Demographic data
n = 51, Male: 29, Female: 22, Median age: 38 years (16–78)
EOR
Symptoms
Histology
Location
Preoperative KPS
Postoperative KPS
Total (25) = 49 %
Irritative (38) = 74.5 %
High-grade gliomas (21) = 41.1 %
Frontal (premotor and prefrontal area) (15)
100 (35)
100 (34)
Subtotal (8) = 15.6 %
Neurological deficits (11) = 21.5 %
Low-grade gliomas (23) = 45 %
Supplementary motor area (6)
90 (10)
90 (10)
Partial (11) = 21.5 %
Headaches (2) = 4 %
Other (7) 13.7 %
Wernicke’s area (4)
80 (6)
80 (3)
Biopsy (7) = 13.7 %
Broca’s area (5)
70 (0)
70 (4)
Left temporal lobe (3)
Insular (2)
Parietal post-rolandic (3)
Subcortical (3)
Frontotemporal (4)
Frontoparietal (4)
Temporoparietal (2)
The World Health Organization (WHO) brain tumor classification was used to establish tumor histology and a thorough neurological examination conducted immediately after surgery, the postoperative period, as well as 3 months later. Extent of resection (EOR) was evaluated on postoperative volumetric sequence MR images, within 72 h of surgery or at 3 month follow-up visit. EOR was subdivided into the following categories: total (100 %), subtotal (90–99 %), partial (50–89 %), and biopsy (<50 %). In the last months, we have incorporated the use of an intraoperative MRI (iMRI) protocol to improve EOR in low-grade gliomas (Fig. 9.5).
9.3.2 Results
The presented series includes 51 patients (29 male and 22 female) ranging in age from 16 to 78 years (median age 38 years). Motor mapping under general anesthesia was performed in 36 patients, and awake surgery for language and motor mapping was performed in 15 patients. Preoperative clinical evaluation revealed irritative symptoms in 38 patients (74.5 %), motor and language deficit in 11 patients (21.5 %), and headache in 2 patients (4 %). Most frequent tumor location was in the premotor area in 12 patients (23.5 %). EOR was total in 25 patients (49 %), subtotal in 8 patients (15.6 %), partial in 11 patients (21.5 %), and biopsy in 7 patients (13.7 %). Histology results reported high-grade glioma in 21 patients (41.1 %), low-grade glioma in 23 patients (45 %), and other tumors in 7 patients (13.7 %). Results of preoperative and postoperative neurological examination using the MRC scale are shown on Fig. 9.7. No cases of intraoperative and immediate postoperative mortality occurred. New motor deficits or worsening of preexisting ones was observed in 14 patients (27.4 %), and 7 patients (46.6 %) developed new language deficit or worsening of preexisting deficit immediately after surgery. Three months later, having completed intensive rehabilitation therapy, motor deficits persisted in 8 patients (15.6 %) and language deficits in 2 patients (13.3 %).
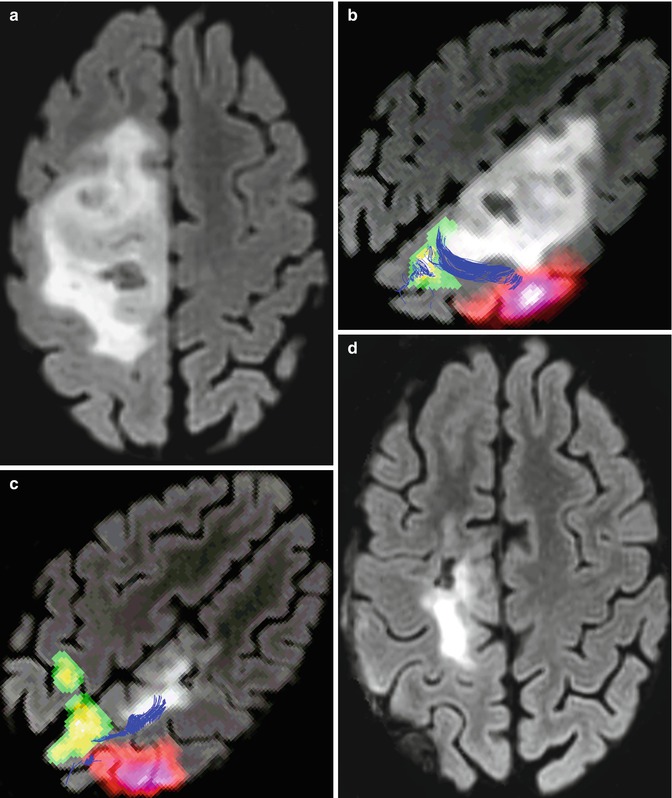
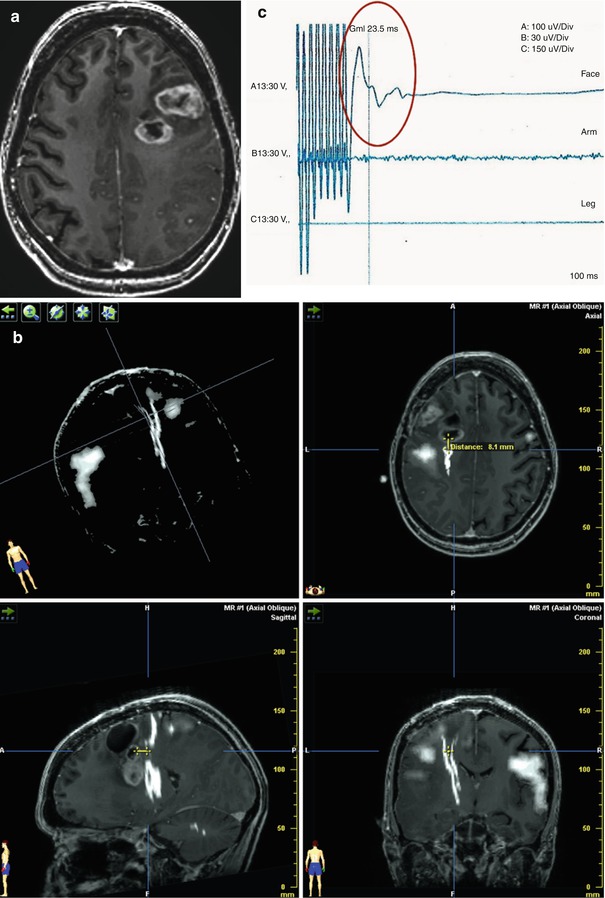
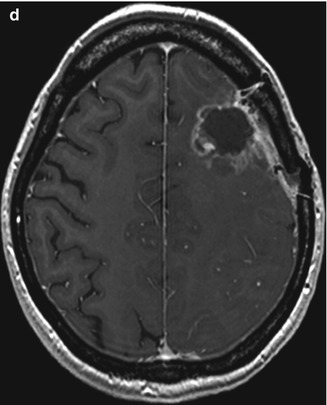
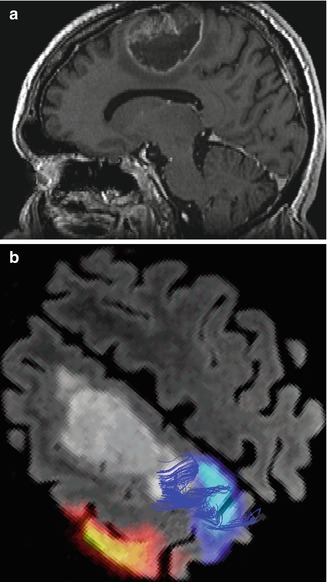
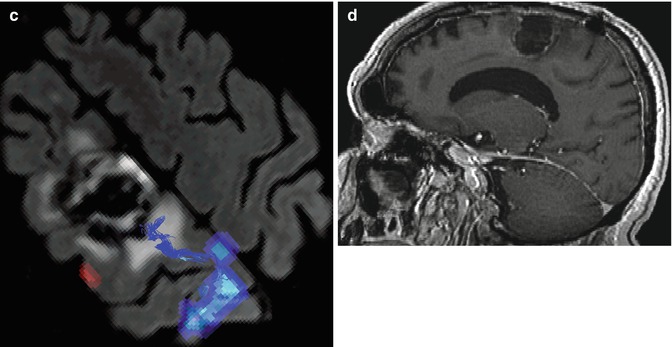
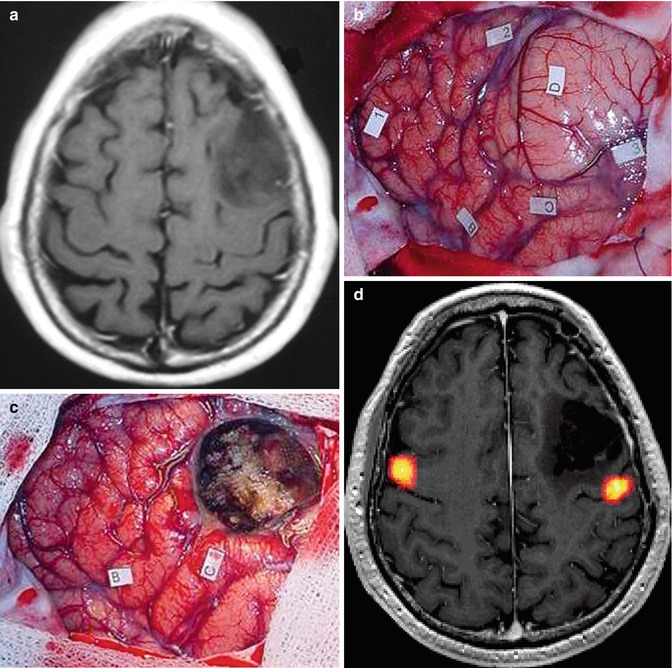
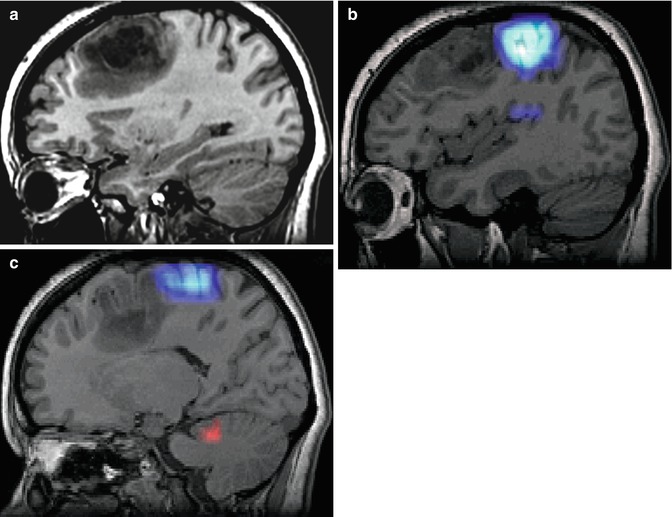
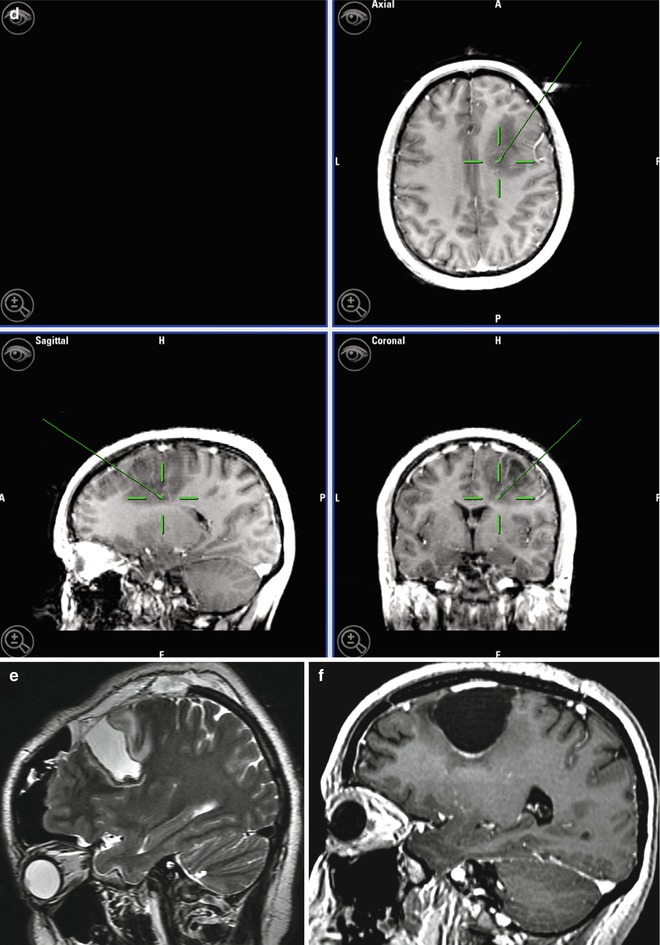
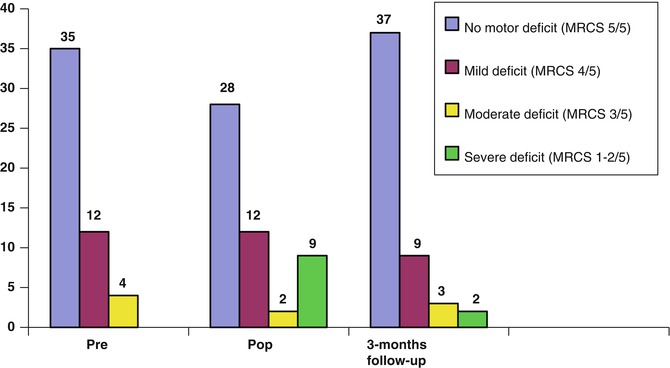

Fig. 9.1
A 31-year-old woman with right frontal oligodendroglioma, without motor deficit. (a) Preoperative axial 3D Cube-FLAIR MRI showing pre-rolandic low-grade gliomas; (b) preoperative fMRI and DTI fusion demonstrating a relationship between the tumor, cortical motor areas, and corticospinal tract (green, foot activation area; pink, hand activation area; blue, corticospinal tract); (c) immediately after surgery, the patient developed severe left-side motor deficit (1/5 MRCS of the leg and 3/5 of the arm). Six months after surgery, arm motor deficit had recovered completely, and mild deficit of foot persisted (MRCS 4/5). fMRI and DTI showing contralateral primary foot region recruitment (green); (d) postoperative axial 3D Cube-FLAIR showing subtotal resection


Fig. 9.2
A 28-year-old woman with left frontal glioblastoma. (a) Preoperative axial T1-weighted MR image with gadolinium showing a high-grade lesion. (b) Intraoperative neuronavigation image demonstrating the distance between point of response to DES and tract as 1 cm. Preoperative tractography and fMRI were loaded into the neuronavigator (hazy white, functional area corresponding to the hand; stark white, functional area corresponding to the corticospinal tract); (c) EMG response of facial muscles evoked during subcortical stimulation (red circle). No response in arm or leg; (d) Postoperative axial T1-weighted MR image depicting resection cavity. Patient developed mild right facial palsy after surgery, with subsequent partial improvement


Fig. 9.3
A 57-year-old man with left frontal glioblastoma who consulted because of complex partial seizures. (a) Preoperative sagittal T1-weighted MR image with gadolinium showing a premotor frontal lesion; (b) preoperative fMRI and DTI fusion depicting the relationship of the tumor to cortical motor areas and the corticospinal tract (navy blue, foot activation area; red, hand activation area; blue, corticospinal tract); (c) after surgery, patient developed right-side motor deficit (2/5 MRCS leg and 3/5 MRCS arm). Recovering almost entirely after 3 months, with mild fine motor deficit persisting in the hand. Postoperative fMRI and DTI showing the relationship of the corticospinal tract and the resected region (blue, corticospinal tract; navy blue, foot activation area; red, hand activation area); (d) postoperative sagittal T1-weighted MR image with gadolinium showing the resected area

Fig. 9.4
A 62-year-old man with left frontal oligodendroglioma operated on awake. (a) Preoperative axial T1-weighted MR image showing low-grade glioma involving the left frontal lobe; (b) intraoperative view before tumor resection, tag number 2, 3, and C. Pars opercularis of the inferior frontal gyrus (tag B). 1 False positive, D Lesion; (c) intraoperative imaging after tumor resection. B and C Anomias; (d) postoperative axial projection of 3D T1-weighted MR image with fMRI activation during phonological language task (yellow) showing subtotal resection performed under cortico-subcortical stimulation. Patient developed postoperative speech deficit which improved after 3 months. At the last follow-up, patient presented only minor stress-related speech slurring


Fig. 9.5
A 33-year-old woman with right frontal oligodendroglioma. (a) Preoperative sagittal T1-weighted MR image showing lesion; (b) fMRI depicting the relationship of the tumor with hand activation area (navy blue); (c) fMRI showing the proximity of the tumor and the cortical area of the foot (navy blue), radiological artifact (red color); (d) screen shot during intraoperative navigation demarcating inferior limit of resection; (e) sagittal iMRI showing the posterior tumor remnant. Observe brain shift in the frontal pole; (f) After surgery the patient developed left-side motor deficit (MRCS 4/5), recovering completely 2 months later. Postoperative sagittal T1-weighted MR image of the surgical resection

Fig. 9.6
Cortical bipolar electrical stimulation in a patient with a premotor lesion

Fig. 9.7
Motor deficit levels (in number of patients). Pr preoperative, Pop postoperative, MRCS Medical Research Council Score
9.3.3 Intraoperative Side Effects
9.3.3.1 Seizure
Stimulation can generate electrical seizures in up to 30–40 % of cases and clinical seizures in 8 %, usually focal [5]. Longer stimuli and rolandic location of the tumor can produce seizures more frequently [45]. Phenytoin prophylaxis was not used in patients free of preoperative seizures. In our series, 2 patients (3.9 %) developed intraoperative seizures (1 patient under general anesthesia and 1 patient undergoing the procedure awake). Seizure control was achieved applying cold saline solution irrigation on the surgical field, or through administration of propofol, while intravenous anticonvulsants were also administered. It is important to bear in mind just how uncomfortable seizures can be for patients operated on awake, with their head fixed to the frame (Fig. 9.6). To prevent intraoperative seizures, use of ECoG recording during surgery has been recommended, to detect the presence of “afterdischarge.” However, for many cases in this series, tailored craniotomy did not allow simultaneous ECoG recording, without hindrance of surgical maneuvers.
9.3.3.2 Intraoperative Neurological Dysfunction
One patient (1.9 %) in the series presented worsening of motor function during surgery; although resection was discontinued immediately, the patient never recovered from the deficit. Intraoperative neurological changes influence postoperative neurological outcome. Kim et al., evaluating clinical results of 309 cases of awake craniotomy, demonstrated postoperative worsening of the neurological status in 70 % of patients with intraoperative neurological changes versus only 27 % in the group of patients without such findings [26].
9.3.3.3 Agitation/Fear
One patient (1.9 %) was unable to tolerate the procedure because of agitation requiring sedation with propofol, and only a biopsy was performed since mapping could not be completed.
9.4 Discussion
The main objective of intrinsic brain tumor treatment is maximal surgical resection with essential neurological function preservation. Currently, there is a trend suggesting that EOR will impact glioma survival rates, regardless of histological tumor grade [6, 47]. Berger has reported that the greater the extent of resection (EOR) in low-grade gliomas (LGG), the better the long-term survival [3, 24, 43]. Maximal EOR improves survival by decreasing the pool of oncological cells potentially degenerating to higher-grade lesions [14, 15, 36, 40, 46]. Low-grade tumors usually affect young people, triggering seizures. Neurological examination is usually normal, making preservation of function mandatory. Patient’s lifestyle at the time of consultation and likelihood of long survival must be taken into account when deciding on treatment strategies. For high-grade gliomas (HGL), there is also evidence supporting EOR as a statistically significant predictor of overall survival [40]. Preservation of neurological function is also very important in such cases even if patient will ultimately have shorter life expectancy, as they will need to receive immediate adjuvant therapies in the best possible physical condition.
Eloquent areas include regions of the brain which, when damaged, can produce significant neurological impairment, affecting patient quality of life [42]. In the series reported by Chang et al., the majority of presumed eloquent-area tumors were located in the sensory-motor (41 %), language (48 %), and subcortical (47 %) regions [8]. Precise intraoperative identification of such areas is mandatory in these patients. Due to considerable interindividual anatomo-functional variability, and to distortion of the brain cortex caused by the tumor, anatomical criteria alone are not always reliable in accurately localizing motor and language areas [13, 26]. Moreover, the infiltrative nature of low-grade gliomas generates different reorganization patterns and compensation of cortical areas, making precise intraoperative identification essential [38, 40].
Preoperatively, cortical motor and language areas can be delineated using fMRI, mapping local task-related changes in brain tissue vascular response, based on the effect of deoxyhemoglobin on MRI signals. At the subcortical level, DTI depicts the relationships of subcortical tracts with the lesion and the surrounding edema [2]. Functional neuronavigation integrates multimodal data, including volumetric MRI, fMRI, and DTI, into the neuronavigation system to guide in the surgical approach. However, some issues may reduce the accuracy of the technique. The fMRI region of activation is dependent on the statistical threshold selected; the lower the threshold, the greater area of activation and vice versa, and fMRI sensitivity and specificity are 81 % and 53 % for language areas [40].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








