteristic posterior leukoencephalopathy predominantly affecting the parietooccipital white matter, often the cerebellum and brainstem (Karampekios et al., 2004), and occasionally other areas as well (Vaughan & Delanty, 2000).
Differential Diagnosis
There are clinical situations in which the BP is elevated and the patient has findings that suggest hypertension-induced target organ damage wherein the findings are unrelated to the elevated BP. Table 8-4 lists conditions that may mimic a hypertensive emergency. A less aggressive approach to lowering of the BP is indicated in such patients. Particular caution is warranted after a thrombotic stroke, when a rapid decrease in BP may shunt blood away from the ischemic area and extend the lesion (Grise et al., 2012).
TABLE 8-4 Conditions that May Mimic a Hypertensive Emergency

In addition to these two specific presentations, hypertension may be life threatening when it accompanies other acute conditions wherein a markedly elevated BP contributes to the ongoing tissue damage (see Table 8-1). The role of hypertension in most of these conditions is covered in Chapter 4, and some of the other specific circumstances (e.g., pheochromocytoma crises, eclampsia) are covered in their respective chapters.
THERAPY FOR HYPERTENSIVE EMERGENCIES
The majority of patients with the conditions shown in Table 8-1 require immediate reduction in BP. In those patients with hypertensive encephalopathy, if the pressure is not reduced, cerebral edema will worsen and the lack of autoregulation in ischemic brain tissue may result in further increases in the volume of the ischemic tissue, which may cause either acute herniation or more gradual compression of normal brain.
On the other hand, the shift to the right of the curve of cerebral autoregulation in most patients who develop encephalopathy exposes them to the hazards of a fall in CBF when systemic pressure is lowered abruptly by more than approximately 25%, even though these levels are not truly hypotensive (Immink et al., 2004; Strandgaard & Paulson, 1996) (see Fig. 8-3).
Initiating Therapy
With encephalopathy or evidence of progressive myocardial ischemia, no more than a very few minutes should be taken to admit a patient to an intensive care unit, set up intravenous access, and begin frequent monitoring of the BP, usually with an intra-arterial line. The initial blood and urine samples should be obtained, and antihypertensive therapy should begin immediately thereafter.
Monitoring Therapy
Abrupt falls in pressure should be avoided, and the goal of immediate therapy should be to lower the diastolic pressure only to approximately 110 mm Hg. The reductions may need to be even less if signs of tissue ischemia develop as the pressure is lowered. Most of the catastrophes seen with treatment of hypertensive emergencies were related to overly aggressive reduction of the BP (Jansen et al., 1987). On the other hand, careful reduction of elevated BP is usually beneficial in those with an intracranial hemorrhage (Anderson et al, 2013; Koga et al, 2012).
Particular care should be taken in elderly patients and in patients with known cerebrovascular disease, who are even more vulnerable to sudden falls in systemic BP (Fischberg et al., 2000). In patients with recent ischemic stroke, the American Stroke Association recommends cautious reduction of BP by 10% to 15% if systolic levels are above 220 mm Hg or diastolic above 120 mm Hg (Adams et al., 2007). Adverse effects have been seen even with gradual reduction of BP in those with an ischemic stroke (Sandset et al, 2012).
If the neurologic status worsens as treatment proceeds, urgent computed tomography of the brain should be obtained, and, if potentially life-threatening cerebral edema is identified, osmotic diuresis with mannitol, often plus intravenous furosemide, can be effective.
Parenteral Drugs
Table 8-5 lists the choices of parenteral therapy now available. All are capable of inducing hypotension, a risk that mandates careful monitoring of BP. They are covered in the order shown in Table 8-5.
TABLE 8-5 Parenteral Drugs for Treatment of Hypertensive Emergency
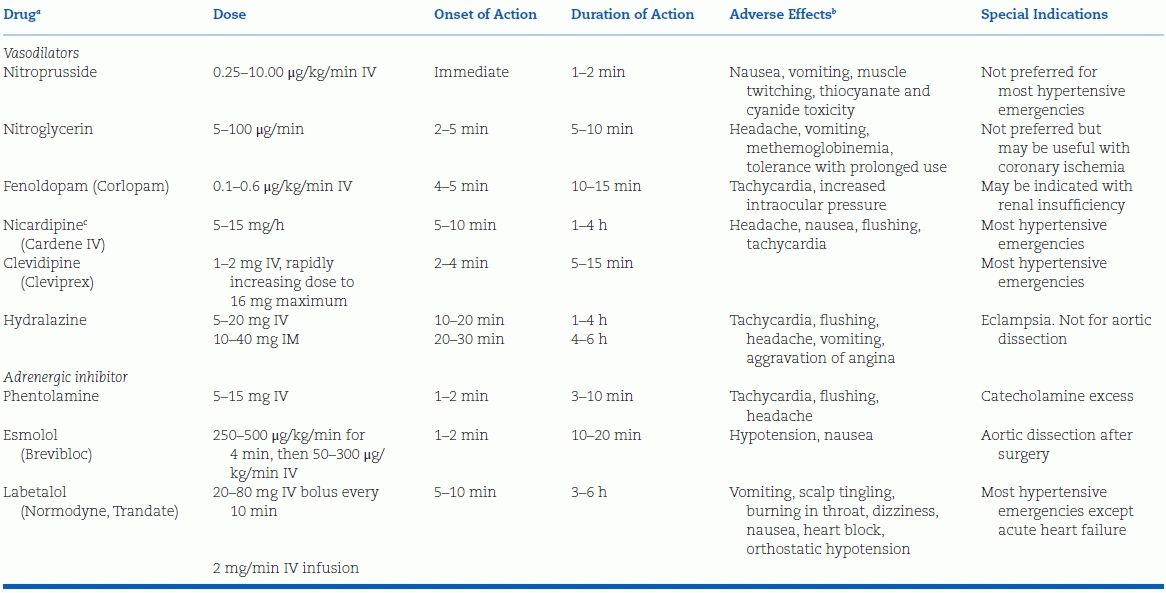
aIn order of rapidity of action.
b
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








