CHAPTER 338 Indications and Techniques for Cranial Decompression after Traumatic Brain Injury
Reduction of death and disability from traumatic brain injury (TBI) is the goal of cranial decompressive surgery. Secondary insults and injury, including brain edema, elevated intracranial pressure (ICP), and decreased cerebral perfusion pressure (CPP), are the leading causes of in-hospital deaths after TBI.1 The primary goals of treatment of TBI are therefore to control brain edema and maintain adequate blood flow and delivery of oxygen to the injured brain tissue. Adherence to published prehospital, intensive care, and surgical guidelines for the management of severe TBI has resulted in improved survival and outcomes by mitigating the deleterious effects of secondary insults and injury. ICP monitoring guides the use of specific therapies such as drainage of cerebrospinal fluid (CSF), sedation, mild hyperventilation, or hyperosmolar euvolemia with the administration of hypertonic saline or mannitol (or both) for control of ICP and CPP. Although these therapies may provide adequate treatment for many patients, there is a cohort of patients in whom cerebral edema will continue to propagate despite “maximal medical management” and culminate in increased cellular injury and death and ultimately in poorer outcomes. These patients are possible candidates for decompressive surgery to assist in the control of ICP.
Background
The concept of cranial decompression is by no means new and perhaps dates as far back as Neolithic times when trephination is thought to have been practiced. In the modern medical era, decompressive surgery has been used intermittently with variable success since Bergmann first described the technique in 1880, followed by Cushing’s descriptions of subtemporal decompressive craniectomy for control of ICP.1–3 Reports of high morbidity and mortality rates were common in the 1960s to 1980s, but a variety of techniques were used, primarily after clinical deterioration.4–16 With the development of aggressive intensive care management of TBI patients, decompressive surgery has garnered renewed interest in the past decade or so because of improved results.1,2,17–31 The setting in which decompressive craniotomy or craniectomy (DC) is used has evolved tremendously in recent years, most notably with respect to prehospital care, neuroanesthesiology, neuroradiology, neurointensive care, and rehabilitation. Advances in these areas have contributed to a reduction in mortality and improved outcomes for patients with severe TBI.32,33
DC has been used in nontrauma settings as well. Uncontrolled ICP after aneurysmal subarachnoid hemorrhage has been treated with DC in a variety of settings, including signs of cerebral edema during aneurysm clipping; increased ICP and epidural, subdural, or intracerebral hematoma after aneurysm surgery; and cerebral edema and elevated ICP without radiologic evidence of cerebral infarction after aneurysm surgery.34,35 DC has also been reported for the treatment of cerebral edema after cerebral infarction.36–44 Cerebral edema and mass effect after an infarction further compromise CPP, oxygenation, and metabolism and lead to refractory intracranial hypertension and eventually transtentorial herniation, the leading cause of death in these patients.35 In experimental models, DC has been shown to improve CPP, survival, and neurological outcome, as well as reduce the volume of infarction after extensive ischemic stroke.45 These beneficial effects have been attributed to increased collateral circulation, reduction in tissue edema, and improvements in oxygenation and energy metabolism in the ischemic penumbra.35 DC for other indications such as brain tumor, meningitis, acute encephalitis, toxoplasmosis, encephalopathy secondary to Reye’s syndrome, subdural empyema, and cerebral venous and dural sinus thrombosis has also been described.35,46–52
The rationale for DC is straightforward. The incidence of elevated ICP requiring therapy after severe TBI is 65%, and half of the patients who die of severe TBI die with uncontrolled ICP.53,54 The total time that ICP is elevated greater than 20 mm Hg correlates directly with outcome, and mortality is increased when ICP is not controlled.55–59 Therapeutic approaches for decreasing ICP include reduction of the volume of intracranial contents (blood, brain, or CSF), reduction of cerebral metabolic demands, or an increase in cranial volume via DC. DC has been proved to decrease ICP.19,60–62 Decompressive surgery also leads to a rightward shift of the pressure-volume curve and therefore to massive increases in compliance, as well as a decrease in the amplitude of ICP waves and an increase in compensatory reserve.25,63,64 This results in an increase in cerebral blood flow and subsequently CPP and cerebral microcirculation, which allows rebalancing of cerebral inflow-outflow regulation.61 Brain tissue oxygenation (PbtO2) also improves with durotomy at DC.61,62 Because reduction of ICP is associated with improved outcome and DC is associated with reduction of ICP, it stands to reason that DC should also be associated with improved outcome, given an acceptable risk-benefit ratio and appropriate patient selection.
Indications
Most authors agree that patients with bilaterally fixed and dilated pupils, a Glasgow Coma Scale (GCS) score of 3, brainstem injury, and central herniation are universally poor candidates for DC because of the known association of these findings with poor outcomes.16,65 The postresuscitation GCS score, especially the motor score, is one of the most important factors to consider in patient selection.63,66 Care should be taken to exclude possible influences on GCS scores such as intoxication, hypoxia, hypotension, and paralytics or sedatives. Younger patients generally have better outcomes; however, age alone should not be used as an exclusion criterion.65,18,67 The presence of midline shift on computed tomography (CT) of the brain is highly predictive prognostically. The degree of shift is inversely related to outcome, and elevated ICP is presumed.68,69 Absent or compressed cisterns are also predictive of elevated ICP and a poor outcome.68,70 The decision to proceed to DC must therefore take into account the preoperative CT appearance, clinical factors, and measured neuromonitoring trends.
The timing of decompressive surgery is important prognostically; once ICP becomes unmanageable and signs of brainstem compression are noted, DC may be lifesaving, but at the expense of severe neurological impairment. Early decompression (within 4 hours of injury) results in profound decreases in mortality and improvement in functional outcome at 6 months.18 Decompressive surgery should be considered very shortly after failure of maximal medical therapy to control ICP. Initial management includes elevation of the head to 30 degrees, use of sedation with or without paralytics, adjustment of ventilation parameters to maintain Paco2 at 30 to 35 mm Hg, maintenance of normothermia, maintenance of hyperosmolar euvolemia (with the administration of mannitol and hypertonic saline), avoidance of hyperglycemia, support of CPP with volume or pressors, and drainage of CSF. Occasionally, pentobarbital therapy or hypothermia may be used before proceeding to surgery. ICP in the low twenties may be tolerated without proceeding to DC if CPP or PbtO2 (or both) is judged to be adequate. However, ICP elevations or CPP or PbtO2 depressions in the face of maximization of the aforementioned parameters are not tolerated for long periods before surgery.
Technique
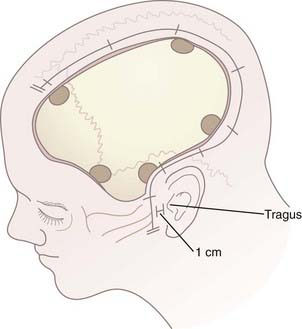
After hair clipping extending just across midline and as far posteriorly as possible, the hemicranium is prepared, marked, and injected with 1% lidocaine with epinephrine to facilitate hemostasis before draping. For a unilateral craniotomy, a standard large question mark or reverse question mark incision is used. The skin incision should start 1 cm in front of the tragus at the zygomatic arch and extend posteriorly above the auricle, upward over the parieto-occipital area, and forward to the frontal region to the hairline. The superior limb should approach the midline, and the posterior limb should be sufficiently posterior to allow creation of an adequately sized bone flap. Although the exact dimensions of the bone flap may vary according to the size and shape of the cranium, the scalp exposure should allow access to specific bony landmarks. For example, the inferior exposure at the temporal region must allow the temporal craniectomy to be extended to the floor of the temporal fossa after the bone flap has been removed (Fig. 338-1; see later also). Scalp hemostasis is facilitated with the use of Raney clips. Bovie cautery is then used to divide the temporalis fascia and muscle in line with the scalp incision. The temporalis muscle, which is often quite edematous, may be reflected anteriorly and inferiorly with the cutaneous flap and both secured with fishhooks after protecting the musculocutaneous flap with rolled sponges underneath. An epinephrine-soaked laparotomy sponge is frequently used on the galeal surface of the cutaneous and muscle flaps to aid in hemostasis. For bifrontal DC, a standard Souttar incision is used.
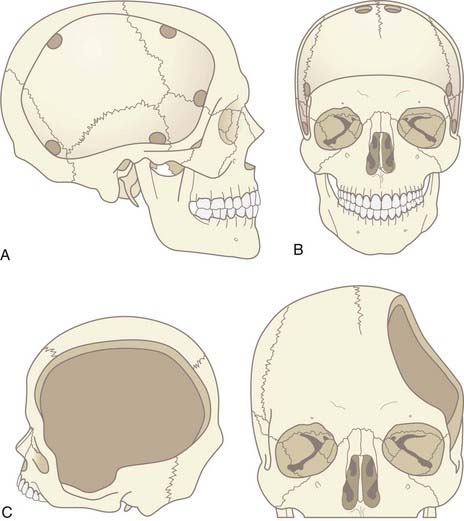
The craniotomy itself for unilateral DC must encompass a large enough area to prevent brain herniation and strangulation, typically from just lateral to the superior sagittal sinus, frontally to the midpupillary line, inferiorly to the floor of the temporal fossa, and posteriorly to the parieto-occipital area (Fig. 338-2A). Bifrontal openings should span from the anterior cranial fossa floor to the coronal suture posteriorly and to the temporal fossa floor bilaterally (Fig. 338-2B). The frontal sinus may be entered, and if so, it should be cranialized. Visibility of the mesencephalic cisterns on postoperative CT correlates with the distance from the craniectomy to the base of the cranium,18 and the size of the bone flap correlates with the degree of reduction of ICP19 (Fig. 338-2C).
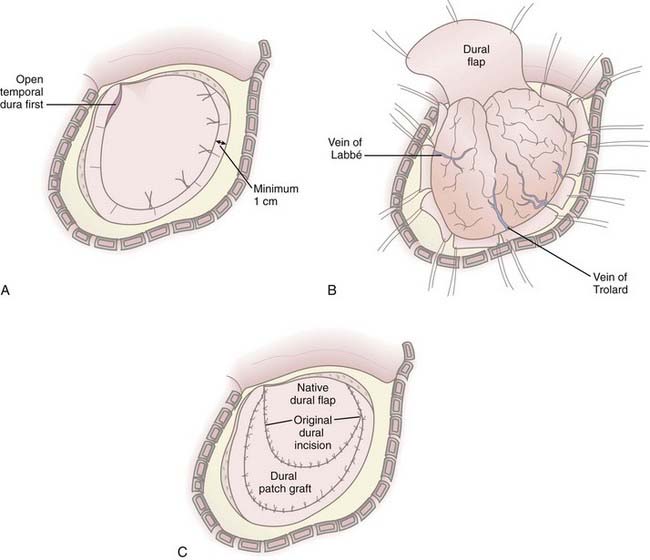
In cases in which it is determined that the bone flap will be left out after evacuation of a mass lesion or after primary DC, durotomy must be performed; otherwise, the operation is ineffective in reducing ICP.61,66,71 Durotomy is akin to fasciotomy in other body compartments. A variety of dural openings have been described. For unilateral operations we use a “C-shaped” dural opening mirroring the scalp incision, with the dural base along the frontal fossa floor (Fig. 338-3A and B). Some have advocated slit dural openings, stellate openings, or other curvilinear openings. For bifrontal procedures, ligation and division of the anterior sagittal sinus, as well as the falx at its most anterior extent, are advocated to allow maximal relief of constriction on the frontal lobes. Whatever the dural opening technique, it is imperative that the temporal dura be opened to decompress the temporal lobe or lobes.
Duraplasty using autograft or allograft may be performed or the dura left open. Several options for dural graft material are available: fascia lata, pericranium, bovine pericardium, collagen matrix products, and synthetic dural substitutes. We perform duraplasty with a collagen matrix or bovine pericardium dural substitute to provide brain protection and avoid adherence of the galea to the underlying brain tissue because return to surgery will be mandated for survivors (Fig. 338-3C). Duraplasty also restores more normal CSF circulation and may prevent or minimize the development of postoperative hygromas and leakage of CSF from the wound. The duraplasty is constructed such that a wide patch graft is created over the herniating cerebral hemisphere or hemispheres, but the following must be avoided: constriction of underlying brain tissue by dural folds and wrinkles and redundant dura or patch graft material resulting in excess graft tissue volume (remembering that in cases of profound cerebral edema, loss of autoregulation, and poor compliance, even the smallest excess volume in the intracranial compartment can contribute to significant increases in ICP). We have used an additional layer of nonbiologic, nonincorporated, synthetic dural substitute as a subtemporalis barrier at the time of DC, which can be readily identified and removed during reimplantation of the bone flap. This has dramatically decreased the dissection time at reoperation and reduces the associated risks of traction on the underlying brain.
A vascular tunneling technique has been described by Csokay and colleagues to alleviate venous congestion (and therefore evolution of brain edema) after decompressive surgery.72 In this technique, small supporting pillars made of hemostatic sponge are placed around the superficial vessels supplying the protruding portion of the brain, thus preventing them from being compressed by the pressure between the dural edge and the brain tissue. In our experience, performing a large craniectomy and durotomy with dural slits along the perimeter of the dural opening perpendicular to the dural incision alleviates the need to perform such a maneuver (Fig. 338-3B).
Partial or anatomic lobectomies have also been performed in conjunction with DC for intractable intracranial hypertension; however, the need for this intervention is significantly lessened when DC is performed early after failure of medical management. If performed, it is often used in conjunction with evacuation of cortical contusions or intraparenchymal hematomas and is limited to the frontal and anterior temporal lobes, preferably on the nondominant side. Lobectomy does not preclude a good outcome.73 However, in our experience, lobectomy is seldom necessary and is reserved for instances of a “pulped” or necrotic lobe with malignant cerebral edema at surgery, pulselessness of the hemisphere, vessel occlusion or infarction (which can be related to a blunt vascular injury), profound coagulopathy in the face of multiple intracranial hemorrhages, or any combination of such conditions. There is a high correlation of lobectomy with poor initial GCS score and emergency surgery in our institution.
The bone flap may be stored in an abdominal subcutaneous pocket or in a certified tissue bank. Abdominal storage has been associated with resorption, rhabdomyolysis,74 and infection and of course requires an additional incision and procedure at both storage and retrieval. Contaminated flaps should not be stored in the abdominal wall.
The interval between decompressive surgery and secondary bone flap replacement in the literature varies from 4 weeks up to 12 months, during which time the unprotected brain is exposed to the risk of injury.75 We recommend the use of a soft helmet when out of bed until the bone flap is replaced to reduce the risk for secondary brain injury. The helmet is removed when the patient is safely in bed or in a chair to prevent scalp sweating and friction on the incision. Our typical approach to the timing of staged secondary bone replacement is to replace the flap at around 3 months. When patients with severe TBI arrive for their first neurosurgical follow-up after discharge from the hospital or acute inpatient rehabilitation, elective surgery for replacement of the bone flap is scheduled if no contraindications exist, such as ongoing active infection, ongoing advanced-stage decubitus ulcers, or extremely poor nutritional status. This approach allows in-hospital and rehabilitative treatment of associated injuries, resolution of cerebral edema, and reduction in the risk for infection related to intensive care unit interventions or nosocomial infections. At the same time, avoidance of longer intervals before bone replacement reduces the incidence of scalp contraction (which can contribute to wound dehiscence), temporalis atrophy (which can contribute to temporomandibular joint complaints and cosmetic asymmetry), and development of the syndrome of the trephine (which can cause severe headache). Furthermore, some patients do exhibit neurological improvement once the flap is replaced, especially improvement in motor and speech deficits, reduction in seizure frequency, and cognitive improvements. Early replacement before the development of severe encephalomalacia also eliminates the potential dead space under the flap postoperatively, thereby decreasing the postoperative risk for epidural hematoma, pneumocephalus, and fluid collections. Finally, early replacement provides cerebral protection during the phases when patients are most at risk for further cerebral injury, such as after they are beginning to be actively transferred and are starting to walk again but are still likely to experience imbalance, incoordination, and orthostatic hypotension.
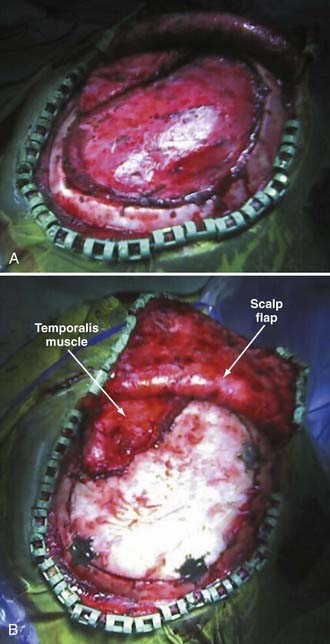
Reimplantation surgery requires meticulous wide sterile preparation of the scalp incision. We use a wide hair clip to identify potential problems from lacerations, abrasions, and other injuries and to facilitate preparation and postoperative wound care. Perioperative antibiotics are administered. The previous incision is used, and care is taken to avoid direct trauma to the underlying brain tissue from local anesthetic injection, scalp incision, and dissection of the cutaneous flap away from the dura. Care must be taken to avoid transfer of heat to the underlying brain from monopolar cautery or pressure on the brain from digital dissection or traction on the cutaneous flap. We use sharp dissection with Metzenbaum scissors and bipolar cautery for hemostasis. The cutaneous flap is gently and consistently lifted upward and gradually retracted as the dissection proceeds, and the bone edges are identified and dissected free of scar tissue with a Penfield No. 1 dissecting tool. Removal of previously placed epidural tack-ups is often necessary to achieve anatomic reseating of the bone flap. There is frequently a ridge of scar tissue at the perimeter of the bone flap that may be shrunken back with bipolar cautery (Fig. 338-4A). The border of the temporalis muscle is identified by sharp dissection, and the muscle is dissected off the underlying dura, to which it is frequently quite adherent unless a barrier has been placed as previously described. It is often not necessary to dissect the temporalis entirely because the bone flap may not fill the cranial defect completely if portions of the temporal bone were rongeured and discarded at the original surgery. Central epidural tack-ups are used to aid in postoperative hemostasis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree