Fig. 10.1
(a) Rib vertebral angle difference (RVAD). (b) Phase of rib head: phase 1. (c) Phase of rib head: phase 2 (Redrawn from Ref. [49])
Ceballos et al. [10] corroborated Mehta’s findings reporting 92 % of their resolving curves having an RVAD of 20° or less. Of the remaining 8 % with an RVAD greater than 20°, all showed improvement at the 3-month follow-up. Robinson and McMaster [57] in 1996 found that the curves that progressed among their 109 patients had a mean initial RVAD of 31°, while those that resolved had a mean of 9° on initial examination.
Mehta [49] recognized a special radiographic feature among the less common (and more aggressive) double major and lumbar curve patterns. She recognized that the RVAD at the apical thoracic vertebra was frequently less than 20° and found that there is significant asymmetry at the 12th vertebra. Here, she found the rib on the concave side becoming more vertical than the rib on the convex side, making the RVAD negative. The 12th rib is initially part of the upper curve but becomes the apex of a secondary curve developing caudally to the first. Consequently, the rib that is on the concavity of the upper curve drops secondary to the progression of the vertebral rotation and increases in magnitude of the caudal curve.
10.4.2 The Role of Advanced Imaging and Neural Axis Abnormalities
The role of advanced imaging in infantile and juvenile scoliosis is directly related to the presence of neural axis abnormalities. As IIS and JIS are a diagnosis of exclusion, all attempts must be made to identify possible etiologies. The incidence of neurological abnormalities has been reported as high as 20 % in patients under the age of 10 years [20, 37, 39, 49]. Lewonowski et al. [39] reported a magnetic resonance imaging (MRI) study of 26 consecutive patients with idiopathic scoliosis under the age of 10 years. They found five patients (19 %) with neuropathology and only two patients with atypical curves. Four of their patients were infantile, and two patients had abnormal findings: a 4-month-old boy with a terminal lipoma and a 3-year-old girl with a syrinx.
Gupta et al. [24] conducted a prospective and retrospective MRI study to evaluate the prevalence of neural axis abnormalities in patients 10 years of age or younger with idiopathic scoliosis and a normal clinical examination. In the prospective arm, he followed 34 patients with a mean age of 9 years and found abnormalities in six patients (18 %). Within this group, six patients were infantile and three patients had identifiable neuropathology. Among the 64 retrospective patients, 20 % were found to have neural axis pathology.
Most recently, Dobbs et al. [17] in multicenter study identified 11 of 46 infantile scoliosis patients with neural axis abnormalities. All patients were clinically asymptomatic and had curves of 20° or less. Five patients had an Arnold-Chiari type-I malformation, three with syringomyelia, one with a low-lying conus, and one with a brain tumor. Of these ten patients, eight required surgical intervention. On the basis of the findings of this paper and other reports, it is our recommendation that all patients with IIS or JIS with a curve of 20° or less have both a brain and a complete spine magnetic resonance imaging (MRI).
Other imaging modalities exist to aid in management and provide continued relevant information in the care of these children. Computed tomography (CT) scans can be helpful for preoperative evaluation in selected patients where the spine will be instrumented. Pedicular anatomy and bony anomalies are made very clear. CT scans can also be used to assess the three-dimensional lung volumes and can be a marker of treatment; however, their use must be weighed against the risk of significant associated radiation exposure.
10.5 Management Themes (Fig. 10.2)
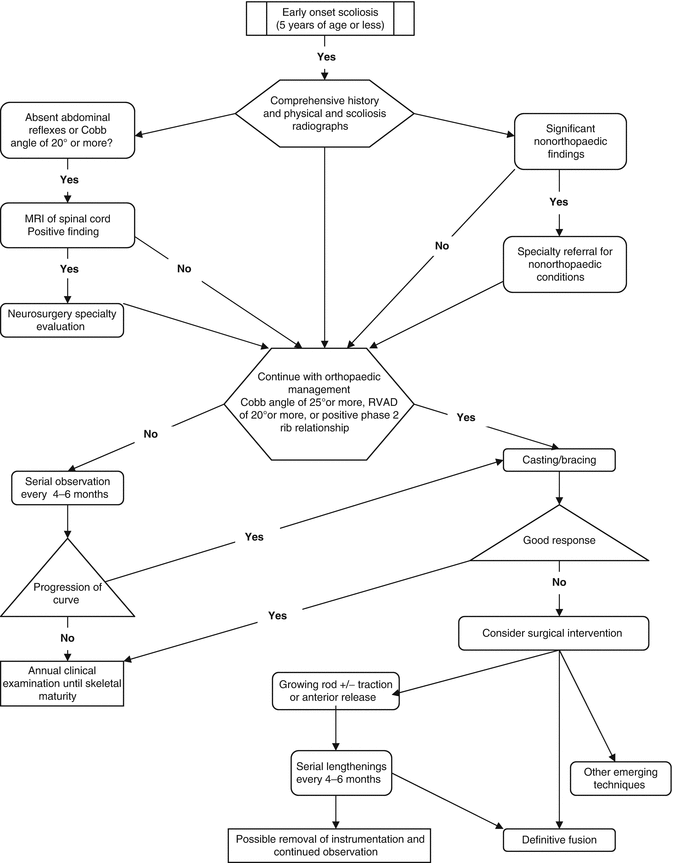
Fig. 10.2
Treatment algorithm for infantile and juvenile idiopathic scoliosis (Adapted from Ref. [23])
10.5.1 Selecting Surgical Candidates
Management of children with early onset idiopathic scoliosis is based on anticipated or actual curve progression. Mehta’s [49] prognostic criteria, as discussed earlier, are very helpful in identifying curves at risk. Curves with an RVAD of less than 20° and a Cobb angle of less than 25° are at low risk of progression. These patients are safely treated with observation; however, they should be followed clinically every 4–6 months for progression. Once the curve has resolved, the follow-up interval can be extended to 1–2 years. We recommend following these patients to maturity to ensure that there is no recurrence during the adolescent growth spurt. Diedrich et al. [14] reported on 25-year follow-up of infantile scoliosis, validating the use of RVAD, and demonstrated that there was no advantage to supine plaster bed treatment over physiotherapy, in regard to time to resolution or functional outcome.
Infants with an RVAD of 20° or more or a phase 2 rib–vertebral relationship and a Cobb angle between 20° and 35° have a higher risk of progression. This group of patients should be followed closely at 4- to 6-month intervals for clinical and radiographic evaluation. Active treatment should be initiated when progression of Cobb angle of 5° or more is documented over 1 year [1]. Active treatment at this point is usually in the form of casting or bracing, which will be discussed thoroughly in separate chapters.
10.5.2 Surgical Treatment: Historic Perspective
The goals of surgical treatment of idiopathic early onset scoliosis are multifold: to stop curve progression and to allow for maximum growth of the spine, lungs, and thoracic cage. Surgery is often recommended in children with progressive curves of 46° or more; however, there are other factors that influence decision making. The risks and benefits of curve correction vs. continued growth should be considered by the treating surgeon to make a final decision. This statement reflects the current trend toward more aggressive operative management since the techniques for fusion-less surgery have become refined and the natural history of this disease, more clearly understood.
Historically, the goals of surgery were a straight shortened spine rather than a deformed spine of near normal length. Isolated posterior spinal fusion in this age group quickly went out of favor, after Dubousset et al. [18] described the crankshaft phenomenon. This phenomenon seen in skeletally immature patients describes progression of deformity following posterior spinal fusion due to continued anterior growth of the spine. Sanders et al. [59] further correlated open triradiate cartilage and Risser 0 to high risk of crankshaft in the presence of an isolated posterior spinal fusion. Anterior arthrodesis was therefore recommended, in addition to posterior fusion to prevent crankshaft. Anterior and posterior fusion, however, results in a significant amount of height loss and thoracic underdevelopment. As discussed earlier, Dimeglio [15] nicely outlined spinal growth throughout childhood with two noticeable peaks of growth (0–5 years and 10–15 years). Using his formula for calculating normal growth, expected loss of height can be determined for patients treated with anterior–posterior fusion. Winter [68] similarly described a formula for calculating amount of projected height loss. To calculate projected shortening in centimeters, multiply 0.07 × number of segments fused × number of growth years remaining. These data are very valuable in educating family and caretaker of the potential ramifications of fusion in this very young patient population. It should also be noted that the effect of fusion on the spine could have morbid effects on lung and thoracic cage development. This has been a motivating factor over many decades to devise other surgical methods that avoid circumferential fusion.
Over 45 years ago, Roaf [56] attempted to modulate spine growth, much like one would modulate an angular deformity in a pediatric lower extremity with hemiepiphysiodesis. He proposed that the spinal deformity was the result of asymmetric growth between the convex (faster growing) and concave (inhibited) sides of the curve. His technique of modulation involved ablation of the convex epiphyseal cartilage and adjacent discs at the vertebrae near the apex of the curve. Only 23 % of his treated patients showed improvement of Cobb angle, while 40 % showed little or no improvement (Cobb angle <10° change). Marks et al. [45] built upon this idea and used hemiepiphysiodesis and simultaneous Harrington internal fixation. No significant improvement was measured in 13 consecutive patients with 12 demonstrating progression of deformity.
Harrington [27], in 1962, described a fusion-less technique in 27 idiopathic and postpolio patients, placing a single distraction rod on the concavity of the curve connected to hooks at both ends. The hooks and rods were placed after a subperiosteal approach to the spine. The idea was to instrument the spine without arthrodesis in an attempt to preserve spinal growth, correct deformity, and control the residual deformity. Although no longitudinal results were reported, he believed that children under 10 years could be managed with instrumentation alone and those 10 years and older required arthrodesis.
Moe et al. [50] modified the technique described by Harrington and limited subperiosteal exposure to the site of hook placement and passed the rod subcutaneously. Furthermore, they modified the rod to have a smooth, thicker central portion to prevent scare formation to the threads and allow for sagittal contouring. Patients were lengthened when a loss of Cobb angle >10° occurred. Of the two patients treated with idiopathic infantile scoliosis, both were reported as having a notable decrease in curve magnitude. They, furthermore, reported a complication rate of 50 %, including rod breakage and hook dislodgement from the rod or the lamina.
In 1997, Klemme et al. [35] reported on 20-year experience of the Moe technique. Sixty-seven patients were followed from initial instrumentation to final fusion, with an average of 6.1 procedures per patient. Curve progression was arrested or improved in 44 of 67 patients with an average curve reduction of 30 %. Of the remaining 23 patients, 12 were neuromuscular, and the curves progressed on average 33 %.
In 1977, Luque and Cardosa [42] described their technique of fusionless treatment of scoliosis with segmental spinal instrumentation. In 1982, Luque [41] modified this technique by adding sublaminar wires and replacing the Harrington rod with L-shaped rods, later to be known as the Luquetrolly. His initial series included 48 paralytic patients who grew by an average of 4.6 cm over the immobilized segment with an average curve correction of 78 %. This system became less favored after reports that subperiosteal exposure and sublaminar wire passage created scar tissue and weakened the lamina, which made revision and later definitive fusion difficult. There were also several reports of spontaneous fusion and substantially less growth preservation than predicted. These findings were attributed to the exposure that was required at each level to pass wires [54].
Patterson et al. [53] combined segmental spinal instrumentation with anterior apical convex growth arrest and fusion in 9 of 13 patients who had previously undergone surgery at an average age of 5 years and 5 months. Curve correction averaged 46 % at 2-year follow-up. Less curve deterioration was identified in those patients who had anterior apical growth arrest compared to those who had segmental instrumentation alone.
In 1999, Pratt et al. [55] performed a retrospective review of patients treated with Luque trolley instrumentation with and without convex epiphysiodesis in 26 patients. Eight were treated with Luque trolley alone, and all showed significant curve deterioration. Of those treated with combined convex epiphysiodesis and Luque instrumentation, the Cobb angle worsened in seven of 13, remained unchanged in four and improved for two. Growth was found to be 49 % among those predicted in the Luque trolley alone group and 32 % among those undergoing combined surgery.
Blakemore et al. [6] further reported periodic lengthening with a submuscular rod with and without apical fusion. Apical fusion was performed on curves 70° or more and in those whose curves were stiff on bending radiographic testing. The rod was placed within the muscle above the spine periosteum, placing the rod closer to the spine for better contour and alignment without inducing spontaneous fusion. He reported on 29 children, ten idiopathic, all treated in a Milwaukee brace postoperatively. Mean Cobb angle improved from 66 to 38° immediately postoperatively with most recent follow-up showing a slight deterioration to 47°. Complication rate was 24 % including hook dislodgement (5), rod breakages (3), and superficial wound infection (1).
10.5.3 Current Approaches to Surgical Management
Once the decision for surgery has been made, several factors have to be considered before choosing the correct surgical approach. The rigidity of the curve plays an important role in decision making, as curves that have little flexibility will not likely be as amenable to a growing construct alone. In this situation, there may be a role for anterior release prior to posterior fusionless surgery. Marks, in unpublished results, discussed the use of annulectomy vs. nucleotomy as anterior release options. No long-term results exist, however, to make any definitive recommendations (D Marks, 2009, personal communication).
The next decision to make is which lengthening procedure is ideal for the patient. Salari et al. [58] recently reported on the results of a survey sent to 40 qualified surgeons on ideal treatment of 11 different case scenarios of infantile scoliosis. Seventeen surgeons responded with a wide variation in treatment recommendations for each patient scenario. The most common treatment selected was a dual growing-rod construct (56.7 %), followed by nonoperative management (16.6 %), SHILLA (15.5 %), VEPTR (7 %), fusion or resection, and immediate fusion (4 %). This study is important to highlight the lack of standardized treatments offered to our patients by highly qualified surgeons [57].
The next two sections briefly describe the various fusionless surgeries. They are subdivided into two categories: distraction-based growing rods and growth-directed surgery. VEPTR, a form of distraction-based growing rod, will be discussed in a separate chapter.
10.5.4 Distraction-Based Growing Rods
The unpredictability and high implant-related complication rate associated with single rod distraction techniques led Akbarnia and Marks [3] to popularize a dual growing rod technique, building on concepts formulated by Asher (Fig. 10.3a–d). Subperiosteal dissection is limited to the proximal and distal foundations (anchor sites). Hooks or pedicle screws are placed on both ends over two or three spinal levels. Foundation sites are fused using local bone graft supplemented with synthetic graft. Upper and lower contoured 3/16 in.-diameter rods are placed submuscularly on both sides of the spine. The rods are joined on each side with extended tandem connectors placed at the thoracolumbar junction to avoid disturbing sagittal balance. The first lengthening is typically performed at the index procedure. A distractor designed to fit within the longitudinal opening in the tandem connector is used at time of lengthening that typically occurs at 6-month intervals starting with the index surgery. The intent of the original lengthening is to obtain modest correction of the scoliotic curve without unduly stressing the foundations. We have found approximately 50 % correction of coronal Cobb angles at the original surgery. More aggressive lengthening can be performed starting with the first lengthening after fusion. Somatosensory-evoked potential monitoring is performed during each lengthening. Lengthening can be performed as outpatient surgery with appropriate anesthesia and nursing support. Bracing is utilized until fusion is achieved at the foundation sites.
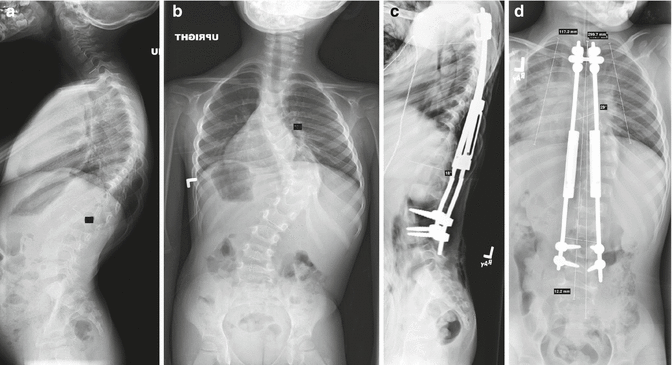

Fig. 10.3
(a, b) Severe progressive scoliosis in a 4-year-old patient with idiopathic infantile. (c, d) Post-initial surgery radiographs
Recent advances in technology have led to the development of magnetically controlled growing rods (MCGRs). The index surgery for placement of the MCGR is similar to placement of dual growing rods with the exception that the implant consists of a single preassembled rod [11]. The use of MCGR has become the preferred method of treating IIS and JIS when feasible, to avoid repeat exposure to anesthesia and surgery traditionally associated with growing rod surgery.
Akbarnia et al. [2] reviewed 13 patients with no previous surgery and noncongenital curves who were followed to final fusion. They found a mean spinal growth of 5.7 cm during a 4.4-year treatment period. The curve improved from 81 to 36° after initial surgery and to 28° at final fusion. T1–S1 length improved from 24 to 29 cm after initial surgery to 35 cm at final fusion. Those patients lengthened at 6-month or less intervals experienced significantly more growth and curve correction than those lengthened less frequently [2].
A recent report by Sankar et al. [60] reviewed 782 growing rod surgeries in 252 patients where neuromonitoring was performed. Surgeries included 252 primary rod implantations, 170 implant exchanges, and 362 lengthenings. Neuromonitoring changes occurred in two primary implant surgeries (0.8 %), one implant exchange (0.6 %), and one lengthening (0.3 %). The change noted in the case of implant exchange also resulted in a clinical deficit, which resolved within 3 months. The monitoring change that occurred in the lengthening was in a child with an intracanal tumor that also had a change during the primary surgery. The final recommendation was that the overall rate of neuromonitoring change seen in primary and implant exchange surgeries justifies its use. No definitive recommendations could be made for lengthenings because of sample size.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







