Infections of the Central Nervous System
Ana Rubio
This chapter reviews infectious diseases of the central nervous system (CNS) as seen in forensic neuropathology. Worldwide, few areas of pathology present such a geographic diversity in mortality and morbidity as do infectious diseases; moreover, in the past 50 years, CNS infections have become more prominent. Innovations in prophylaxis and more aggressive medical and surgical treatments have changed the epidemiology of infections in the developed world.1 Some of the entities with the highest mortality and morbidity in the developing world, including bacterial (tuberculosis), viral (poliomyelitis), protozoal (malaria), or parasitic (cysticercosis) infections affecting the brain, are only rarely encountered in inhabitants of developed countries, with the exception of immigrants and travelers who have visited developing areas or the tropics. This chapter does not represent the global impact of CNS infections, but focuses on what is seen in the United States. We include neither a detailed description of specific microorganisms nor characteristic pathologic findings (which are covered in texts and reviews of infectious diseases and neuropathology2, 3, 4, 5, 6, 7, 8, 9, 10). Instead, we attempt to provide guidelines for the diagnosis, management, and reporting of suspicious or unsuspected cases of CNS infection reaching the medical examiner’s office.
To enter the brain, pathogens must cross the blood-brain barrier and overcome the innate defense mechanisms within the brain. An infection can spread to the brain through direct extension from a neighboring focus such as sinusitis or mastoiditis. More frequently, however, pathogens enter the brain from the bloodstream either bypassing the blood-brain barrier by transcellular penetration through the endothelium (Listeria monocytogenes and Rickettsia), via paracellular entry (Trypanosoma), or through infected cells carrying the pathogen (L. monocytogenes, Mycobacterium tuberculosis, and human immunodeficiency virus [HIV]), which has been called the “Trojan horse” entry.11, 12, 13
CNS infections can be classified according to the causative agent (e.g., bacteria, virus, fungus, parasite, prion), characteristics of the host (e.g., age, geographic, economic, or sociopolitical environment; state of health or immunocompetency), gross anatomic involvement (e.g., whether affecting the brain parenchyma, leptomeninges, dura, dura-related spaces), or type of histopathologic reaction (e.g., acute versus chronic, hemorrhagic versus bland). We have found that a classification based primarily on the anatomicpathologic involvement (combined gross and microscopic findings) is most helpful for diagnosis, especially in cases unsuspected clinically.
EPIDEMIOLOGY
In forensic settings, clinically confirmed CNS infections are rare, because those cases would not be usually referred to a medical examiner’s office. Most commonly, infection is first detected or suspected at autopsy. In our experience, CNS infections are the cause of death in a minority of medical examiner’s cases. Of 4, 478 natural deaths certified at the Office of the Chief Medical Examiner of the State of Maryland in a 3-year period (2003 to 2005), 378 (8.4%) had an infectious cause listed as the main cause of death. In 33 cases (8.7% of all infectious cases and 0.7% of all natural deaths), the cause of death was a primary CNS infection. Among the 33 CNS infections, there were 20 cases of bacterial meningitis, seven cases of cryptococcal meningitis, two cases of encephalitis, two cases of viral meningoencephalitis, one case of poliomyelitisrelated complications, and one case of acute disseminated encephalomyelitis. In addition, 40 deaths were coded as acquired immune deficiency syndrome (AIDS) or complications of AIDS, and 55 cases had bacterial, viral, or fungal sepsis (unpublished review of the Office of the Chief Medical Examiner of the State of Maryland).
When performing an autopsy on a suspected infectious case, the appropriate collection and handling of fluids and tissue samples for cultures and other diagnostic techniques are of paramount importance. Collection of samples and interpretation of the results require experience and clinicopathologic correlation.14 Table 18.1 lists some of the circumstances that increase the risk of CNS infection. A high level of awareness is necessary for the rapid diagnosis
of contagious cases, when quick feedback to the local health department and information to close contacts may prevent spreading. The most likely scenarios of CNS infections in forensic settings are as follows:
of contagious cases, when quick feedback to the local health department and information to close contacts may prevent spreading. The most likely scenarios of CNS infections in forensic settings are as follows:
TABLE 18.1 Increased Susceptibility to CNS Infections | ||
|---|---|---|
|
Sudden and unexpected death in previously healthy individuals (e.g., bacterial meningitis in young college students).
An incidental finding, whether or not the cause of death (e.g., cerebral abscesses in intravenous drug users with endocarditis who are brought to the office primarily for toxicologic testing, opportunistic infections in individuals with AIDS).
Spreading to the brain of systemic or localized infections (i.e., pulmonary bronchiectasis, sinusitis, mastoiditis, osteomyelitis, postsurgical or posttraumatic infections).
Complications of the clinical course in vulnerable subjects (e.g., infection of devices such as ventriculoperitoneal shunts, children with malformations of the brain).
Incidental findings during the autopsy of individuals with traumatic death.
The proband of a new disease or the arrival of an old disease in a new area (e.g., Lyme disease, West Nile encephalitis).
Rarely, infections that are the result of criminal actions (e.g., intentional contamination, terrorism).
Medical examiner offices are especially positioned to see uncommon presentations of common diseases and, in exceptional cases, even to detect new diseases, including emerging infectious diseases15 resulting from either evolution of the infectious agent (enterovirus 70 and 71 inducing polio-like paralytic diseases), movement of the agent into new geographic areas (Lyme disease, West Nile encephalitis), or entry of the agent into new hosts across species (variant Creutzfeldt-Jakob disease from bovine spongiform encephalopathy, AIDS, and others). Autopsies have been crucial for the detection and description of index cases of local epidemics or diseases spread from localized geographic areas.16, 17, 18, 19, 20
ANATOMIC CLASSIFICATION OF CNS INFECTIONS
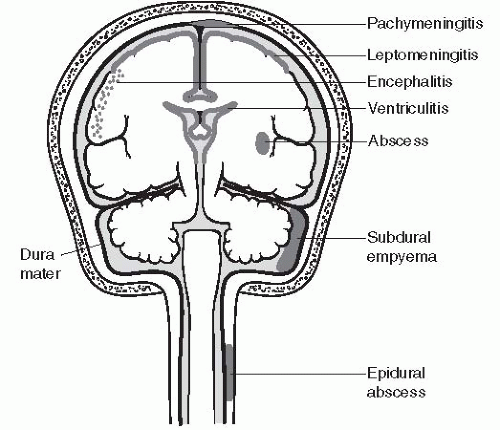
Usually, specific CNS infections are limited to specific anatomic compartments.21 The infectious agent may reach its target by a hematogenous route (e.g., embolism), by spread of local infection (e.g., paranasal sinuses), or by advancing to the CNS through peripheral nerves (e.g., rabies, herpes). The following is a brief review of infections by anatomic boundaries (Fig. 18.1).
Epidural Abscess
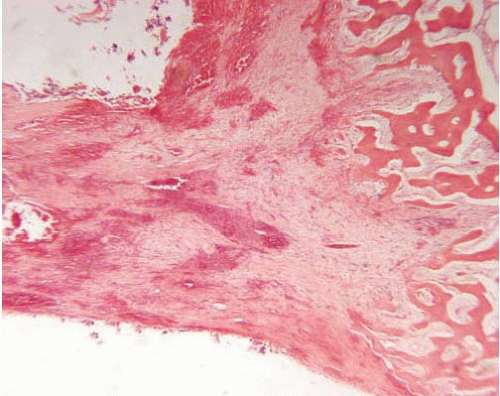
Epidural abscess is an infection localized to the space between the bone and the dura mater, and is found more commonly in the spine than in the skull. The majority of incidents originate from the spread of local infection. Predisposing factors include osteomyelitis (Fig. 18.2), surgical manipulation, placement of shunts, epidural injections, sinusitis, or trauma.
Microorganisms
Microorganisms are almost exclusively bacterial (Staphylococcus aureus, Staphylococcus epidermidis, or Gram-negative bacteria). Rarely, abscesses are caused by M. tuberculosis in subjects with Pott’s disease (spinal tuberculous osteomyelitis).
Gross Appearance
There is collection of purulent exudate within the epidural space. Because the cranial dura is attached to the inner table of the calvaria, intracranial epidural abscesses acquire a typical biconvex shape, as the collection of pus forces the separation of dura and bone (similar to that of epidural hematomas, see Chapter 6). In the spine, where the dura is naturally located away from the bone, separated by fat and soft tissue, the infection spreads along the vertical axis more freely and is usually found along several vertebrae and surrounding nerve roots.
Microscopic Findings
When the process is fully developed, a purulent inflammatory exudate with necrosis and neutrophilic infiltration is followed in time by granulation tissue development and fibrosis.
Pachymeningitis
Pachymeningitis is an inflammation of the dura mater. Isolated infectious pachymeningitis is rare and is most commonly caused by M. tuberculosis; the infection of the dura is more often seen as an integral part of leptomeningitis or epidural or subdural abscesses. Cases are often detected by magnetic resonance imaging (MRI); studies performed in individuals with headache, cranial nerve abnormalities, or seizures may identify a thickened, enhancing dura (hypertrophic pachymeningitis). The etiologic diagnosis of hypertrophic pachymeningitis (whether infectious, neoplastic, autoimmune or, as is most commonly the case, idiopathic) can only be accurately made by histologic studies.22, 23
Subdural Abscess (Empyema)
Subdural abscess is a localized infection between the dura mater and the leptomeninges. It is most commonly located intracranially and supratentorially. It is usually unilateral, as the falx prevents the spread to the opposite side. As in epidural abscesses, most cases result from local spread, most commonly from sinusitis.24 Subjects most commonly present in the second decade of life, and men predominate over women.25 In children, subdural abscess may complicate leptomeningitis. Other causes include previous surgery, head trauma, or spread from overinfection of subdural hematoma. 25 Predisposing factors include sinusitis, ear infections, surgical manipulation, or trauma.
Microorganisms
Gross Appearance
There is purulent exudate adherent to the dura.
Microscopic Findings
Microscopic findings depend on the clinical course; when the abscess is well developed, it consists of a purulent inflammation with necrotic debris walled off by granulation-like tissue. Subdural abscesses in children can remain as subdural hygromas.
Septic thrombophlebitis with venous thrombosis and resulting venous infarctions may result from infectious involvement of the venous sinuses.
Mycotic aneurysms result when the walls of large arteries are invaded by the microorganism (in spite of the name, the cause is bacterial more often than fungal).
Meningitis (Leptomeningitis)
Meningitis is an inflammatory process affecting the leptomeninges. Meningitides are the most common CNS infections encountered in the United States, affecting four to six individuals per 100, 000 adults, with a mortality approaching 20%. Predisposing factors include systemic infections (most commonly pneumonia or sepsis), developmental abnormalities (especially malformations), surgical procedures, 27 or ventriculoperitoneal shunts. Traumatic injuries of the base of the skull may produce recurrent bouts of meningitis. Most occurrences are of bacterial cause and result from hematogenous spread of organisms that have colonized mucosal surfaces. The classic triad of fever, neck stiffness, and altered mental status is only present in approximately 40% of cases.28 Age, environment, and host characteristics determine the incidence and specific agent. Individuals who survive the acute illness may have long-term complications, including hydrocephalus and infarcts, and significant clinical morbidity. Viral meningitides (aseptic meningitis, see below) are common, but the majority are not diagnosed and are either clinically silent or have a mild clinical course concurrent with a systemic viral illness.
Microorganisms
Most of the clinically relevant cases are bacterial. Although Pneumococcus, Meningococcus, and Haemophilus influenzae were historically the three most common bacteria, the epidemiology of meningitis has changed dramatically in the last quarter of the 20th century after the introduction of H. influenzae vaccine in the 1990s and the approval of Streptococcus pneumoniae vaccine in 2000.29, 30, 31 The relative incidence of H. influenzae meningitis in pediatric cases has declined from 44% in 1986 to 7% in 199732; the introduction of pneumococcal conjugate vaccine (PCV7) in the routine pediatric vaccination schedule has resulted in a decrease of more than 60% in pneumococcal meningitis, 34, 35 and the majority of current cases in vaccinated individuals are due to serotypes not included in the vaccine conjugates. Other successful strategies contributing to the recent decline in bacterial meningitis in the United States include the prevention of food contamination by Listeria monocytogenes and intrapartum prophylaxis against group B Streptococcus (GBS) infection.36
GBS causes the majority of neonatal meningitis in infants younger than 3 months of age.29, 31 Bacteremia follows delivery, and pneumonia and meningitis present thereafter. GBS meningitis has a protean presentation, and ventriculitis is common in the course of the illness.33 The second most common microorganism is Escherichia coli with K1 antigen. A rare but severe neonatal viral leptomeningitis is produced by the lymphocytic choriomeningitis virus, with congenital infection due to vertical transmission from mother to fetus. The lymphocytic choriomeningitis virus can also be transmitted by rodents, and in addition to affecting young infants, can also affect the immunosuppressed, including transplant recipients.37
Currently, Neisseria meningitidis causes most seasonal, sporadic cases of meningitis in children and adolescents from 2 to 18 years of age33, 40 in the United States. N. meningitidis produces endemic and epidemic meningitis. Rates of epidemic N. meningitidis are two per 100, 000 in the developed world, and the specific strain varies depending on socioeconomic and environmental conditions. Of the five serotypes that cause 90% of the N. meningitidis meningitis, types B and C predominate in the United States, with a recent increase in the frequency of type Y and a similar recent decrease in cases resulting from W-135.41 Development of a universally effective N. meningitidis vaccine is being attempted.40 The N. meningitidis vaccine currently used in the standard immunization protocols does not cover all serotypes, in part because N. meningitidis acquires genetic variability through frequent genetic recombination.36 In addition, the vaccine is not fully effective in children younger than 2 years of age.
In adults, the most common bacterial causes of meningitis are S. pneumoniae and N. meningitidis30, 31 followed by other species of Streptococcus, Staphylococcus, and Listeria.
Meningitis caused by L. monocytogenes is most commonly seen at both ends of the age spectrum, with the highest incidence in older adults; other risk factors include diabetes, malignancies, alcoholism, or liver disease.42
Case 18.1
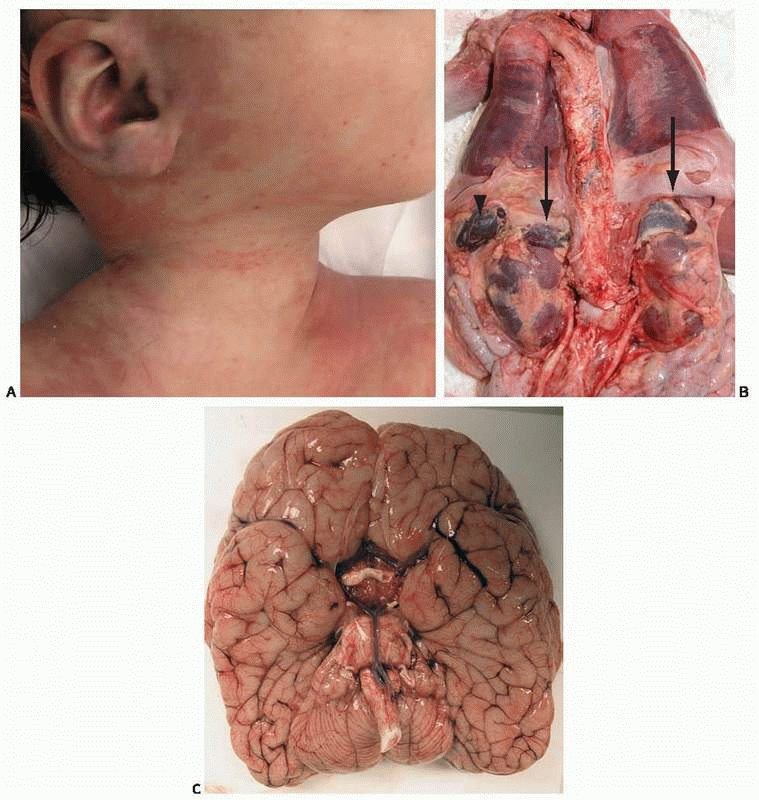
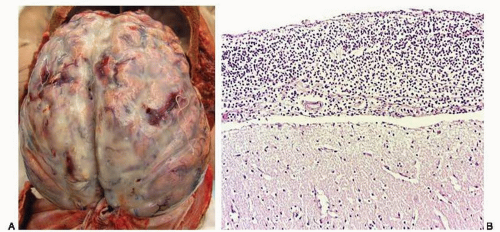
A nearly 5-month-old girl was found unresponsive in her crib at her day care center. Resuscitation efforts were unsuccessful and she was pronounced dead soon afterward. She was generally healthy, but over the last few days she had been “fussy” and had a low-grade fever. She was up-to-date in her immunization routine; a week before death she had received the second injection of the pneumococcal vaccine. External examination at autopsy revealed bilateral eyelid erythema and a faint macular rash, more evident at the end of autopsy (e-Fig. 18.1, Fig. A). The enlarged adrenal glands were hemorrhagic (Fig. B, arrows) with an external appearance similar to that of the spleen (arrowhead). On cross-section the adrenal glands resembled bags of blood. The brain was externally unremarkable (Fig. C) and microscopically did not show inflammation. Emergency Gram stain of the postmortem cerebrospinal fluid revealed Gram-positive diplococci. Cultures were positive for S. pneumoniae.
|
Gross Appearance
There is milky exudate involving the leptomeninges (Fig. 18.3A, e-Figs. 18.3A and B). The thickness of the exudate and the opacity of the meninges are directly related to the duration of the disease (the meninges in very acute infections may appear normal). The involvement is usually bilateral, symmetric, and generalized; in some cases, the exudate can only be seen in the convexities or the basilar surfaces, whereas in other cases, inflammation of the ventricles can be detected easily (ventriculitis). Most cases display vasculitis, and the lesions may be hemorrhagic.
Case 18.2
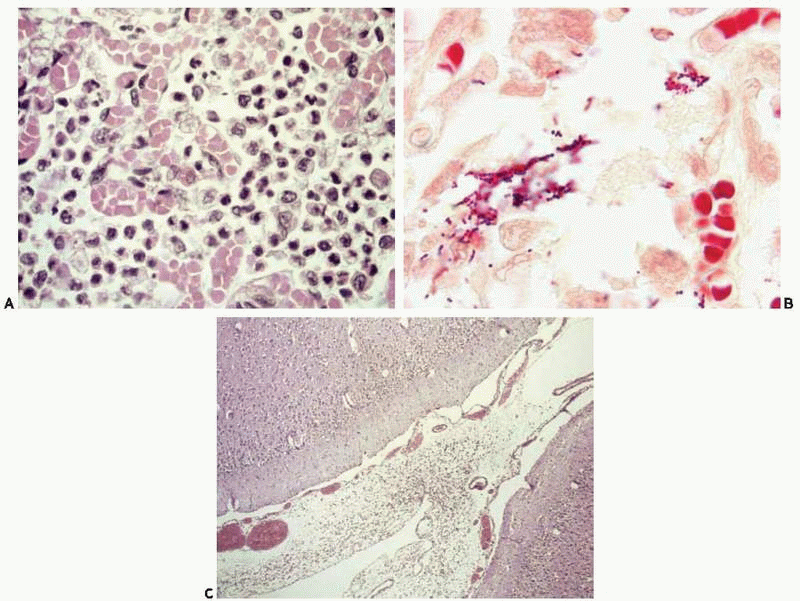
A 10-day-old girl had a nose bleed and became apneic shortly after being breastfed. Resuscitation maneuvers were started at home and the baby was immediately transported to the hospital, where she was pronounced dead soon after arrival. Postmortem blood cultures were positive for GBS. Autopsy revealed patchy consolidation of both lungs. Microscopically, the air spaces were collapsed, and had abundant alveolar macrophages, early hyaline membrane formation, and patchy bronchopneumonia (Fig. A and e-Fig. 18.2). A modified Gram stain was positive for Gram-positive cocci in chains (Fig. B). The brain was grossly unremarkable, but microscopic examination revealed hypercellular leptomeninges with incipient leptomeningitis (Fig. C). Of note, the mother had tested positive for vaginal GBS before delivery and had been treated according to current recommended guidelines. Recent reports38 indicate that early-onset GBS neonatal infection may be prevented by prepartum maternal screening and prophylaxis with antibiotics, but the current guidelines may not prevent late-onset cases, and the risk for neonatal GBS may actually be increased by the maternal treatment.39
|
Microscopic Findings
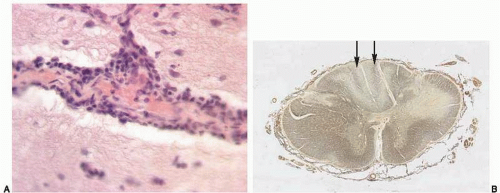
There is purulent exudate (with abundant neutrophils) in the leptomeninges and subarachnoid space (Fig. 18.3B, e-Fig. 18.3C), including Virchow-Robin spaces. Gram stains usually show bacteria. After the initial stage, fibrinous exudate is followed by fibrous resolution.
Leptomeningitis in children may spread into the brain, producing intraparenchymal abscesses, or to the subdural space, producing subdural empyema. Basilar meningitis produced by L. monocytogenes may be accompanied by intraparenchymal nodules in the brainstem. Obstructive hydrocephalus may be a long-term complication of meningitis, resulting from obstruction to cerebrospinal fluid (CSF) drainage resulting from fibrosis around the Pacchioni granulations. Tuberculous meningitis has a basilar topography. It may be the manifestation of primary infection or reactivation of a latent infection. Microscopically, it is characterized by a granulomatous infiltrate with giant cells and vasculitis (endarteritis obliterans).
Multiple types of fungi can cause fungal meningitis, depending on geographic and host characteristics. Blastomycosis resembles tuberculous meningitis, but is commonly accompanied by pachymeningitis, and affects farmers; Candida affects infants, diabetics, or the immunosuppressed. Cryptococcus neoformans involves the basal leptomeninges preferentially and produces a “soap bubbles” appearance as a result of dilatation of perivascular spaces (Fig. 18.4A, e-Fig. 18.4); it is characterized microscopically by the lack of an inflammatory response as a result of the antiphagocytic properties of the carbohydrate capsule43 (Fig. 18.4B). CNS infection
with C. neoformans occurs most commonly in immunosuppressed subjects, and in HIV-infected individuals, it usually coexists with other infections. The gattii variety of C. neoformans can produce infection in immunocompetent individuals.43 Other fungi of importance include those causing histoplasmosis (producing a chronic meningitis in approximately 5% to 10% of subjects with disseminated disease44), cladosporiosis, coccidioidomycosis, pseudallescheriasis, and Absidia.
with C. neoformans occurs most commonly in immunosuppressed subjects, and in HIV-infected individuals, it usually coexists with other infections. The gattii variety of C. neoformans can produce infection in immunocompetent individuals.43 Other fungi of importance include those causing histoplasmosis (producing a chronic meningitis in approximately 5% to 10% of subjects with disseminated disease44), cladosporiosis, coccidioidomycosis, pseudallescheriasis, and Absidia.
Meningovascular syphilis most commonly presents years after the initial infection, and, similar to tuberculous and listerial meningitides, has a basilar topography. Microscopically, meningovascular syphilis is characterized by chronic lymphoplasmacytic inflammation and endarteritis obliterans (with involvement of medium and large arteries and resulting ischemic lesions) and intraparenchymal nodules (gumma) usually attached to the dura. Tabes dorsalis (degeneration of the posterior columns of the spinal cord) may be the end result of meningovascular syphilis affecting the spinal cord (Figs. 18.5A and B).
Eosinophilic meningitis is most likely due to metazoal infection with Angiostrongylus cantonensis or Gnathostoma spinigerum.
Aseptic meningitis (nonpurulent meningitis) is a benign disease usually seen at autopsy as an incidental finding. It is characterized by vascular congestion and mild lymphocytic meningeal infiltrates. The cause is usually viral (enteroviruses cause approximately half of all cases, including echovirus, coxsackie A and B, enterovirus 71, herpes simplex virus (HSV) type 2, mumps, HIV, arboviruses, adenovirus, and measles).36, 45, 46 Less commonly, aseptic meningitis is associated with neoplastic processes or drug reactions.47, 48 Rarely, aseptic meningitis may be caused by Lyme disease.
Recurrent aseptic meningitis (Mollaret meningitis) preferentially affects women and is most commonly caused by HSV2.46 Large
mononuclear cells (Mollaret cells) can be isolated from the CSF during an acute episode. Patients may have recurrent genital herpes.
mononuclear cells (Mollaret cells) can be isolated from the CSF during an acute episode. Patients may have recurrent genital herpes.
Brain Abscess
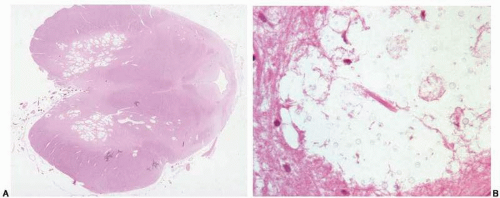
Brain abscess is a localized, purulent infection of the brain parenchyma. Approximately 15% to 20% of cases are of hematogenous origin; abscesses are commonly multiple, located at the gray-white junction of the cerebrum on the middle cerebral artery distribution (Figs. 18.6A-C, e-Figs 18.5A-C), and the focus of origin is the heart, lungs, or rarely, other infectious sources49 (e-Fig. 18.6). More than half of all brain abscesses result from local spread from paranasal sinusitis24 or middle ear infections (most commonly located in the temporal poles or cerebellum). In approximately one-quarter of all subjects, the source is unknown.49, 50 Although mortality has decreased sharply during the last four or five decades, morbidity remains high. Common clinical presentations are acute increased intracranial pressure or focal signs as a result of mass effect. Neuroradiologic images show the stage of the disease, and at the peak of abscess formation, the classic neuroradiologic image is that of a ring-enhancing lesion.21
Microorganisms
Microorganisms most commonly include bacteria, followed by fungi (Aspergillus, Candida, Cladosporium, Pseudallescheria boydii), Toxoplasma, amoeba (Entamoeba histolytica), and metazoa (those causing cysticercosis, hydatidosis, schistosomiasis, toxocariasis).
Gross Appearance
Gross appearance depends on the development of the lesion. The initial stage is characterized by hyperemia and localized softening. After a few days, a distinct necrotic center forms. Well-formed abscesses (older than 2 weeks) are characterized by a fibrous capsule walling off purulent content.
Microscopic Findings
Microscopic findings vary according to the age of the lesion (Fig. 18.7). Initially, acute inflammatory exudate is present, followed by purulent exudate surrounded by granulation tissue. Wellformed abscesses have a fibrotic capsule with necrotic contents surrounded by gliotic tissue and acute and chronic inflammation. If the abscess breaks into the ventricle, intraventricular empyema develops.
Aspergillus, 51 as do other fungi, tends to invade vascular walls and produce necrotizing vasculitis, thrombosis, and hemorrhage. Aspergillus may reach the brain from the maxillary sinuses or from the lungs, the most common reservoirs in humans. The neuropathologic picture is that of combined cerebritis and ischemic/hemorrhagic lesions.51
Toxoplasmic infection most commonly results from reactivation of a latent infection in immunocompromised subjects.52 Grossly, it presents with multiple poorly formed abscesses located at the gray-white junction or the deep gray matter. Microscopically, it is characterized by necrosis, hemorrhage, variegated inflammation with pseudocysts (bradyzoites), or free forms (tachyzoites) that can be visualized with hematoxylin and eosin (Fig. 18.8) or with immunostains.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree