Technique
Author (year)
N. of patients
% of facial restoration (H–B scale)
% of hemitongue atrophy/% of swallowing, chewing, and speech disturbances
II
III
IV
V
VI
HFA
Conley and Baker (1979) [9]
137
65
18
17
100/45
Pensak et al. (1986) [33]
61
42
48
10
100/74
Samii and Matthies (1997) [41]
29
96
4
100/7
HHFA with longitudinal split of the HN (without JIG)
Cusimano and Sekhar (1994) [10]
1
100
100/100
Arai et al. (1995) [1]b
8
100
100/13
HHFAa with JIG
May et al. (1991) [31]
20
80
15
5
10/3
Hammerschlag (1999) [18]
17
22
61
11
0
Manni et al. (2001) [30]
29
21
45
24
7
3
3/0
HHFA with FN transposition
Atlas and Lowinger (1997) [3]a
3
100
0
Sawamura and Abe (1997) [42]
4
75
25
0
Darrouzet et al. (1999) [11]
6
83
17
33/33
Donzelli et al. (2003) [13]
3
33
67
100/0
Rebol et al. (2006) [35]
5
40
20
20
20
0
Roland et al. (2006) [36]b
10
100
0
End-to-side HFA
Ferraresi et al. (2006) [16]c
2
100
0
Koh et al. (2002) [24]b
4
50
50
0
37.3 Indications
Neurotisation techniques, i.e. the use of a donor nerve to reinnervate the facial nerve, are indicated when a direct nerve repair is not possible and facial muscles are viable. More precisely, a neurotisation technique is indicated in the following three situations [40]: (1) loss of the proximal part of the facial nerve at the brainstem in the cerebellopontine angle (CPA); (2) destruction of the facial motor nucleus, as in pontine haemorrhages due to pontine cavernomas; and (3) internal axonotmesis, as can be presumed in cases in which, during a CPA operation, the nerve appeared anatomically in continuity but functional recovery does not occur after 12 months.
Possible donor nerves used for reanimation procedures are the phrenic, spinal accessory, hypoglossal, and contralateral facial nerves [40].
While the spinal accessory–facial nerve anastomosis gives evident reinnervation of the facial muscles, the functional and cosmetic results are poor. In fact, the innervation is only sufficiently present on movement of the shoulder. The gross shoulder movements are not apt to equal the fine and differentiated pattern of facial innervation [40].
Facio-facial (“cross-face”) anastomosis [40, 41] can yield good results and may be used in patients in whom no other cranial nerve can be sacrificed. This procedure entails the use of up to one-half of the plexiform facial nerve branches on the healthy side of the face, which remains without a discernible deficit. These contralateral branches can be connected, using sural nerve grafts, to the corresponding branches of the paralysed side. Since the consequent reinnervation is fairly weak, most authors recommend additional muscle transfers [6, 18, 40, 41].
HFA yields the chance of strong reliable reinnervation and is the most popular operation for facial reanimation (Table 37.1) [40]. When the hypoglossus cannot be sacrificed, either the less powerful facio-facial anastomosis or the alternative types of HFA techniques, aiming to preserve tongue function, can be used.
37.4 Timing
On one hand, facial movement after spontaneous recovery of the facial nerve is generally better than after HFA. On the other hand, several degenerative phenomena occur after facial nerve injury, such as muscular atrophy, nerve fibrosis, pontine nucleus degeneration, and degeneration and loss of plasticity and information in the facial area of the motor cortex [40, 41]. The closer the interruption is located to the proximal nuclei (i.e. at the facial nerve origin at the brainstem), the faster these degenerative processes will occur. Timing is therefore a fundamental issue in HFA, especially after facial nerve injury in CPA, as in vestibular schwannoma surgery. To achieve an optimal result, the reanimation procedure should be performed before the degenerative mechanisms can negatively influence its final result.
Although some investigators initially reported that the onset of facial nerve remission can occur as late as 2 years after tumour resection [34], others [26–28] reported that this is quite rare. Kunihiro et al. [27, 28] noted that 2/3 of their patients recovered from complete facial paralysis spontaneously; signs of recovery appeared most frequently between 3 and 4 months after surgery and never after 12 months.
Though initial reports did not document any difference between early and late repair of the facial nerve [34], it is now generally recommended that the nerve reanimation procedure should be performed within 6 months and, in any case, never scheduled later than 1 year after the onset of the paralysis [28, 31, 41]. Good results are obtained more frequently when patients are operated before 1 year of persistent paralysis, rather than later [26, 40, 46]. Experimental research in guinea pigs revealed that nerve regeneration and rearrangement in central nucleus were better in early HFA [8]. Nevertheless, if a patient is treated as late as 2 years, HFA offers some opportunity of facial muscle reinnervation, but a very satisfying result is less probable [41].
37.5 Surgical Anatomy
The surgical anatomy of the hypoglossal and facial nerves for HFA has recently been extensively studied by Asaoka et al. [2] and Salame et al. [38, 39].
37.5.1 Cervical Segment of the Hypoglossal Nerve [HN]
The HN exits the skull through the hypoglossal canal, which is antero-mesial to the jugular foramen in the posterior cranial base. It continues medial to the internal jugular vein, internal carotid artery, and cranial nerves IX, X, and XI, descending in a plane between the styloid process anteriorly and the transverse process of the atlas posteriorly [20, 38]. Below the level of the mandibular angle, the HN passes medial to the posterior belly of the digastric muscle, which is a reliable guide to identify the nerve (Fig. 37.1). The HN is crossed superficially by the occipital artery. Assuming a horizontal course, the nerve continues forward, infero-medially to the digastric posterior belly to enter the submandibular triangle. This triangle is bounded superiorly by the mandible, anteriorly by the anterior belly of the digastric muscle, and posteriorly by the posterior belly of the same muscle. Whereas the nerve can be either superior or inferior to the digastric tendon [38], it is always superior to the body of the hyoid bone (Fig. 37.1).
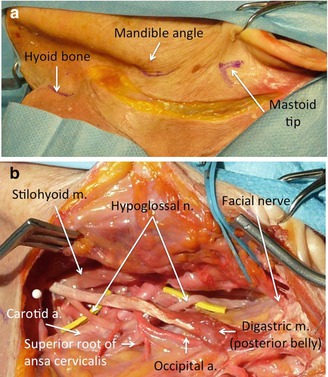

Fig. 37.1
Essential anatomy for hypoglossal–facial nerve anastomosis. (a) The three fundamental landmarks for the skin incision have been marked on the anatomical specimen. (b) The final view before the hypoglossus is prepared for approximation to the facial nerve: the digastric muscle has been isolated together with its tendon, the stylohyoid muscle, and occipital artery; the hypoglossal nerve is usually found at this level (yellow rubber band beneath its course). The facial nerve is encircled by a blue rubber band before its entry in the parotid gland. A white pin is positioned at the level of the hyoid bone
The descending ramus (ramus descendens; descendens hypoglossi), long and slender, quits the hypoglossal where it turns around the occipital artery and descends in front of or in the sheath of the carotid vessels [20]; it gives a branch to the superior belly of the omohyoideus and then joins the communicantes cervicales from the second and third cervical nerves, just below the middle of the neck, to form a loop, the ansa hypoglossi.
The shortest distance between the HN and the bifurcation of the FN trunk (pes anserinus) is approximately 16–17 mm [1, 38]. Asaoka et al. [1] reported that the length of the FN from the external genu to the pes anserinus was 30.5 ± 4.4 mm. Salame et al. [39] found that the length of the FN trunk from the stylomastoid foramen (SMF) to the bifurcation was 16.44 mm. The minimal distance between the HN immediately before entering the submandibular triangle and the SMF was 33.54 ± 12.71 mm [38].
According to Salame et al. [38, 39], in formaldehyde-fixed cadavers, the diameter of the HN just before its entrance into the submandibular triangle was 2.69 ± 0.36 mm and its cross-sectional area was 6.88 mm2. The diameter of the ansa hypoglossi, 10 mm after its separation from the HN, was 1.62 ± 0.36 mm.
37.5.2 Facial Nerve [FN] Trunk
The FN exits from the skull through the SMF, bound medially by the styloid process and laterally by the mastoid tip. The distance between the mastoid tip and the SMF was 17.22 ± 3.18 mm.
The FN is relatively superficial and Kempe [21] has pointed out the common error of looking for the nerve too deeply. In fact, the FN runs under the deep cervical fascia, the mean minimal distance from the skin surface being 22.4 ± 3.8 mm [39].
The FN trunk runs anteriorly in an oblique caudal–external direction with a slight upward concavity. It traverses the styloid process, the posterior belly of the digastric muscle, the external carotid artery, the posterior division of the retromandibular vein, and the ramus of the mandible. The number of branches arising from the FN between the SMF and the parotid gland varies from two to four. The posterior auricular nerve and the branch to the digastric muscle are the most commonly found and they originate approximately 3 and 3.6 mm from the SMF [39]. The stylomastoid artery usually supplies the FN trunk and enters into the skull through the SMF.
As reported by Salame et al. [39], in 45 out of 46 dissections, the FN terminated in a bifurcation within the parotid gland. The length of the FN trunk from the SMF to its bifurcation was 16.44 ± 3.20 mm (range, 12.20–18.68 mm). The distance between the bifurcation and the mastoid tip was 16.11 ± 3.92 mm (range, 12.10–25.10 mm). The distance between the bifurcation and the mandibular angle was 33.35 ± 4.41 mm (range, 26.46–54.88 mm) [39].
According to Asaoka et al. [1], the mean number of myelinated axons in the FN is approximately 75 % of that in the HN; the myelinated axons in the FN have a smaller calibre than those in the HN. The cross-sectioned area of the FN is then usually smaller than the HN, and the difference can significantly increase when the FN has been severed and has undergone degeneration [1].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








