Intoxications
Mary Ripple
Juan C. Troncoso
Ana Rubio
Barry Levine
One of the basic principles of toxicology is that any substance can be toxic in the appropriate concentration. Exposure to a toxicant can occur by a variety of routes of administration, including oral ingestion; inhalation; intravascular, subcutaneous or intramuscular injection; or transcutaneously. Whether or not a poison is the cause of death depends on a variety of factors, including dose, solubility, route of administration, degree of exposure, absorption, metabolism, distribution, and rate of elimination.
Intoxications can be either acute or chronic. Acute and chronic exposures cause a variety of symptoms and can have long-lasting or permanent effects. Often, we can detect the acute or late effects of these toxicants in the examination of the brain or nervous system. Although few toxicants leave morphologic footprints of their acute effects on the brain, more leave evidence either of their direct chronic effects or associated metabolic derangements. Among the former, we will focus on carbon monoxide, a fairly common toxicant, which illustrates well the concept of acute toxicity, whereas alcohol and narcotics are good examples of the latter category.
The nervous system can be the primary target for a toxic substance, or indirect, secondary damage can be inflicted. Neurons, astrocytes, and oligodendrocytes have selective vulnerability to different agents. Pathologic changes of toxic encephalopathy may include cerebral edema and neuronal, axonal, or white matter injury. Both the central and peripheral nervous systems can be affected and may result in seizures and abnormalities of consciousness, sensation, motor function, and behavior.1
Autopsy findings in poisoning deaths are usually nonspecific, and the diagnosis is usually reached by toxicologic analysis as determined by circumstances elucidated during the case investigation. That investigation at best should offer a clue to the inciting agent and should involve a complete medical and social history and interviews with family, friends, witnesses, and physicians. Scene findings, such as the presence of controlled drug substances (Fig. 22.1), evidence of drug use (Fig. 22.2), and drug paraphernalia (Fig. 22.3), and reported symptoms of loss of consciousness, seizures, tremor, hallucinations, and dementia suggest poisoning. Major suspicion should be raised in cases of conspicuous psychophysical changes that cannot be clinically explained by illness. Autopsy findings of unusual smells; recent injection sites or track marks (Figs. 22.4, 22.5 and 22.6); skin popping marks; tongue bites; discolored lips, teeth, or stomach contents; heart valve vegetations; enlarged lymph nodes of the porta hepatis; a nodular or fatty liver; ascites; sequelae of human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), and multiple tablets in the stomach or proximal small bowel suggest drug or alcohol
abuse. Nonspecific findings of visceral congestion, pulmonary edema, or cerebral edema may be the only conditions that are present, however. If a morphologic central nervous system (CNS) finding at autopsy cannot be attributed to any known disease, then a toxic agent must be considered.2 In addition, microscopic findings that suggest drug abuse include hepatitis, cirrhosis, liver steatosis, endocarditis, and foreign body granulomas with birefringent foreign material in the lungs or lymph nodes.
abuse. Nonspecific findings of visceral congestion, pulmonary edema, or cerebral edema may be the only conditions that are present, however. If a morphologic central nervous system (CNS) finding at autopsy cannot be attributed to any known disease, then a toxic agent must be considered.2 In addition, microscopic findings that suggest drug abuse include hepatitis, cirrhosis, liver steatosis, endocarditis, and foreign body granulomas with birefringent foreign material in the lungs or lymph nodes.
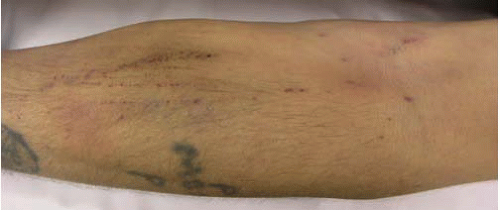
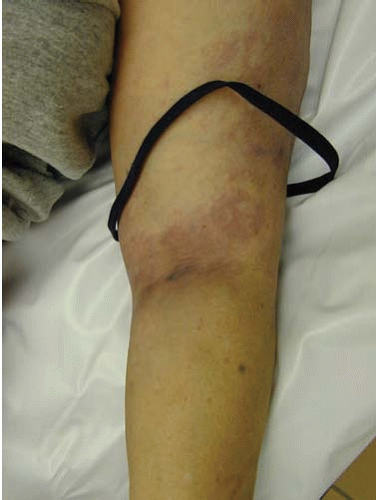 FIG. 22.2. The antecubital fossa of the arm with signs of recent venipunctures and a tourniquet, a common finding in acute heroin intoxication, is shown. |
 FIG. 22.3. This tank contained helium, which was inhaled by the decedent to get euphoric (“high”). The helium displaces the oxygen from the breathing air and causes asphyxia and death. |
Postmortem forensic toxicologic analysis starts with the collection of the proper specimens at autopsy. When available, heart blood, peripheral blood, vitreous humor, urine, bile, liver, kidney, and stomach contents should be collected on all cases. Proper chain of custody needs to be followed in specimen receipt and accessioning. The analytical process includes analyte separation, identification, confirmation, and quantification. Separation includes volatilization and acid/neutral and basic drug liquid/liquid or solid-phase extraction. Initial screening of urine, bile, or blood uses identification techniques that include color tests and ultraviolet spectrophotometry, gas or liquid chromatography, or immunoassay. If the initial drug screen is positive, the presence of the drug must be confirmed by another analytical technique for the substance to be reported as positive. Gas chromatography/mass spectroscopy is the gold standard confirmatory technique used in toxicology. Drug standard curves and internal standard compounds are used to calculate the concentration of drugs in the blood. The quantified level of a drug in the blood should only be interpreted as a cause of death after careful correlation with investigative and autopsy findings and consultation with the toxicologist. The determination of the manner of death also requires interpretation of the drug levels in the context of the scene findings and presence of other disease processes or injury.3
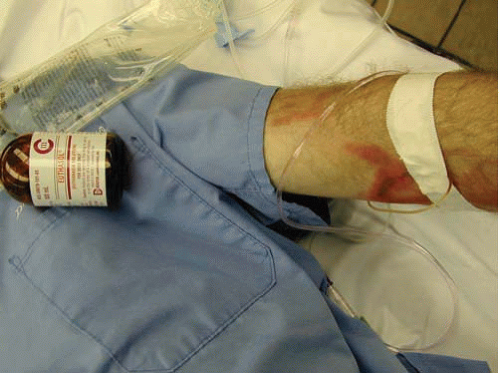
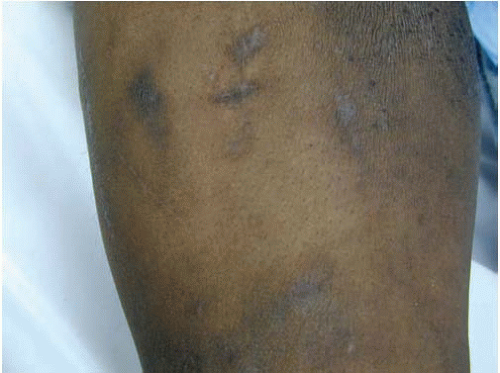 FIG. 22.5. Anterior aspect of the arm of an intravenous drug user showing chronic scars of repeated venipunctures, also known as track marks. |
Toxic substances are frequently the cause of death in forensic autopsies and may occur as a consequence of accidents, suicides, or homicides. Approximately 600 to 700 drug intoxication deaths occur per year in Maryland (approximately 10% of autopsies), primarily accidental overdoses, followed by suicides (Fig. 22.7) and a few homicides. Accidental drug overdose and the effects of alcohol are frequent findings in forensic pathology and neuropathology. Accidental poisoning can also involve children or can result from occupational or environmental exposure or iatrogenic errors. Homicidal poisoning is rare and usually involves an assailant who has some knowledge of medicine. These assailants either use their knowledge to euthanize a debilitated individual or they have a psychiatric or financial reason to eliminate an individual. It is the responsibility of the medical examiner or coroner to investigate a death thoroughly to determine cause and manner of death. In that investigation, a complete neuropathologic examination by a forensic neuropathologist is often necessary.
CARBON MONOXIDE
Intoxication with carbon monoxide (CO) is common and may be the cause of more than half of all fatal poisonings reported worldwide.4 CO is formed from the incomplete combustion of organic materials. The most common circumstances of fatal exposure to CO include fires, malfunctioning stoves and heaters, or work-related accidents in improperly ventilated areas, thus explaining the increased frequency of accidents in the cold season. The second most common cause is the exhaust of internal combustion engines, of which automobile engines are by far the most common culprits. We are already seeing deaths in subjects exposed to the exhaust of the increasingly popular gas-powered domestic electricity generators. CO deaths are usually accidental or suicidal. Rare instances of homicidal CO intoxication occur, and the determination is based on investigation. These instances usually involve arson fire-related deaths. In Maryland in 2005, there were 16 CO intoxication deaths, of which seven were suicides and nine were accidental. In that same year, there were 59 smoke inhalation deaths, most of which (90%) were accidents, but there were three suicides (5%), two homicides (3%), and one undetermined death (2%).
Although this chapter focuses on cases of CO toxicity that are fatal or leave the victim with morphologic brain lesions, the reader should be aware that low-level exposure to CO has the potential to produce significant morbidity (for review see Raub et al.4), and the neuropathologic lesions can be ascertained when the surviving individual dies of unrelated causes.5, 6, 7
Mechanisms of Tissue Damage
CO is a colorless, tasteless, odorless, and nonirritating gas that has a 200 to 300 times greater affinity for hemoglobin than oxygen (O2). It displaces O2 and forms a compound known as carboxyhemoglobin (COHb). The presence of COHb in red blood cells results in decreased capacity of the blood to carry O2, reduced availability of O2 to tissues, and resultant tissue hypoxia and damage. CO also binds to myoglobin, catalase, and cytochrome c oxidase and inhibits adenosine triphosphate synthesis.8 This impairment of cellular respiration and other cellular functions generates oxidative radicals. A third, recently described mechanism of brain injury in CO poisoning is the marked elevation of nitric oxide in brain tissue as a result of neuronal nitric oxide synthase activity linked to calcium influx through N-methyl-D-aspartate channels.9 Clinically, hypotension and functional anemia occur, leading to oxygen deficiency and global ischemia. Notably, these mechanisms of tissue injury are frequently compounded by impaired perfusion secondary to CO-induced hypoxic cardiac dysfunction.2
Scene Investigation
Fire-related death investigations by the medical examiner’s or coroner’s offices and law enforcement agencies are integral in determining the manner of death, as autopsy findings would be the same in an individual dying from smoke inhalation in a fire set by another individual (arson/homicide), a fire set intentionally to kill oneself (suicide), or in an accidental fire. At the scene of a fire-related death, it is important to know whether the fire department tested for atmospheric oxygen, CO, or other chemicals, such as cyanide or benzene. Other useful information includes whether oxygen was administered by health care personnel and any initial COHb saturation levels obtained at the hospital. Important scene findings are the location of the victim, the presence or absence of accelerants, other living or dead victims, and the origin and cause of the fire. These investigations can be time-consuming and exhaustive, but they are necessary to determine the manner of death. The investigation of non-fire-related CO intoxication deaths can also be complex, and in some cases, the manner of death is not obvious. The scene may be that of an obvious suicide in which the decedent left a suicide note and connected a hose from the car exhaust to the inside of the car. In these cases, the heat from the exhaust can accelerate decompositional changes, causing postmortem skin slippage. Additional investigation must also include whether the vehicle’s key was in the “on” position and whether there was gasoline in the tank. More than one victim, including pets, suggests an accidental manner, often caused by a defective heating source, but investigation is necessary to rule out arson. The scenes of individual victims found dead in their home may be quite benign and suggest a natural death. In those cases, autopsy would be likely to show some indication of CO intoxication, and toxicology would confirm whether it occurred. Identification of intoxication in such instances also necessitates identification of the source so it can be removed from the environment to protect the lives of other individuals. In addition, one of the most important questions to be asked in a CO-related death investigation is, “Was the individual alive at the time of exposure?” Autopsy, including toxicologic analysis of COHb saturation, will help determine whether the individual died before the fire started. Clinical symptoms of CO intoxication include headache, nausea, vomiting, faintness, drowsiness, tachypnea, tachycardia, mental confusion, weakness, loss of consciousness, convulsions, respiratory failure, and death.10
General Autopsy Findings
Concentrations of COHb saturation greater than 30% confer a bright, cherry-red color to blood, mucous membranes, nail beds, skin, and all tissues.10 Other confounders causing pink discoloration
of the skin include cyanide, cold temperature, and early decomposition. Cherry-red lividity might not be discernible in all cases at the time of autopsy. In addition, tissues from these cases often impart a distinct red or pink discoloration to the formalin fixative.11 In fire victims, soot may be present on the skin and clothing and internally in the mouth, nose, upper and lower airways, and stomach. Thermal injury of various degrees to the skin or airway may or may not be present. In cases of CO exposure not due to fire, there may be no external or internal features normally attributed to CO exposure, and the determination of the cause of death relies solely on toxicology. In many cases, STAT toxicology screens are ordered to determine immediately whether CO intoxication occurred. The results of these STAT levels, in conjunction with investigation, determine the need for a complete autopsy. In addition, autopsy identifies disease processes, injury, and other intoxication that might have contributed to death.
of the skin include cyanide, cold temperature, and early decomposition. Cherry-red lividity might not be discernible in all cases at the time of autopsy. In addition, tissues from these cases often impart a distinct red or pink discoloration to the formalin fixative.11 In fire victims, soot may be present on the skin and clothing and internally in the mouth, nose, upper and lower airways, and stomach. Thermal injury of various degrees to the skin or airway may or may not be present. In cases of CO exposure not due to fire, there may be no external or internal features normally attributed to CO exposure, and the determination of the cause of death relies solely on toxicology. In many cases, STAT toxicology screens are ordered to determine immediately whether CO intoxication occurred. The results of these STAT levels, in conjunction with investigation, determine the need for a complete autopsy. In addition, autopsy identifies disease processes, injury, and other intoxication that might have contributed to death.
Toxicology
The primary route of exposure to CO is through the lungs. CO is absorbed into the circulation and rapidly distributes into the blood, and is not metabolized. The half-life of CO is 5 to 6 hours at a normal atmospheric oxygen concentration of 21%, 30 to 90 minutes at 100% oxygen, and 30 minutes under hyperbaric oxygen treatment.3
The percent saturation of CO is defined as the percentage of hemoglobin that combines with CO to form COHb. In the atmosphere, a concentration of 5 to 10, 000 parts per million (ppm) can produce COHb saturations of 73% to 76% in 2 to 15 minutes. The recommended maximum allowable exposure limit set by the federal Occupational Safety and Health Administration is 35 ppm over 8 hours. Nonsmokers have blood COHb levels of 1%to 3%and smokers have levels ranging from 5% to 10%. Levels greater than 10% are considered to be toxicologically significant, with headache a predominant initial symptom at levels of 10% to 40%, and hallucinations, severe ataxia, and tachypnea occurring at levels greater than 40%. Lethal COHb saturation is considered at levels greater than 50%. Levels less than 50% saturation can be fatal in some circumstances, such as in individuals with preexisting heart or lung disease, individuals exposed to other intoxicants either produced in a fire (e.g., cyanide) or taken exogenously (e.g., CNS depressants), individuals with increased oxygen demand, or individuals who have received oxygen therapy prior to death.10
Sodium fluoride-preserved blood is the preferred postmortem specimen; however, in decomposed or fragmented bodies, the spleen or tissue fluids are also acceptable specimens. Blood in suspected cases is screened using either spectrophotometric or microdiffusion methods. Quantification of percent saturation of CO may be performed by spectrophotometric methods; however, these methods are dependent on total hemoglobin. In specimens with low total hemoglobin or in tissue specimens, a hemoglobin-independent method using gas chromatography/flame ionization can be used. After separation from the hemoglobin, CO is detected. The percent COHb is then calculated by saturating another aliquot of the blood with CO and reanalysis. The CO content divided by the CO capacity provides the percent COHb saturation.3 As in all cases, a complete toxicologic analysis for alcohol and drugs is also warranted to determine additional intoxication contributory to death.
Neuropathology
Many individuals exposed to high concentrations of CO die before receiving medical assistance, and their brains will show the effects of acute intoxication. Others will receive medical care, survive for variable periods of time, and develop the pathologic and neurologic late effects of CO intoxication.12
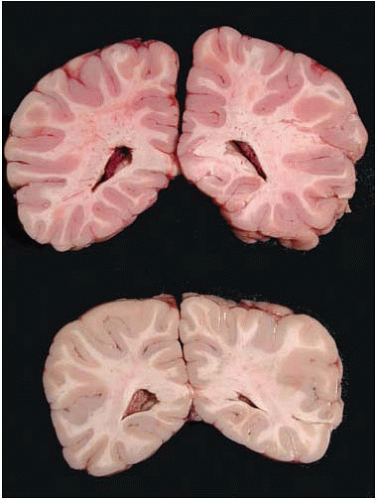
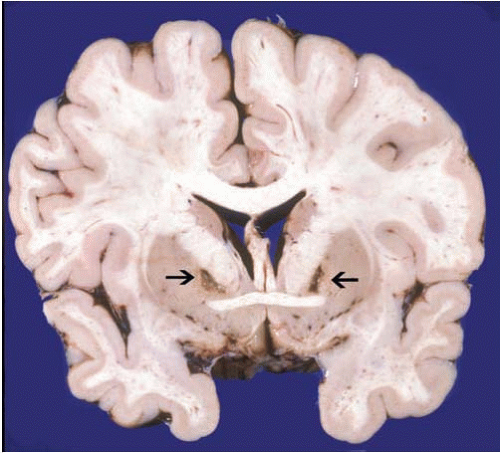
In acute CO poisoning, the forensic neuropathologist has the opportunity to determine the cause of death by macroscopic examination because of cerebral edema and the intense pink discoloration of brain tissues and dura (Fig. 22.8). This discoloration, evident in both fresh and fixed specimens, fades as the surface of the tissue is exposed to the oxygen in the air and O2 slowly displaces CO from COHb. For this reason at times, external examination of the brain may not be remarkable, and it is only when the brain is sliced that the pink discoloration becomes strikingly evident. Then, within a few minutes, the discoloration fades. The most common confounder at brain cutting is a poorly fixed brain, which also will look somewhat pink. We can tell the two scenarios apart because the discoloration of CO affects gray and white matter uniformly, and then fades, whereas the discoloration in a poorly fixed specimen affects predominantly the deep white matter and remains after sectioning. Acute CO toxicity has no microscopic changes, because of the lack of time for the development of morphologic manifestations of cell death and tissue injury.
There is poor correlation between COHb saturation after exposure and the degree of neurologic injury that follows nonfatal CO exposures.3 If a subject survives an acute exposure for a period of time (at least 12 hours) and then dies, the brain will show the widespread neuronal loss and gliosis characteristic of hypoxia and ischemia, including pseudolaminar cortical necrosis, loss of nerve cells in the hippocampi, and loss of Purkinje cells in the cerebellum. In addition, there may be prominent involvement of certain regions, such as bilateral necrosis with cavitary lesions of the globus pallidum ormarked degeneration of the pars reticulata of the substantia nigra. These lesions have been attributed to the direct toxic effect of CO because of the high cellular content of iron in these affected regions. Of note, however, these lesions are common in, but not exclusive to, CO toxicity and may be seen in other instances of brain hypoxia and ischemia. Another common sequela of CO poisoning is white
matter disease. CO causes demyelination of the white matter and white matter necrosis.13 Intermittent exposure leads to progressive encephalopathy and demyelination, with oligodendroglial swelling and proliferation of astrocytes that resembles multifocal leukoencephalopathy.2 The mechanism of delayed leukoencephalopathy in CO toxicity is not well understood, but recent studies have proposed an immune-mediated pathogenesis.14
matter disease. CO causes demyelination of the white matter and white matter necrosis.13 Intermittent exposure leads to progressive encephalopathy and demyelination, with oligodendroglial swelling and proliferation of astrocytes that resembles multifocal leukoencephalopathy.2 The mechanism of delayed leukoencephalopathy in CO toxicity is not well understood, but recent studies have proposed an immune-mediated pathogenesis.14
CYANIDE
Cyanide is a well-known poison that, despite popular belief in the media, is rarely used as an agent of murder. It is potent and rapidly acting and can cause death within minutes of ingestion or inhalation. It is most often encountered in subjects in the medical examiner’s office as a product of the incomplete combustion of certain synthetic materials in house fires in conjunction with CO.3 Hydrogen cyanide is used as a fumigant and in the production of resin monomers. It is also released as a byproduct in blast furnaces, coke ovens, cigarettes, and the burning of nitrogen-containing materials such as wool, silk, a crylonitriles, polyurethane, and other polyacrylic fibers and materials.3 The potassium and sodium salts of cyanide hydrolyze in water, making them strong bases that cause tissue damage on contact. In addition, when they come into contact with acid, they produce hydrogen cyanide gas. They are used in photography, metal working, and chemical laboratories, and in the illicit manufacture of phencyclidine. Other forms are used in the production of resins and synthetic materials, pharmaceuticals, herbicides, and dyes, and in leather tanning. In addition, several plants contain cyanogenic glycosides, including the pits of apricots, peaches, apples, pears, and plums. Cyanide has a characteristic odor of bitter almonds, but only approximately 50% of the population has the genetic ability to smell it. In Maryland, cyanide poisoning is rare and averages between one and two cases per year, with the manner of death usually being suicide or homicide.
Mechanisms of Tissue Damage
Cyanide is a chemical asphyxiant that stops cellular respiration by binding to the ferric ion of the heme group of cytochrome c oxidase and inhibiting the electron transport chain, resulting in cellular hypoxia and metabolic acidosis. The primary target of cyanide is the brain, with resulting central suppression of respiration, but there is evidence that it is also toxic to the heart. Because cyanide does not directly cause necrosis of the CNS, it is believed that heart failure, hypotension, and the resulting brain ischemia are responsible for necrotic lesions of brain tissues.2, 3
Scene Investigation
Investigation into possible cyanide-related deaths can be difficult; some suspicion of poisoning from either the scene findings or information retrieved from family or acquaintances of the deceased is usually needed. Autopsy findings can be relatively nonspecific in cyanide poisoning, and if there is no initial suspicion in the investigation, specific toxicologic testing for cyanide might not be ordered. Investigation into certain types of deaths might suggest cyanide involvement, as the availability of cyanide is limited (e.g., fire-related deaths in which an individual has soot in the upper and lower airways without lethal levels of CO, suggesting that cyanide or other noxious chemicals were released in the fire, or where fire department personnel discovered high levels of atmospheric cyanide at the scene; or suspected homicidal, suicidal, or accidental poisonings in which the victim or suspect works in a laboratory or industry that uses cyanide). Other sources in homicidal and suicidal poisonings have included ordering through the Internet. In the past, cyanide was used as an agent of suicide for politicians and in espionage and also in mass poisonings such as the Jonestown massacre and in Nazi concentration camps. Clinical symptoms of acute toxicity are nonspecific, reflecting cellular hypoxia, and include dyspnea, headache, tachypnea, dizziness, gasping breaths, arrhythmias, seizures, coma, and death, with unconsciousness occurring in seconds to minutes.2, 3
General Autopsy Findings
Overall autopsy findings in cyanide poisoning are nonspecific. Cyanide can cause a bright, cherry-red color to blood, mucous membranes, nail beds, skin, and all tissues. Other confounders causing pink discoloration of the skin include CO, cold temperature, and early decomposition. Cherry-red lividity might not be discernible in all cases at the time of autopsy. In fire deaths, the only feature distinguishing cyanide poisoning from CO intoxication might be the lack of a lethal COHb level and presence of soot in the airways. In poisonings, the odor of bitter almonds might be discernible for those with the genetic ability to smell it. The mucosal surface of the stomach, esophagus, or oral mucosa may show alkaline burns if salts of cyanide were ingested.
Toxicology
Absorption of cyanide depends on the type and route of administration. The estimated lethal dose of hydrogen cyanide is 100 mg. Time is of the essence if cyanide is suspected, and analysis for cyanide in the blood must be performed as soon as possible, as it rapidly degrades into less toxic components.3 Many methods are used to analyze for cyanide. Initial steps include separation from the biologic matrix, most commonly by acidification to release hydrogen cyanide, which can be measured directly. Other methods include trapping cyanide for detection by microdiffusion reactions. The trapped cyanide can then be analyzed by colorimetric methods with or without spectrophotometry, gas chromatographic methods with nitrogen-phosphorus and electron capture detection, or microdiffusion coupled with direct potentiometric measurement by using ion-specific electrodes.3 Cyanide levels less than 0.25 mg/L are generally considered to be normal, and in the absence of other more relevant autopsy findings, concentrations greater than 2 to 3 mg/L are considered to be lethal.
Neuropathology
Morphologic changes are similar to those of CO toxicity but usually lack the marked pallidal involvement of CO. These changes cannot be distinguished from global ischemia and show marked congestion, with occasional perivascular and subarachnoid hemorrhage.2 Rare cases of long survival show loss of Purkinje cells, gliosis of the cerebral cortex, scattered petechial hemorrhages, bilateral necrosis of the globus pallidus, and circumscribed white matter necrosis that is indistinguishable from cardiac hypotension in critical illness.2 In addition, chronic cyanide poisoning can be accompanied by striatal degeneration with parkinsonism and dystonia.2 Few autopsy studies document the neuropathology in subjects surviving more than 24 hours after acute cyanide intoxication.15 Changes describe macroscopic softening of globus pallidus, pseudolaminar necrosis of the cerebral cortex, and degeneration of Purkinje cells in the cerebellum. In a subject who survived for 19 months after attempted suicide with cyanide, lesions of the cerebral cortex were present, predominantly in the watershed distribution, putamen, globus pallidum,
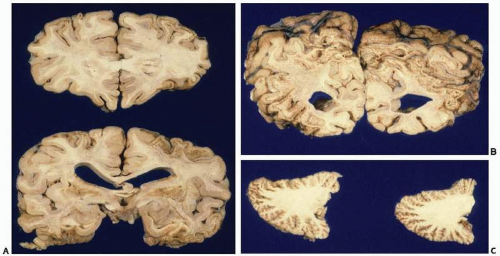
subthalamic nucleus, substantia nigra, and cerebellum. However, the hippocampal formations were spared. We examined the case of a 17-year-old who died four days after homicidal cyanide intoxication. The most remarkable feature was pseudolaminar necrosis of the cerebral cortex, in addition to widespread necrotic neurons through basal ganglia, hippocampus, and cerebellum (Figs. 22.9 and 22.10).16 Studies in experimental animals indicate that the neuropathologic lesions of cyanide intoxication are due to hypoxic-ischemic injury rather than to direct cyanide histotoxicity.
subthalamic nucleus, substantia nigra, and cerebellum. However, the hippocampal formations were spared. We examined the case of a 17-year-old who died four days after homicidal cyanide intoxication. The most remarkable feature was pseudolaminar necrosis of the cerebral cortex, in addition to widespread necrotic neurons through basal ganglia, hippocampus, and cerebellum (Figs. 22.9 and 22.10).16 Studies in experimental animals indicate that the neuropathologic lesions of cyanide intoxication are due to hypoxic-ischemic injury rather than to direct cyanide histotoxicity.
DRUGS OF ABUSE
Investigation of probable drug-related deaths involve the evaluation of witness statements, the presence of drug paraphernalia or drugs at the scene (with positive identification by forensic crime lab testing), documentation of history of prior abuse or overdose, complete social andmedical history, details of prescribed medications, and participation in drug treatment programs. Autopsy findings in drug intoxication deaths must rule out other lethal disease processes or injury.
Cocaine
Cocaine is an alkaloid from the Erythroxylum coca plant that has been used as a psychotropic drug for 2, 000 years.3 As a stimulant drug of abuse, it can be snorted or used intravenously as the hydrochloride salt, or smoked as the freebase crack. Cocaine sold on the street can be diluted or cut with mannitol, lactose, sucrose, lidocaine, procaine, benzocaine, caffeine, ephedrine, phenylpropanolamine, 3 or diltiazem.17 Cocaine can cause sudden death as a result of action of the drug on the CNS or its direct action on the myocardium.10 The state of Maryland averages approximately 200 cocaine-associated drug intoxication deaths per year, not including homicides, suicides, or accidents where the presence of cocaine is an incidental finding.
Mechanisms of Tissue Damage
Cocaine is still used in medicine, primarily in eye surgery, as a topical anesthetic. Cocaine blocks the reuptake of the neurotransmitters
norepinephrine, dopamine, and serotonin and causes an increase in release of catecholamines. It also acts on α-adrenergic receptors to induce vasoconstriction and ischemia.2, 10 Studies have also suggested that effects of cocaine include the formation of nitric oxide, an increase in N-methyl-D-aspartate receptor density, alterations in D1 and D2 dopamine receptors, alterations in cerebral blood flow, increase in cerebral glucose metabolism, and interactions with the serotonin transporter and γ-aminobutyric acid receptor.18
norepinephrine, dopamine, and serotonin and causes an increase in release of catecholamines. It also acts on α-adrenergic receptors to induce vasoconstriction and ischemia.2, 10 Studies have also suggested that effects of cocaine include the formation of nitric oxide, an increase in N-methyl-D-aspartate receptor density, alterations in D1 and D2 dopamine receptors, alterations in cerebral blood flow, increase in cerebral glucose metabolism, and interactions with the serotonin transporter and γ-aminobutyric acid receptor.18
Scene Investigation
Because chronic heavy use of cocaine may cause paranoid psychosis and because cocaine has been associated with excited delirium, investigation may reveal erratic behavior before collapse. The typical scenario in excited delirium is a hyperthermic, violent, paranoid, or aggressive individual who dies suddenly, immediately after attempts at restraint or confrontation with law enforcement. Autopsy shows no lethal injury or neuropathologic changes, presence of cocaine or another stimulant in the blood, usually in low concentrations, and moderate to severe heart disease.10 Another potential scenario is that of an individual who collapses shortly after arriving at the airport from a foreign country and exhibits some if not all of the above symptoms. In this case the individual might be a “drug mule” or “body packer.” Yet another scenario is that of an individual who swallows a packet of drugs to avoid detection by the police. In both of these scenarios, the swallowed packet of drugs can rupture and lead to death. Other clinical symptoms of acute cocaine toxicity include elevation of arterial blood pressure, pulmonary dysfunction, arrhythmias, myocardial infarction, stroke, and seizures.3, 19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree