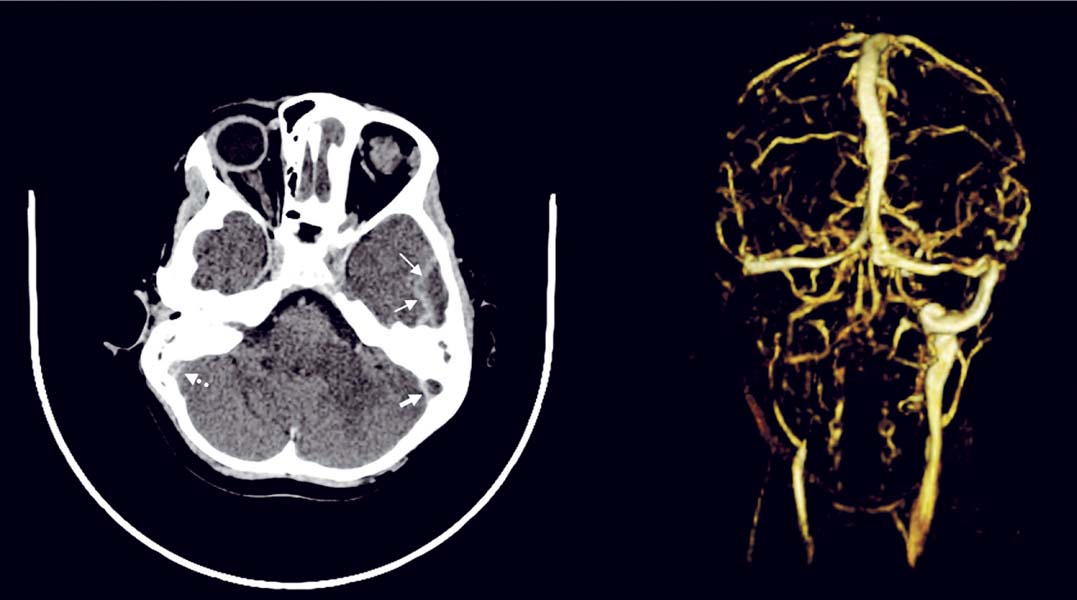
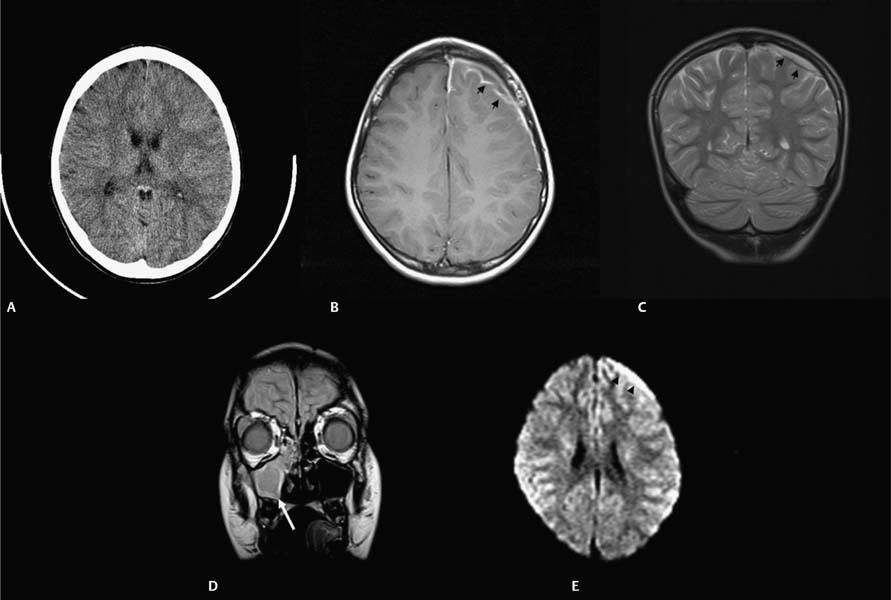
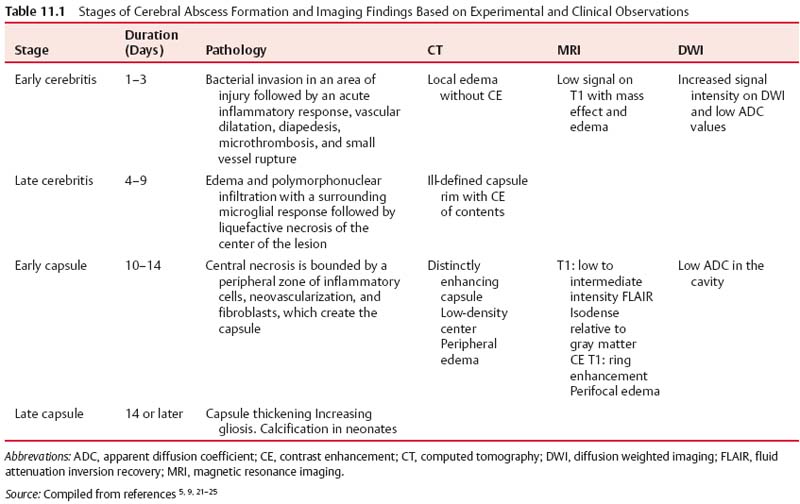
11 Intracranial Suppuration A previously healthy 4-year-old girl developed a mild upper respiratory tract infection 5 days prior to presentation with frontal morning headache, vomiting, earache, and fever varying from 38.0 to 39.5°C (100.4–103.1°F). Clinical examination revealed a febrile, alert, and neurologically intact child with chronic otitis media, left-sided conductive hearing loss, and retroauricular tenderness. Myringotomies and left mastoid drainage were performed. Mixed anaerobic organisms and Pseudomonas species were cultured (Fig. 11.1). Fig. 11.1 Axial computed tomography (CT) scan with contrast (left) with thin arrows outlining the extradural temporal and subtemporal abscess collection. Solid thick arrow shows the filling defect in the left transverse-sigmoid sinus complex compared with the patent sinus (right, dotted thick arrow). An alert 14-year-old male patient presented with frontal headache, nasal congestion, and a first-ever generalized seizure. Following the postictal period, clinical examination revealed him to be neurologically intact with mild fever (38.5°C [101.3°F]) and to have purulent nasal discharge. Fig. 11.2 (A) CT scan without contrast shows obliteration of the cortical subarachnoid space over the left frontal lobe, suggesting a subtle mass effect on the left hemisphere. (B) Axial and (C) coronal magnetic resonance imaging (MRI) confirmed that the mass was due to a thin extra-axial collection (arrowheads). (D) Lower left coronal MRI showing mucosal thickening in a fluid-filled maxillary sinus (arrow). (E) Diffusion axial image with high-intensity signal in the collection (arrowheads). Endoscopic sinus drainage of the right maxillary sinus grew mixed organisms, predominantly aerobic and anaerobic Streptococcus (Fig. 11.2). Surgery of intracranial infections laid the foundation for clinical neurosurgery as it is known today. The first successful craniectomies and craniotomies for nontraumatic mass lesions were performed for these disorders. Although chronic infections in bone and intra- and extradural spaces were controllable with surgery, acute infections, particularly in the subdural space, had to await the availability of effective antimicrobial agents to be effectively treated.1With sulfonamides and penicillin, in addition to surgical drainage, subdural empyema became potentially curable. Guided by neuroradiologic investigations, initially angiography, and now computed tomography (CT) and magnetic resonance imaging (MRI), the focus of treatment has shifted to early recognition, accurate and specific bacteriologic diagnosis, and directed surgical intervention. The neurosurgical management of intracranial suppuration is designed to (1) obtain adequate specimens of puru-lent material for accurate bacteriologic diagnosis and to direct antimicrobial therapy where accurate cultures cannot be obtained by other means; (2) evacuate sizable collections of liquefied pus from any and all extra- and intradural spaces affected and to lower intracranial pressure and minimize cerebral distortion; (3) débride sequestered bone; (4) decompress and, if needed, irrigate the ventricular system in the event of rupture of the abscess into the ventricular system; and (5) control hydrocephalus, if present. In the absence of these indications, neurosurgical intervention may not be necessary. The rationale and nature of neurosurgical intervention necessary to manage patients with intracranial suppuration are dependent upon the principles outlined in the following sections that describe the impact of current neuroimaging for early diagnosis and assessment of the response to therapy, the need for accurate bacteriologic diagnosis and administration of effective antibiotics, and the requirement to evacuate sizable collections of pus. The pathologic and imaging evolution of brain abscess is outlined in Table 11.1. Experimental studies in primates suggest that brain abscess formation occurs in an area of preexisting necrosis or injury caused by direct traumatic injury, hemorrhage, infarction secondary to thrombophlebitis, or relative hypoxia in watershed areas.2,3 Seeding with organisms sets the stage for the development of septic cerebritis occurring within the white matter. Cerebritis is characterized by an acute inflammatory response, vascular dilatation, diapedesis, microthrombosis, and small vessel rupture. Edema and polymorphonuclear infiltration with a surrounding microglial response occur. The center of the lesion then undergoes liquefaction. By 10 to 13 days, early encapsulation is seen in experimental brain abscesses.4,5 A peripheral zone of inflammatory cells, neovascularization, and fibroblasts surround a central necrosis. The latter cells produce a dense collagenous capsule. The capsule is surrounded by edema and reactive gliosis. Capsule formation is thinnest adjacent to the ventricle and thicker on the side of the more luxuriantly vascularized cortex. Grant, in a clinical study of patients with brain abscess during the preantibiotic era, demonstrated the presence of a capsule in every patient after a period of 6 weeks.6 In immune-suppressed animals, there is a decrease and delay in collagen formation, a reduction in polymorphonu-clear leukocytes and macrophages, a longer persistence of organisms, and an increase in gliosis.9 Early cerebritis is characterized on CT scan by an ill-defined, low-density change within the parenchyma. Enhancement occurs after contrast medium administration. Subsequently, imaging will show the classic ring-enhancing lesion with surrounding edema. Ring enhancement may or may not correlate with capsule formation. Differentiation of capsule formation from enhancing cerebritis may be made using delayed (30–60 minutes) scanning after contrast medium administration. On CT, ring contrast enhancement reflects the degree of neovascularization and capsule maturation. Calcification is common in abscesses in neonates.7,8 The administration of corticosteroids to a patient whose lesion is in the cerebritis stage may result in definite reduction in contrast medium enhancement. Contrast medium enhancement in well-encapsulated lesions is not reduced.4 Increased uptake of contrast material by the ependyma may indicate the presence of primary ventriculitis or that due to intraventricular rupture. CT and MRI are required for accurate preoperative localization of the epidural, subdural, and/or parenchymal collections and for monitoring resolution of the infection. Contrast medium enhancement of the dura, empyema wall, and abscess capsule is usually seen, and complicating cerebral edema, venous infarction, and parenchymal abscess formation may be revealed.10–12 CT may not be diagnostic, revealing only hemispheral swelling in subdural empyema;13 however, contrast medium–enhanced MRI will usually reveal the extra-axial collections, as well as areas of underlying tissue and vascular injury.10 Whether imaging is done using CT or MRI, coronal and sagittal views are helpful in revealing collections at the skull base and at the vertex and in defining the relationship of the intracranial collection (s) to the involved sinuses. MR images have typical characteristics on T1 weighting (central hypointensity surrounded by a ring of enhancement after gadolinium administration) and T2 weighting (hyperin-tense area of pus, surrounded by hypointense capsule, enveloped within surrounding edema).14,15 MR spectroscopy has an evolving role in the differentiation of abscess from tumor,16,17 in the differential diagnosis of brain lesions in patients with acquired immunodeficiency syndrome (AIDS),18 and in staging abscess development.19 The use of diffusion weighted imaging (DWI) and the corresponding apparent diffusion coefficient (ADC) maps has greatly improved the ability to differentiate cerebral infections from other cystic cerebral lesions presenting as nonspecific ring enhancement20 and to demonstrate treatment success and failure.21–25 DWI is based on detecting the microscopic motion of water molecules and depends mostly on the water located in the extracellular space. ADC maps are used to quantify the degree of water motion. The degree of viscosity, the causative organism, and the level of protein in the abscess cavity may affect DWI findings and ADC values. Cerebral abscesses contain inflammatory cells, a matrix of proteins, cellular debris, and bacteria in high-viscosity pus; all of these factors restrict water motion.21 Restricted water motion in cerebral abscesses has increased signal intensity on trace DWI and low ADC values. Because water movement is less restricted in necrotic tumors, most show mildly increased diffusion with low to intermediate signal intensity on trace DWI and high ADC values.21 In one series, the sensitivity of DWI for the differentiation of brain abscesses from nonabscesses was 96%; specificity, 96%; positive predictive value, 98%; negative predictive value, 92%; and accuracy of the test, 96%.24 Changes in the signal intensity have been correlated to successful treatment.21,22 Persisting or reappearing high signal intensity in the abscess cavity on trace DW images and low ADC values indicating restricted diffusion were seen in cases of treatment failure and were correlated with pus accumulation. Changes in the capsule interface may also be important.23,26 Sensitivity and specificity have been reported by Reddy and colleagues.24 Nonpyogenic parasitic and fungal abscesses may show increased ADC in the cavity compared with bacterial abscesses.22,27 Although overlap in values occurs with bacterial abscesses, other features are important in distinguishing fungal abscesses, including multiplicity, size, deep nuclear location, and signal heterogeneity on T2 and DWI. On DWI, subdural empyema exhibits areas of high signal similar to that produced by most brain abscesses, with the high signal resulting from the high viscosity of the infected fluid.21 DWI may be used as a simple method of making a reliable diagnosis if an area of abnormal high signal is found. Sterile effusions sometimes give signal similar to that of empyema, and benign effusions often appear as areas of low signal similar to that of cerebrospinal fluid (CSF). The degree of enhancement of dura, empyema, or abscess wall on CT or MRI varies over the course of treatment and is a reflection of the inflammatory response and not whether the collection has been sterilized. In limited case observations, the DWI signal intensity from epidural abscesses was variable.28 In infants with meningitis-associated subdural empyema, cranial ultrasonography (or CT or MRI) usually reveals unilateral or bilateral frontoparietal extra-axial collections with echogenic boundaries.29,30 Plain film studies supplement the information obtained by CT (and MRI) in patients with otorhinologic-associated intracranial suppuration, by demonstrating opacification of one or more sinuses13 and/or revealing a foreign body, skull fracturing, pneumocephalus, or gas within the abscess cavity. Bacteriologic identification can be obtained from various sources in the acutely unwell patient (Table 11.2). Blood cultures may reveal the causative organism in epidural abscess or subdural empyema but are rarely positive in brain abscess.31 In patients with sinogenic suppuration, pus collected at the time of sinus or ear drainage will usually reveal the causative organisms unless antibiotic treatment has been started. The culture of purulent material obtained at the time of surgical drainage provides the best opportunity for micro-biologic diagnosis. Proper handling of the specimen and appropriate aerobic and anaerobic culture techniques result in positive cultures in the majority of cases. Gram staining of the pus can guide therapy in the presence of a negative culture.
Conservative Management
Cases
Case 1
Case 2
Principles of Management
Impact of Neuroimaging: Early Diagnosis and Assessment of Response to Therapy
Accurate Bacteriologic Diagnosis and Appropriate Antimicrobials
Pathogen Identification
Underlying Condition | Usual Organisms | Initial Antibiotic Choice* |
Otorhinological disease | Aerobic and anaerobic Streptococcus S. aureus Other anaerobes Pseudomonas aeruginosa | Penicillinase-resistant synthetic penicillin + third-generation cephalosporin + metronidazole† or meropenem‡ |
Head injury or operation | Staphylococcus aureus Gram negatives | Penicillinase-resistant synthetic penicillin + third-generation cephalosporin or meropenem‡ |
Meningitis in infancy | Usually sterile unless secondarily infected | Third-generation cephalosporin ± vancomycin |
Unknown | Unknown | Penicillinase-resistant synthetic penicillin + third-generation cephalosporin + metronidazole |
Drug Doses |
|
|
Cloxacillin | 200 mg/kg/day divided q6hr |
|
Vancomycin | 60 mg/kg/day divided q6hr |
|
Cefotaxime | 300 mg/kg/day divided q8hr |
|
Ceftriaxone | 100 mg/kg/day divided q12hr |
|
Ceftazidime | 225 mg/kg/day divided q8hr |
|
Metronidazole | 30 mg/kg/day divided q8hr |
|
Meropenem | 120 mg/kg/day divided q6hr |
|
• Previous third-generation cephalosporin therapy has been given
• Prolonged hospitalization
• Prolonged ventilatory support
• Burns
The spectrum of organisms cultured from sites of intracranial suppuration has changed (Table 11.2). This is a reflection of improved bacteriologic isolation techniques, aggressive treatment of primary infections, and a decreased incidence of nonmissile compound brain wounds. In most series, the incidence of Staphylococcus aureus has decreased and that of anaerobes has increased.32
Cultures from suppuration associated with sinusitis commonly show the growth of organisms responsible for chronic sinusitis, including aerobic and/or anaerobic Streptococcus (made up of the Viridans group streptococci and the Streptococcus anginosus group) or other anaerobes. S. aureus, aerobic and anaerobic Streptococcus, Pseudomonas aeruginosa, facultative gram-negative organisms, and other anaerobes are causative in those infections of mastoid or middle ear origin, whereas those secondary to trauma, surgery, or primary calvarial osteomyelitis are due to Staphylococcus, Streptococcus, or gram-negative organisms. Skin flora of low pathogenicity, including coagulase-negative Staphylococcus, Corynebacterium, and Propionibacterium acnes, have been reported in postoperative/traumatic epidural abscesses.33
Reflecting their role in the pathogenesis of sinusitis, anaerobes play a definite part in the pathogenesis of subdural suppuration.34 Anaerobic or microaerophilic Streptococcus, Bacteroides species, Fusobacterium, Peptostreptococcus, and Clostridium perfringens35 have been reported. Salmonella has been reported in the immunologically compromised host. Subdural fluid collections complicating meningitis in infants, although usually sterile, may culture the organism responsible for the meningitis. Specimens from subdural empyema are sterile in up to 25% of patients.34,36
Anaerobic organisms isolated from brain abscess pus include Bacteroides, Peptostreptococcus, Fusobacterium, Veillonella, Propionibacterium, and Actinomyces. Aerobic organisms include Staphylococcus, Streptococcus, Enterobacteriaceae, and Haemophilus. In at least one third of brain abscesses, the pus will culture multiple organisms. This is particularly common in otitic abscesses. In patients with cyanotic congenital heart disease, Viridans Streptococcus, anaerobic Streptococcus, and occasionally Haemophilus species are seen. No growth is reported from up to 25 to 30% of properly handled specimens.37–39
The bacteriologic diagnosis often points to the originating infection. S. aureus abscess formation occurs most commonly after compound skull fracturing, after operative intervention, or as a result of a retained foreign body.40 Gram-negative organisms, including Bacteroides and Haemophilus, are commonly isolated from otogenic abscesses. Most brain abscesses that complicate paranasal sinus infection will culture aerobic and/or anaerobic streptococci.
The nature of the offending organism may influence the development of the abscess capsule. Bacteroides excretes a collagenase that inhibits capsule formation, resulting in the development of small daughter abscesses. Propagation of edema is aided by bacterial production of hyaluronidase and heparinase.
Brain abscess formation in the neonate is usually caused by Proteus mirabilis or Citrobacter diversus.41 Individual case reports and series have also reported Salmonella, Serratia, Enterobacter, and Staphylococcus as causative organisms. Meningitis caused by Citrobacter, Proteus, Serratia, or Enterobacter is complicated by abscess formation in a high percentage of patients.42,43
Fungal brain abscesses most commonly develop in patients with impaired host defenses, particularly those with AIDS, or who are immunologically suppressed after organ transplantation or chemotherapy for malignancy. Candida, Aspergillus, Nocardia, and Cryptococcus are the most common responsible fungal organisms. Pathogenic fungi, acquired through either inhalation or implantation, can result in brain abscess formation. This is most commonly seen with Histoplasma, Coccidioides, and Blastomyces and the zygomycetes (Mucor and Rhizopus).
Toxoplasma gondii is the leading cause of brain abscess in patients with AIDS. Entamoeba histolytica brain abscess usually occurs in the presence of involvement in extracranial sites. Helminthic brain abscess is rare.39,41
Antibiotic Treatment
Systemic antibiotic treatment (see Table 11.2) should be started as soon as the diagnosis of epidural abscess, subdural empyema, or brain abscess is considered. Antibiotics administered before surgery, although achieving some penetration of bone, are unlikely to interfere with culture information from the pus.
The microbial spectrum of brain abscess varies with the primary site of infection. If this is known, the initial choice of antibiotics can be directed to the most likely organisms. For abscesses arising as a result of sinusitis where streptococcal species are the most likely organisms, penicillin or a third-generation cephalosporin (e.g., cefotaxime) and metronidazole provide basic coverage. The majority of infratentorial subdural empyemas are otogenic in origin,36 and because chronic otitis media or mastoiditis is often associated with P. aeruginosa and Enterobacteriaceae, coverage should include a third-generation cephalosporin, such as ceftazadime. Metastatic abscesses require a regimen based on the likely site of primary infection. In particular circumstances where multiresistant, gram-negative organisms 11 Intracranial Suppuration131 might be suspected (see Table 11.2), a carbapenem antibiotic, such as meropenem, can be used as monotherapy, as it also covers S. aureus and anaerobes.
S. aureus is commonly identified in an abscess after trauma. Compound wounds, in particular those contaminated with soil, may contain facultative gram-negative organisms or Clostridium species. In these situations, a penicillinase-resistant penicillin and a third-generation cephalosporin provide initial coverage. Postoperative brain abscess is rare and usually due to S. aureus or S. epidermidis. Vancomycin should be considered, given the resistance of nosocomial organisms to penicillinase-resistant penicillins. Abscesses secondary to animal bites contain a mixed flora, including Pasteurella multocida and a variety of Haemophilus species.44
In the absence of a likely primary source of infection, the initial choice of antibiotics should include agents to cover Streptococcus (aerobic and anaerobic), other anaerobes, and the less common Staphylococcus. A penicillinase-resistant synthetic penicillin, a third-generation cephalosporin, and metronidazole should be instituted empirically pending results of Gram staining and culture.
The optimal duration of parenteral antibiotic therapy is not known, although 6 to 8 weeks of intravenous therapy has been empirically recommended. Certainly, treatment should be continued until systemic evidence of infection has resolved and the abscess collections have been shown to be decreasing in size.45–49 In some situations a shorter course may be sufficient. Depending on the organisms cultured, their sensitivity to the prescribed antibiotic regimen, and the response to therapy, some authors recommend an additional 2 to 3 months of oral antimicrobial therapy to prevent recurrence.43
Evacuation of Sizable Collections of Liquefied Pus
The need to evacuate collections of pus is dependent upon the patient’s clinical condition, degree of cerebral distortion, and the need for bacteriologic diagnosis. Neurosurgical drainage is not always required. Drainage of the epidural abscess may occur spontaneously or as a result of sinus drainage procedures.50–52 When necessary, and with the aid of modern imaging and stereotaxic localization, a more conservative approach can be taken to bone removal to achieve diagnostic and therapeutic drainage by targeting collections with precision. The extent of bone removal is determined by the degree of osteomyelitis and bone sequestration, as in some epidural abscesses; the need to reach the subdural space widely, as in hemispheral, parafalcine, and infratentorial subdural empyema; or the need to address multiple separated lesions within the brain and subdural space concurrently.
Neurosurgical Intervention
When Is Neurosurgical Intervention Not Required?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree