FIGURE 40.1 A 69-year-old man had new onset of right-sided visual blurring and right-sided numbness. Neuroimaging studies demonstrate left occipital and right medial temporal infarcts; the right side temporal infarct was harder to be visualized on CT. (Courtesy of Dr. Lotfi Hacein-Bey.). ADC, apparent diffusion coefficient; CT, computed tomography; DWI, diffusion-weighted imaging; FLAIR, fluid attenuated inversion recovery.
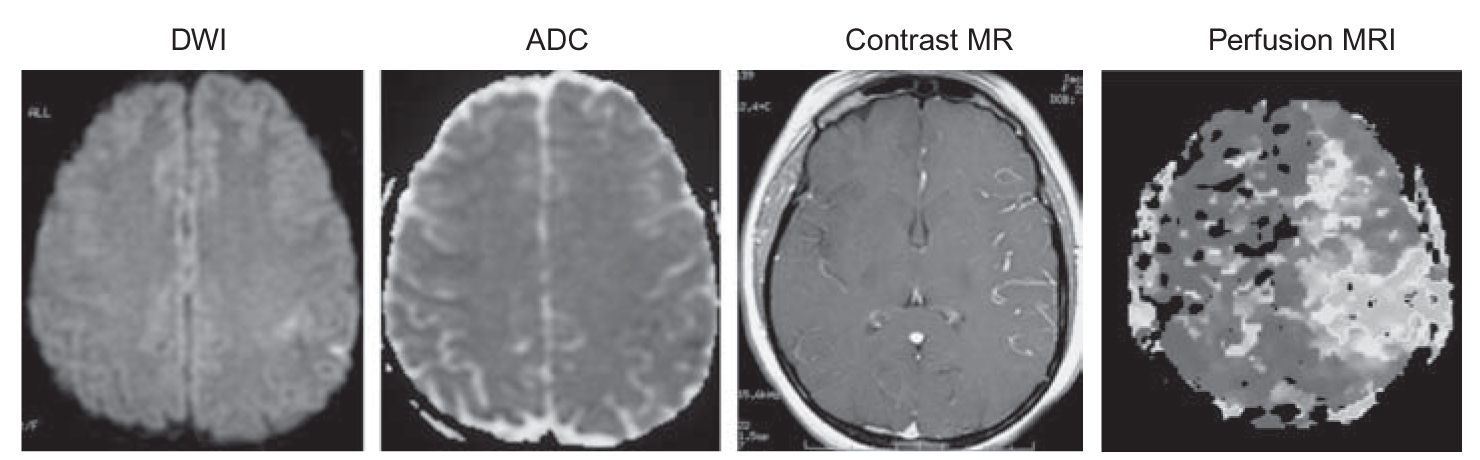
FIGURE 40.2 Perfusion/diffusion mismatch. Acute left ICA occlusion in a 29-year-old woman. There are very small diffusion defects in the left posterior frontal lobe. Perfusion MRI shows that the full left MCA territory is at risk. (Courtesy of Dr. Lotfi Hacein-Bey.). ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; ICA, internal carotid artery; MCA, middle cerebral artery; MRI, magnetic resonance imaging. (See color plates.)
Cardiac investigations to determine whether emboli have cardiac sources are advised in selected circumstances. Two-dimensional echocardiography for older patients with ischemic stroke is limited to patients with clinical clues of heart disease. Two-dimensional echocardiography should be considered for all patients younger than 45 years with otherwise unexplained ischemic stroke. Transesophageal echocardiography should be used for selected individuals, particularly for evaluation of mitral and aortic prosthetic valves or vegetations, whenever there is a need for better visualization of the left atrial appendage or interatrial septum, or when a right-to-left shunt is suspected.
Most patients with ischemic stroke have extracranial (EC) or intracranial (IC) cerebrovascular atherosclerosis. Cerebrovascular atherosclerosis primarily affects the carotid bulb, carotid siphon, middle cerebral artery (MCA) stem, origin, and IC segments of the vertebral arteries, and basilar artery. Ischemia results from thrombotic vascular occlusion, embolization of atherosclerotic debris, or hemodynamic disturbances causing focal hypoperfusion in areas in which the circulation is inadequate.
A. Ischemic stroke resulting from large-artery atherosclerotic disease. Large-artery atherothrombotic infarctions almost always occur in patients who already have significant risk factors for cerebrovascular atherosclerosis, such as arterial hypertension, cigarette smoking, diabetes mellitus, dyslipidemia, asymptomatic carotid bruits, asymptomatic carotid stenosis, and TIAs.
1. A TIA is defined as a transient episode of neurologic dysfunction caused by focal brain or retinal ischemia without infarction. The conventional boundary in differentiating between a TIA and stroke has been 24 hours. Yet, most TIAs last only a few minutes. As TIAs may be associated with variable rates of infarction on diffusion-weighted MRI, a new “tissue based definition” of TIA is now commonly used: brief episodes of neurologic dysfunction caused by focal retinal or brain ischemia with symptoms typically lasting <60 minutes, and without evidence of acute infarction.
TIAs are most often caused by thromboembolism associated with large-artery atherosclerosis, cardioembolism, or small-vessel (lacunar) disease. TIAs are important harbingers of subsequent stroke and are often associated with lesions on brain imaging. TIAs involving the anterior or carotid circulation should be distinguished from those involving the posterior or vertebrobasilar circulation.
a. The following symptoms are considered typical of TIAs in the carotid circulation: ipsilateral amaurosis fugax, contralateral sensory or motor dysfunction limited to one side of the body, aphasia, contralateral homonymous hemianopia, or any combination thereof.
b. The following symptoms represent typical TIAs in the vertebrobasilar system: bilateral or shifting motor or sensory dysfunction, complete or partial loss of vision in both homonymous fields, or any combination of these symptoms. Isolated diplopia, vertigo, dysarthria, and dysphagia should not be considered TIAs, but in combination with one another or with any of the symptoms just listed, they should be considered vertebrobasilar TIAs.
c. Preceding TIAs occur in approximately 30% to 50% of patients with atherothrombotic brain infarction, in 15% to 25% of lacunar infarctions, and in 10% of cardioembolic infarctions.
d. A TIA is a risk factor for stroke. The independent risk of a subsequent stroke is at least three times greater for patients with histories of TIAs than for those who have not had TIAs. The ABCD2 score is useful for stroke risk stratification in patients with TIAs: Age >60 years == 1 point; Blood pressure >140/90 mm Hg == 1 point; Clinical unilateral weakness == 2 points; speech-only impairment == 1 point; Duration >60 minutes == 2 points, <60 minutes == 1 point; Diabetes == 1 point. Scores of 4 or greater in the ABCD2 score indicate moderate to high stroke risk.
2. Atherosclerosis tends to occur in areas of reduced flow shear such as the posterior aspect of the carotid artery bulb. It primarily affects the larger EC and IC vessels. Approximately 80% of ischemic strokes occur in the carotid or anterior circulation, and 20% in the vertebrobasilar or posterior circulation (Fig. 40.3).
3. The mechanism of large-artery atherothrombotic infarction is either artery-to-artery embolization or in situ formation of a thrombus in the setting of preexisting arterial stenosis. Artery-to-artery embolism or low CBF is a common mechanism of cerebral ischemic events. Embolism from ulcerated carotid atherosclerotic plaques is the most common cause of cerebral infarction. In situ thrombosis occurs in the proximal carotid, distal vertebral, and basilar arteries. Such a circumstance may arise in association with hypercoagulable states. When the internal carotid artery (ICA) occludes, it can also cause low-flow ischemic events depending on status of the collateral circulation.
B. Ischemic strokes resulting from small-vessel or penetrating artery disease (lacunes). Long-standing arterial hypertension affects primarily the smaller penetrating IC vessels. It induces hypertrophy of the media and deposition of fibrinoid material into the vessel wall (fibrinoid necrosis), which eventually leads to occlusion. Lacunes are small ischemic infarcts in the deep regions of the brain or brainstem ranging in diameter from 0.5 to 15.0 mm resulting mainly from lipohyalinosis of penetrating arteries or branches related to long-standing arterial hypertension—chiefly the anterior choroidal, middle cerebral, posterior cerebral, and basilar arteries. Diabetes mellitus and EC arterial and cardiac sources of embolism are found less frequently.
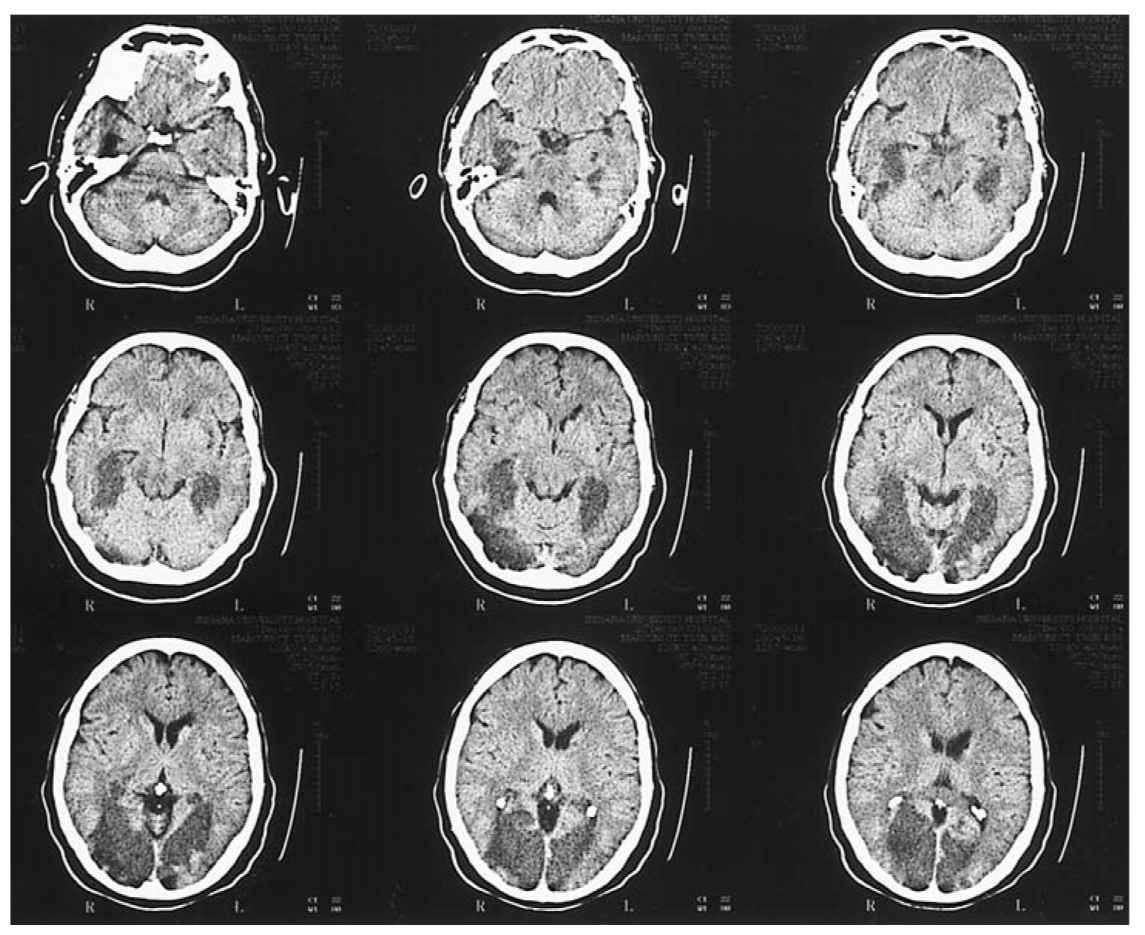
FIGURE 40.3 Axial unenhanced CT shows areas of low attenuation involving the medial aspects of both temporal lobes, both occipital lobes, the midbrain, and the pons in a patient with a diagnosis of basilar arterial occlusion. CT, computed tomography.
C. Ischemic stroke resulting from cardioembolism. Cardioembolic strokes are associated with substantial morbidity and mortality. Embolism of cardiac origin accounts for approximately 15% to 20% of all ischemic strokes. Emboli from cardiac sources frequently lodge in the MCA territory, are often large, and often have the worst outcomes. Although most types of heart disease can produce cerebral embolism, certain cardiac disorders are more likely to be associated with emboli (Table 40.1). Low or uncertain embolic risk disorders include mitral valve prolapse, mitral annulus calcification, aortic valve calcification, calcific aortic stenosis, bicuspid aortic valve, atrial flutter, patent foramen ovale, atrial septal aneurysms, valvular strands, and a Chiari network. Identification of a cardiac source of potential embolism is helpful for management. However, finding a potential cardiac embolic source is not by itself sufficient to diagnose cardio embolic cerebral infarction because many cardiac problems can coexist with cerebrovascular atherosclerosis.
D. Ischemic stroke resulting from hemodynamic mechanisms. Another mechanism of ischemic CNS damage is decreased systemic perfusion pressure that causes diminished blood flow to the brain in a diffuse manner. This occurs most commonly in the setting of cardiac pump failure or systemic hypotension. Border-zone ischemia is often explained by the combination of two frequently interrelated processes—hypoperfusion and embolization. This type of insult is most critical in border-zone territories, or so-called watershed areas, in the most distal regions of supply of the major arterial territories. Border-zone ischemia can result in several characteristic syndromes depending on whether the ischemia is in the border-zone territory of all three major arterial systems (anterior, middle, and posterior cerebral arteries), the territory between the anterior and middle cerebral arteries, or the territory between the middle and posterior cerebral arteries. Watershed infarcts are often bilateral, but can be unilateral when preexisting ipsilateral vascular disease causes focal hypoperfusion in the most distal territory. Other mechanisms whereby watershed infarcts develop include microemboli and hematologic abnormalities.
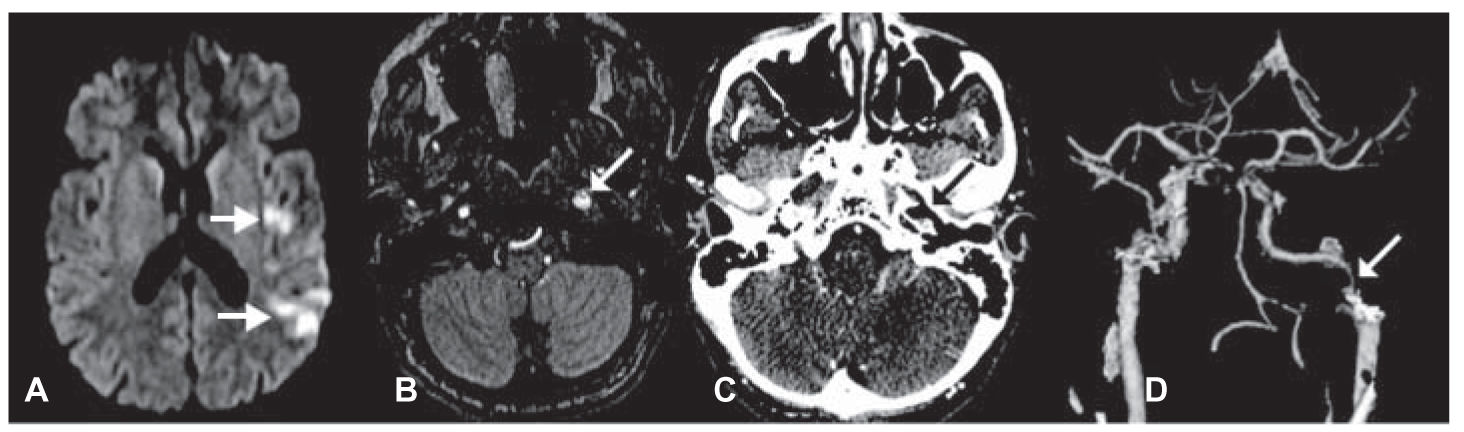
FIGURE 40.4 A 47-year-old man with acute onset of aphasia and right upper extremity weakness from left hemispheric ischemic injuries demonstrated on DWI MRI (A, arrowheads). Source image from MRA shows enlargement of the left ICA immediately inferior to the petrous canal (B, arrow) because of aneurysmal dilatation of the false lumen by an intimal flap. CTA, axial image (C) and three-dimensional reconstruction (D) show the same findings. CTA, computed tomographic angiography; DWI, diffusion-weighted imaging; ICA, internal carotid artery; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging. (See color plates.)
E. Ischemic stroke resulting from nonatherosclerotic vasculopathies. Several nonatherosclerotic forms of vasculopathy are predisposing factors for ischemic stroke. These vasculopathies include, among others, cervicocephalic arterial dissection (Figs. 40.4 and 40.5A–C), Moyamoya, fibromuscular dysplasia, and cerebral vasculitis. Together, these uncommon conditions represent 5% of all ischemic strokes. They are relatively more common among children and young adults.
F. Ischemic stroke resulting from hypercoagulable disorders. Alterations in hemostasis have been associated with an increased risk of ischemic stroke. These conditions include deficiencies in the anticoagulant proteins such as antithrombin, protein C, protein S, activated protein C resistance, factor V Leiden mutation, prothrombin gene (G20210A) mutation, and heparin cofactor II; disorders of fibrinogen or the fibrinolytic system; methylene-tetrahydrofolate reductase gene mutation; lipoprotein (a) disorders; and secondary hypercoagulable states encountered in patients with malignancies, pregnancy/puerperium, the antiphospholipid antibody syndrome, nephrotic syndrome, polycythemia vera, sickle cell disease, thrombotic thrombocytopenic purpura (TTP), and paroxysmal nocturnal hemoglobinuria. These disorders account for 1% of all strokes and for 2% to 7% of ischemic strokes in young patients.
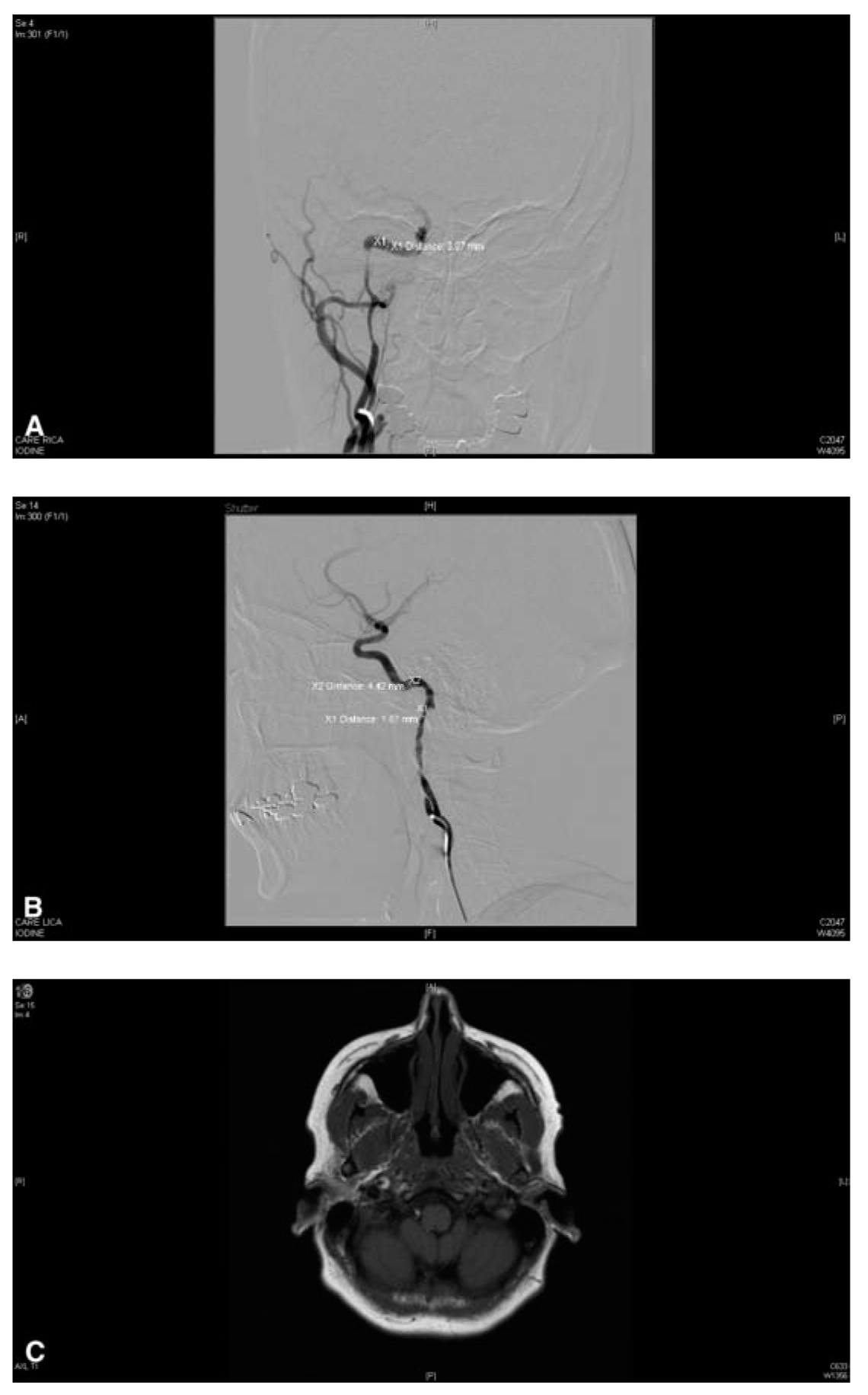
FIGURE 40.5 A: Angiogram of a 51-year-old woman with prior history of head trauma depicts a right ICA chronic dissection with luminal irregularities, severe stenosis, and delayed IC flow. B: Angiogram of acute on chronic left ICA dissection with luminal irregularities, severe stenosis, and small elevated flap predisposing patient to thrombus formation and ischemic stroke, treated with anticoagulation. C: Axial T1 weighted fat saturated MRI image shows right ICA high signal crescent sign, characteristic for arterial dissection. IC, intracranial; ICA, internal carotid artery; MRI, magnetic resonance imaging.
G. Ischemic stroke of undetermined causation. Despite extensive evaluation, in as many as one-third of ischemic strokes, a cause cannot be determined. This percentage is possibly higher among patients younger than 45 years (![]() Video 40.1). It is possible that some of these strokes are caused by cardioembolic or hematologic events not readily demonstrable.
Video 40.1). It is possible that some of these strokes are caused by cardioembolic or hematologic events not readily demonstrable.
1. If no cause of ischemic stroke is discovered, patients should undergo prolonged ambulatory cardiac monitoring in an attempt to uncover paroxysmal atrial fibrillation (PAF). Studies in patients with pacemakers or cardioverter-defibrillators revealed PAF is more prevalent than persistent atrial fibrillation and carries the same stroke risk as persistent atrial fibrillation (AF). Technologic advances have made it possible to monitor for PAF episodes for months and even years and quantitate the duration and frequency of PAF. Modes of prolonged cardiac monitoring are either noninvasive relying on surface electrodes (Holter-sensitive and specific for PAF detection), external loop recorder, ambulatory telemetry, or invasive (implantable loop recorder, dual-chamber pacemaker/defibrillator). Yield of detecting PAF increases with duration, device sensitivity, PAF definition, and strict patient selection, specifically targeting those patients who have suffered cryptogenic ischemic strokes. Patient risk factors for PAF include older age, cryptogenic stroke, vascular risk factors based on CHADS2 or CHA2DS2-VASC score or vascular disease, left atrial enlargement, left atrial appendage dysfunction, premature atrial complexes on electrocardiography (ECG), and single or multiple cortical and subcortical areas of restricted diffusion on MRI. Oral anticoagulation would be the treatment of choice for PAF but the decision should be made based on risk stratification scores assessing risk of stroke (CHADS2, CHA2DS2-VASC scores) and hemorrhage (HAS-BLED and HEMORRHAGES scores). Questions that remain uncertain mandating further research include which is the best monitoring device to use, duration of monitoring, patient selection, and risk of stroke associated with brief (seconds) episodes of PAF.
PREVENTION
The prevention of strokes follows three main avenues—control of modifiable risk factors, pharmacologic therapy, and surgical intervention. Knowledge and control of modifiable risk factors are paramount in prevention of primary and secondary strokes. Treatable or modifiable risk factors include arterial hypertension, diabetes mellitus, cigarette smoking, hyperlipidemia, excessive alcohol intake, obesity, and physical inactivity. Other risk factors include age and gender, cardiac disease, TIAs, previous strokes, asymptomatic carotid bruit or stenosis, high hemoglobin level or hematocrit, increased fibrinogen level, use of oral contraceptives, and possibly race and ethnicity.
A. Hypertension predisposes to ischemic stroke by aggravating atherosclerosis and accelerating heart disease. Approximately, 60 million Americans have arterial hypertension. Arterial hypertension is the most important modifiable risk factor for stroke, increasing the relative risk 3- to 4-fold. Blood pressure lowering also reduces the risk of stroke in individuals with isolated systolic hypertension and in elderly subjects. Blood pressure treatment resulting in a decrease in mean diastolic blood pressure of 5 mm Hg over 2 to 3 years is associated with a 40% reduction in risk of stroke. Blood pressure targets are individualized according to age, ethnicity, and coexisting comorbidities. Reduction of blood pressure is more important than the specific antihypertensive agent or modality use.
B. Diabetes mellitus increases the risk of ischemic cerebrovascular disease 2- to 4-fold compared with the risk among persons without diabetes. In addition, diabetes increases morbidity and mortality after stroke. Most persons with diabetes die of atherosclerotic cardiovascular complications, and atherosclerosis causing blockage to the arteries in the heart and brain accounts for >80% of all deaths in diabetes. Rigorous blood pressure control is particularly recommended for diabetic patients. Patients with diabetes who have concomitant hypertension should be treated with a regimen that includes an angiotensin-converting-enzyme (ACE) inhibitor or an angiotensin receptor blocker. There is presently no evidence to suggest that tighter diabetic control decreases the risk of stroke or recurrent stroke.
C. Cigarette smoking is an independent risk factor for ischemic stroke among men and women of all ages. More than 5 years may be required before a reduction in stroke risk is observed after cessation of smoking.
D. Hyperlipidemia. There is a positive association between serum cholesterol and risk of ischemic stroke. Patients with TIAs or ischemic stroke with elevated cholesterol, comorbid coronary artery disease, or evidence of an atherosclerotic lesion should be managed with 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase inhibitors (statins). The statins block the rate-limiting step in cholesterol biosynthesis. These agents not only effectively lower the rate of cholesterol biosynthesis, but a number of cholesterol-independent or pleiotrophic effects allow statins to exert important influences on a number of critical cellular signaling mechanisms. Meta-analysis of randomized trials of statins in primary or secondary preventions has shown a relative stroke risk reduction of approximately one-fifth. In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels, treatment with atorvastatin 80 mg daily significantly reduced the risk of nonfatal or fatal stroke, and the risk of stroke or TIA compared with placebo. A reduction in major cardiovascular events was also observed. Although atorvastatin was associated with fewer ischemic strokes than placebo, hemorrhagic strokes were more frequent in the atorvastatin-treated group. Further data are needed regarding the effective range of HMG CoA reductase inhibitor doses in these patients.
E. Excessive alcohol use. There is a J-shaped association between alcohol consumption and ischemic stroke. Lower doses (upto two drinks a day) offer reduced risk and higher doses elevate the risk. Moderate alcohol intake may elevate high-density lipoprotein concentration.
F. Obesity and physical inactivity. Obesity exerts an independent increase in risk of stroke among men younger than 63 years. This increase is greater among cigarette smokers and patients with hypertension, elevated blood glucose, or hyperlipidemia. There is some evidence that physical activity can reduce the risk of stroke.
TREATMENT
Ischemic stroke is a medical emergency. Any patient with acute ischemic stroke should be admitted to hospital for prompt evaluation (Table 40.2) and treatment. This is best accomplished in an intensive care unit or stroke unit. Management must be individualized according to the pathophysiologic process.
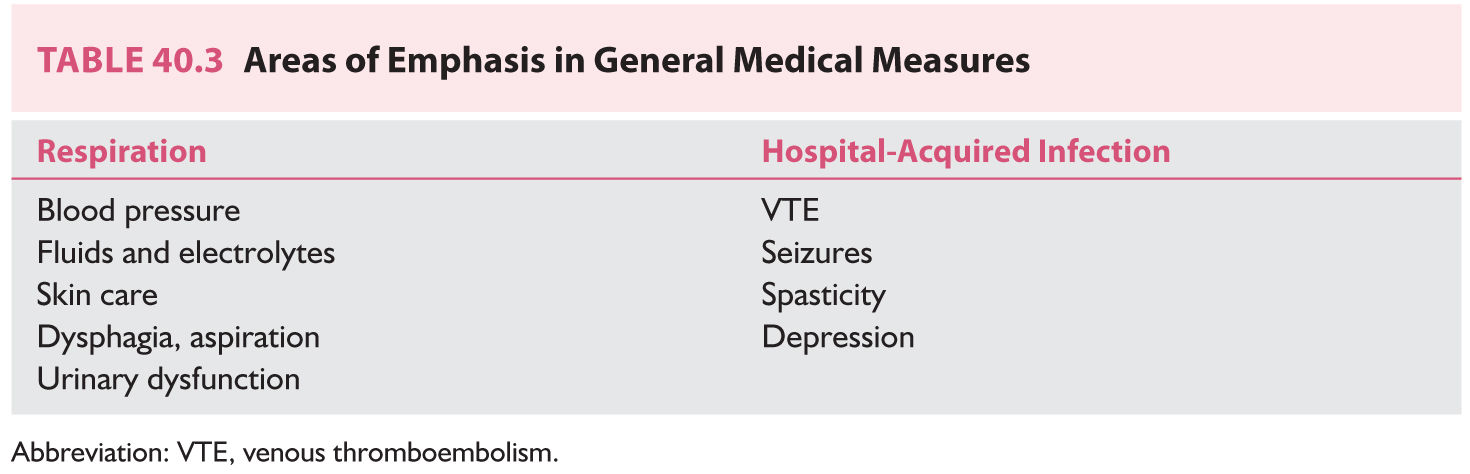
A. General measures. Particular attention should be paid to the following parameters (Table 40.3).
1. Medical measures.
a. Respiratory tract protection and infection. The airway of an obtunded patient should be protected. Some critically ill patients need ventilatory assistance. Aspiration and atelectasis should be prevented. Nosocomial pneumonia frequently complicates stroke and is a leading cause of mortality in the second to fourth weeks following cerebral infarction. Risk factors for nosocomial pneumonia include advanced age, prolonged hospitalization, serious medical comorbidity, immunosuppression, and endotracheal intubation.
Dysphagia is common after stroke. Failure of the swallowing process increases the risks of aspiration, malnutrition, and dehydration. The risk of pneumonia is increased by aspiration, which occurs in as many as 25% of unilateral hemispheric strokes and 70% of bilateral hemispheric or brainstem strokes. A meticulous history and examination of the oral, pharyngeal, and esophageal stages with a modified barium swallow using videofluorography are recommended. Oral ingestion of food or liquids is often precluded in the first 24 to 48 hours. Nasogastric feedings are often necessary. Some patients may need a gastrostomy to maintain adequate nutritional intake.
b. Urinary tract infections. Urinary bladder dysfunction can complicate stroke, particularly basal ganglia, frontoparietal, and bilateral hemispheric strokes. Urinary tract infections are an important cause of hyperpyrexia following stroke. They contribute to almost one-third of stroke-related deaths and are present in almost one-half of patients in autopsy series. Incontinent or comatose patients should be catheterized, preferably with a condom catheter for men or a closed Foley’s catheter for women. Many patients need an indwelling catheter, which is associated with a risk of infection. In addition, even continent patients can have postvoiding residuals that also increase the likelihood of urinary tract infections.
c. Electrolytic and metabolic disturbances.
(1) Electrolytic disturbances. Stroke patients are at risk of electrolyte disturbances resulting from reduced oral intake, potentially increased gastric and skin losses, and derangements in secretion of antidiuretic hormone (ADH). Levels of ADH increase after stroke. In some cases, inappropriate secretion of ADH places the patient at risk of hyponatremia. Possible mechanisms include damage to the anterior hypothalamus and a prolonged recumbent position. In most cases, these alterations do not persist beyond the first week after a stroke.
(2) Hyperglycemia in acute stroke is a common phenomenon and correlates with a poor outcome. Hyperglycemia increases the extent of ischemic brain damage. High-serum glucose levels increase anaerobic metabolism, increase lactic acid production in ischemic brain tissue, and cause cellular acidosis. Hypotonic solutions or fluids containing glucose should be avoided.
d. Venous thromboembolism (VTE) is a common complication among patients with acute ischemic stroke. The risk is highest in the early weeks after ictus, but remains significant in the chronic phase. The frequency of deep venous thrombosis (DVT) ranges from 20% to 40% in hospitalized stroke patients, and approximately 75% of stroke patients may develop DVT without prophylaxis. Moreover, lethal pulmonary embolism may account for upto 25% of the early poststroke deaths. Prophylaxis of VTE is an essential component of stroke center protocols. Prevention includes the use of pressure gradient stockings, pneumatic compression stockings, low-dosage subcutaneous unfractionated heparin (UFH) (5,000 units every 8 to 12 hours), or low-molecular-weight heparin (enoxaparin 40 mg once daily, dalteparin 5,000 units once daily, or tinzaparin 4,500 units once daily).
e. Cardiac events constitute an important cause of death after acute stroke because 40% to 70% of these patients have baseline coronary artery disease. Cardiac manifestations that can occur after acute ischemic stroke include ECG abnormalities, cardiac arrhythmias, elevation of CK-MB or cardiac selective troponin levels, left ventricular dysfunction, and MI. Approximately 15% of deaths following ischemic stroke are from fatal arrhythmia or MI. As many as 30% of patients have ST segment depression on an ECG in the first 48 hours after the event, and 35% have ventricular couplets or tachycardia. Other changes include QT interval prolongation, T-wave inversion, or increases in duration and amplitude of T waves. Strokes restricted to the insular cortex have been associated with arterial hypertension, cardiac arrhythmias, increased risk of myocardial injury, raised catecholamine levels, and an increased susceptibility to sudden death.
f. Cerebral autoregulation is lost during acute ischemic stroke. The blood pressure should be measured frequently during the first few days after ischemic stroke. Transient blood pressure elevation following acute cerebral infarction and normalization over days without treatment are common. Mild to moderate hypertension may be compensatory, and rapid lowering of the blood pressure is generally not recommended. Exceptions to this rule include patients with hypertensive encephalopathy and cerebral ischemia secondary to aortic dissection.
g. Pressure sores. Stroke patients, like other patients with reduced mobility, are at increased risk of having pressure sores. Altered level of consciousness, peripheral vascular disease, and malnourishment are contributing factors. Pressure sores develop most often over bony prominences—sacrum, ischium, trochanters, and the areas around the ankles and heels. The patient’s position should be changed frequently to reduce pressure and shear forces. Flotation beds help reduce the risk. Treatment includes debridement and moist dressing. Surgical treatment is sometimes necessary. Cellulitis in the surrounding skin and systemic infection necessitate antibiotic therapy.
h. Depression. Depression occurs in the first few weeks after stroke, but is maximal between 6 and 24 months afterward. Patients with left frontal strokes appear to be more susceptible than are those with right hemisphere or brainstem strokes, but this remains debatable. Depression also correlates with severity of neurologic deficit and quality of available social support. Therapy for poststroke depression is the same as that for endogenous depression.
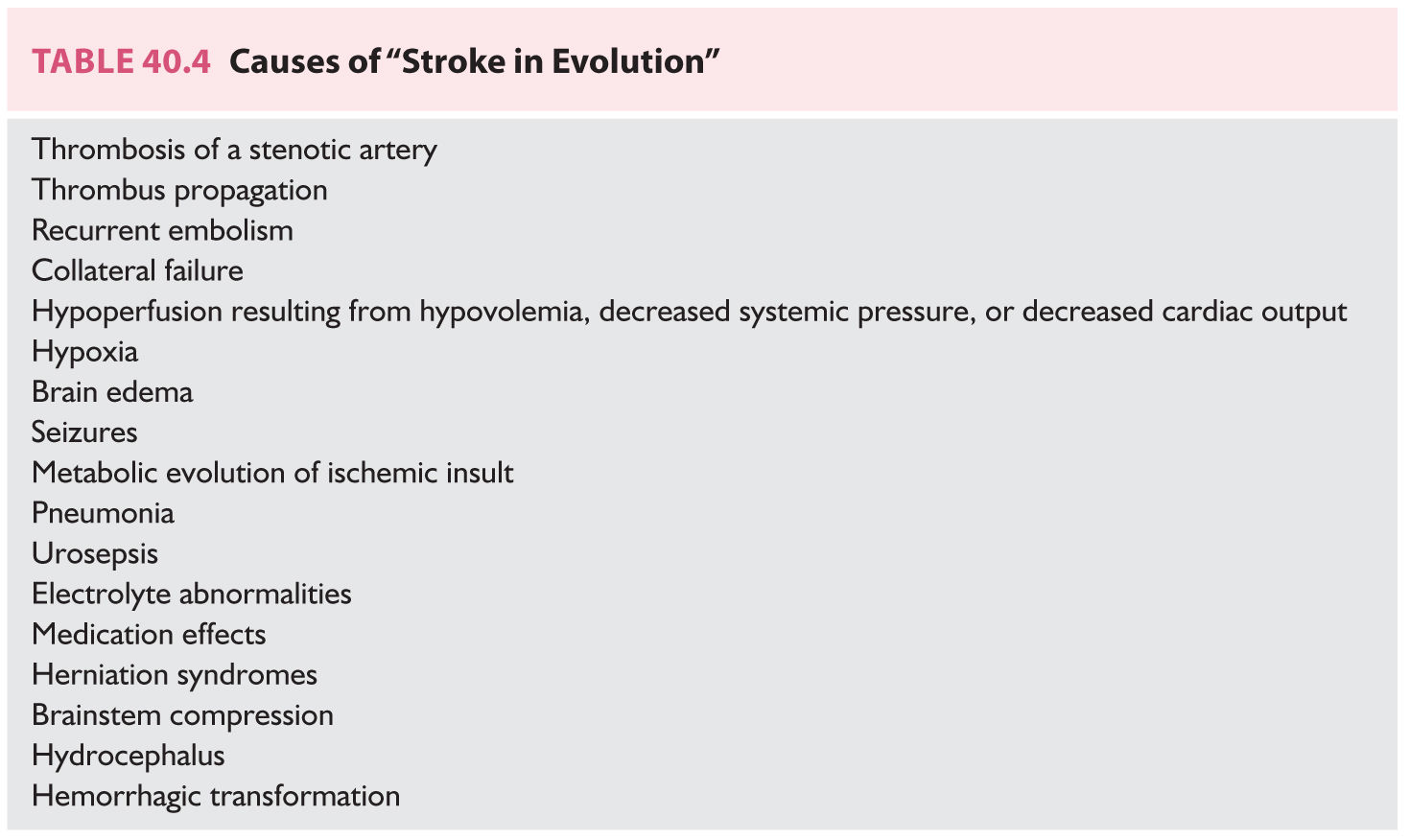
2. Neurologic measures. Approximately 30% of patients with acute ischemic strokes worsen after the initial event, but deterioration after stroke or “stroke in evolution” does not necessarily equate to propagating thrombus or recurrent embolism (Table 40.4). Massive hemispheric infarctions have a high mortality. Large (malignant) MCA territory strokes (5% of all strokes) are associated with poor prognosis. The underlying mechanism of malignant MCA infarction is often either a carotid terminus occlusion or a proximal MCA occlusion. Acute cerebellar infarction may cause brainstem compression with hydrocephalus.
a. Brain edema is the most common cause of deterioration and early death during the first week after acute cerebral infarction. Risk factors for malignant cerebral edema include National Institute of Health Stroke Scale (NIHSS) >20 in left hemispheric strokes (>15 for right hemispheric strokes), carotid terminus thrombus, nausea and vomiting, elevated white blood cells, >50% MCA territory involvement, and additional involvement of anterior cerebral artery (ACA) and posterior cerebral artery territories. Young patients and patients with large infarcts are most affected. Massive cerebral edema complicates approximately 10% of large hemispheric strokes. Edema develops within several hours after an acute ischemic brain insult and peaks around 3 to 5 days. Ischemic brain edema is initially cytotoxic and later vasogenic. Cytotoxic edema involves predominantly the gray matter, whereas vasogenic edema involves predominantly the white matter. No specific pharmacologic agent has been proved effective against ischemic cerebral edema. Corticosteroids have not been proved useful in the management of ischemic cerebral edema and may even be detrimental. Mannitol does not cross the blood–brain barrier and may accentuate compartmentalized pressure gradients between abnormal and normal brain regions. Hypernatremia, hypokalemia, and hypocalcemia can result from excessive osmotherapy. Excessive osmotherapy can also result in intravascular volume depletion and arterial hypotension. Normal saline solution is administered to prevent intravascular depletion. In appreciation of the role of brain tissue shifts, hypertonic saline administration or surgical evacuation of life-threatening supratentorial infarctions by means of hemicraniectomy and duroplasty may have to be considered (see section B.2 under Treatment).
b. Hemorrhagic transformation occurs in approximately 40% of all ischemic infarcts, and of these, 10% show secondary clinical deterioration. Hemorrhagic transformation often occurs in the first few weeks following stroke, most often in the first 2 weeks. Risk factors for hemorrhagic transformation include large strokes with mass effect, enhancement on contrast CT scans, and severe initial neurologic deficits.
c. Seizures occur in 4% to 6% of cases of ischemic infarction, mostly in carotid territory cortical infarcts. Infarcts in the posterior circulation are infrequently associated with seizures. Cardioembolic strokes have been found to be more epileptogenic than atherothrombotic strokes, but several studies have found no significant difference. Seizures associated with lacunar infarcts are extremely rare. Partial seizures are more common than are generalized tonic–clonic seizures. Many seizures occur within 48 hours of onset of symptoms. In general, seizures are self-limited and respond well to antiepileptic drugs. Patients with seizures that occur in the first few days after the ischemic event do not have increased mortality. Status epilepticus is unusual.
3. Rehabilitation. Prevention of complications is the first stage of rehabilitation. Patients who need inpatient rehabilitation are transferred to the appropriate rehabilitation facility. The long-term prognosis for stroke depends on severity and type of neurologic deficit, the cause of the stroke, medical comorbidity, premorbid personality, family constellation, home environment, type of community and available services, and the rehabilitation team. Approximately, 50% to 85% of long-term survivors of stroke are able to walk independently, most of the recovery taking place in the first 3 months. Approximately, two-thirds of long-term survivors eventually become independent for activities of daily living, and approximately 85% of surviving patients eventually return home.
B. Specific measures.
1. Medical therapy. General measures and use of antithrombotic agents (antiplatelet agents or anticoagulants) and thrombolytic agents remain the mainstays of medical therapy for acute ischemic stroke.
a. Antiplatelet agents. Antiplatelet agents such as aspirin, the combination of extended-release dipyridamole plus aspirin, and clopidogrel play a critical role in the secondary prevention of atherothrombotic events. Antiplatelet therapy is highly effective in reducing the risk of recurrent vascular events and is recommended over warfarin for noncardioembolic ischemic strokes. Antiplatelet therapy should be avoided in the first 24 hours following the administration of intravenous (IV) tissue plasminogen activator (tPA) for acute ischemic stroke.
(1) Aspirin. The mechanism of action of aspirin is irreversible inhibition of platelet function through inactivation of cyclooxygenase. Aspirin reduces the combined risk of stroke, MI, and vascular death by approximately 25%. Early (within the first 48 hours) aspirin therapy (160 to 325 mg/day) is recommended in patients with ischemic stroke who are not receiving thrombolytic therapy. The US Food and Drug Administration (FDA) recommends a dose of 50 to 325 mg/day of aspirin in the secondary prevention of noncardioembolic ischemic stroke. The main side effect is gastric discomfort. A subpopulation of patients may be resistant to the antiplatelet effects of aspirin.
(2) Dipyridamole plus aspirin. Dipyridamole is a cyclic nucleotide phosphodiesterase inhibitor. The Second European Stroke Prevention Study (ESPS-2) randomized 6,602 patients with previous TIA or stroke to treatment with aspirin alone (25 mg twice per day), modified-release dipyridamole (200 mg twice per day), the two agents in combination, or placebo. The investigators reported an additive effect of dipyridamole (37%) when coprescribed with aspirin. There was a decrease in stroke rate with combined treatment versus either agent alone (aspirin, 18%; dipyridamole, 16%). Both low-dose aspirin and high-dose dipyridamole in a modified-release form alone were better than placebo. The combination of aspirin and dipyridamole was effective in reducing the rate of nonfatal stroke, but had little effect on the rate of MI or fatal stroke. Addition of the European and Australian Stroke Prevention Reversible Ischemia Trial data to the meta-analysis of previous trials results in an overall risk ratio for the composite of vascular death, stroke, or MI of 0.82 (95% CI 0.74 to 0.91) in favor of the dipyridamole plus aspirin regimen. A randomized clinical trial comparing aspirin plus extended-release dipyridamole versus clopidogrel in more than 20,000 patients found no difference in recurrent stroke or a composite outcome of stroke, MI, or death after 2.5 years of follow-up. The main side effects of dipyridamole are gastrointestinal (GI) distress and headaches.
(3) Clopidogrel. Clopidogrel is a platelet adenosine diphosphate receptor antagonist. In a study enrolling more than 19,000 patients with atherosclerotic vascular disease manifested as either recent ischemic stroke, recent MI, or symptomatic peripheral arterial disease, 75 mg of clopidogrel was modestly more effective (8.7% relative risk reduction) than 325 mg of aspirin in reducing the combined risk of ischemic stroke, MI, or vascular death. The side-effect profile was thought to be relatively benign, with no increased incidence of neutropenia, although a report associated the use of clopidogrel with TTP in 11 patients. Several of these patients were taking concomitant medications. Clopidogrel is a reasonable alternative in patients allergic to aspirin. The addition of aspirin to clopidogrel increases the risk of hemorrhage and is not routinely recommended in patients with ischemic stroke or TIA. However, a recent Chinese study determined the safety and superiority of combination therapy with clopidogrel and aspirin to aspirin monotherapy for reducing the risk of stroke in the first 90 days when administered within 24 hours after onset of minor stroke or TIA. Functional variants in the cytochrome P450 genes can alter the effectiveness of clopidogrel.
(4) Other agents. Cilostazol, a phosphodiesterase III inhibitor, is often used for stroke prevention in Japan and other East Asian countries. Triflusal, which is chemically related to aspirin, is considered to be an acceptable first-line antiplatelet agent in some European countries.
(5) Summary. Aspirin at doses of 50 to 325 mg/day extended-release dipyridamole 200 mg plus aspirin 25 mg twice daily, or clopidogrel 75 mg/day, are all acceptable alternatives for initial therapy.
b. Anticoagulants.
(1) Prevention.
(a) Warfarin. Warfarin inhibits the vitamin K-dependent gamma-carboxylation of factors II, VII, IX, and X. Warfarin is indicated for primary and secondary prevention of stroke among patients with nonvalvular atrial fibrillation (NVAF). Data from a series of AF trials demonstrate an approximate two-third risk reduction in the rate of stroke occurrence when patients are treated to a goal international normalized ratio (INR) range of 2.0 to 3.0. The risk of stroke is the same for patients with chronic or PAF. Warfarin is also indicated for prevention of stroke in patients with rheumatic AF, mechanical prosthetic heart valves, or other selected subgroup of patients with other high-risk cardiac sources of embolism.
(b) New Oral Anticoagulants (NOACS). Dabigatran, a reversible oral direct thrombin inhibitor, and the anti-Xa agents (rivaroxaban, apixaban, edoxaban) are used as alternative agents for adjusted-dose warfarin in the prevention of stroke in patients with NVAF. The RE-LY study was a phase III, prospective, randomized, open-label trial comparing dabigatran (110 or 150 mg twice daily) with warfarin. Dabigatran was the first NOAC approved by the US FDA, has a rapid onset of action (1 to 2 hours), is 80% renally excreted, and contraindicated with concurrent administration of P-glycoprotein inducers. Dabigatran 150 mg twice daily was superior to warfarin for the primary efficacy endpoint of stroke and systemic embolism without significant differences in major bleeding; GI bleeding was more frequent with Dabigatran. Dabigatran 110 mg twice daily was noninferior to warfarin for the primary endpoint and had a 20% reduction in major bleeding. Results were confirmed by the RELY-ABLE study, which assessed the long-term effects of the two doses of Dabigatran at 2 year follow-up. Rivaroxaban, the second NOAC approved by the FDA, was studied in the ROCKET AF trial; Rivaroxaban also has a rapid onset of action (2 to 4 hours), has a renal excretion of 35%, and is contraindicated with cytochrome P450 isoenzymes and P-glycoprotein inhibitors because of increased risk of bleeding. Rivaroxaban was shown to be noninferior to warfarin but failed to show superiority for prevention of stroke or systemic embolism. There was greater GI bleeding in the Rivaroxaban group but less frequent IC and fatal bleeding. Apixaban and edoxaban were the last NOACs approved by the FDA. The ARISTOTLE trial demonstrated superiority of apixaban over warfarin with fewer primary outcomes of both ischemic and hemorrhagic stroke and systemic emboli, but the overall rate of ischemic strokes was similar. GI bleeding was similar among both groups, but apixaban had significantly fewer IC bleeds. All-cause mortality was significantly lower in the apixaban group. Apixaban was also compared to aspirin in the AVERROES, which was prematurely stopped because of a clear benefit of Apxiaban for prevention of ischemic stroke and systemic embolism; major and IC bleeding was similar between both groups. Finally, edoxaban at higher dosing (60 mg once daily) was noninferior to warfarin in stroke prevention but inferior at lower dosing (30 mg once daily); major and IC bleeding was less with edoxaban except for GI bleeding. No trial has been completed comparing the NOACs. Dabigatran 150 mg twice daily is the only NOAC shown to decrease ischemic stroke compared to warfarin. Aside from apixaban, all NOACs were associated with more GI bleeding than warfarin. Dabigatran is renally excreted but apixaban and Rivaroxaban are less dependent on renal elimination. Rivaroxaban and edoxaban are dosed once daily, which may be attractive noncompliant patients. NOACs remain contraindicated in patients with mechanical heart valves, rheumatic heart disease, or severe renal insufficiency.
(2) Treatment.
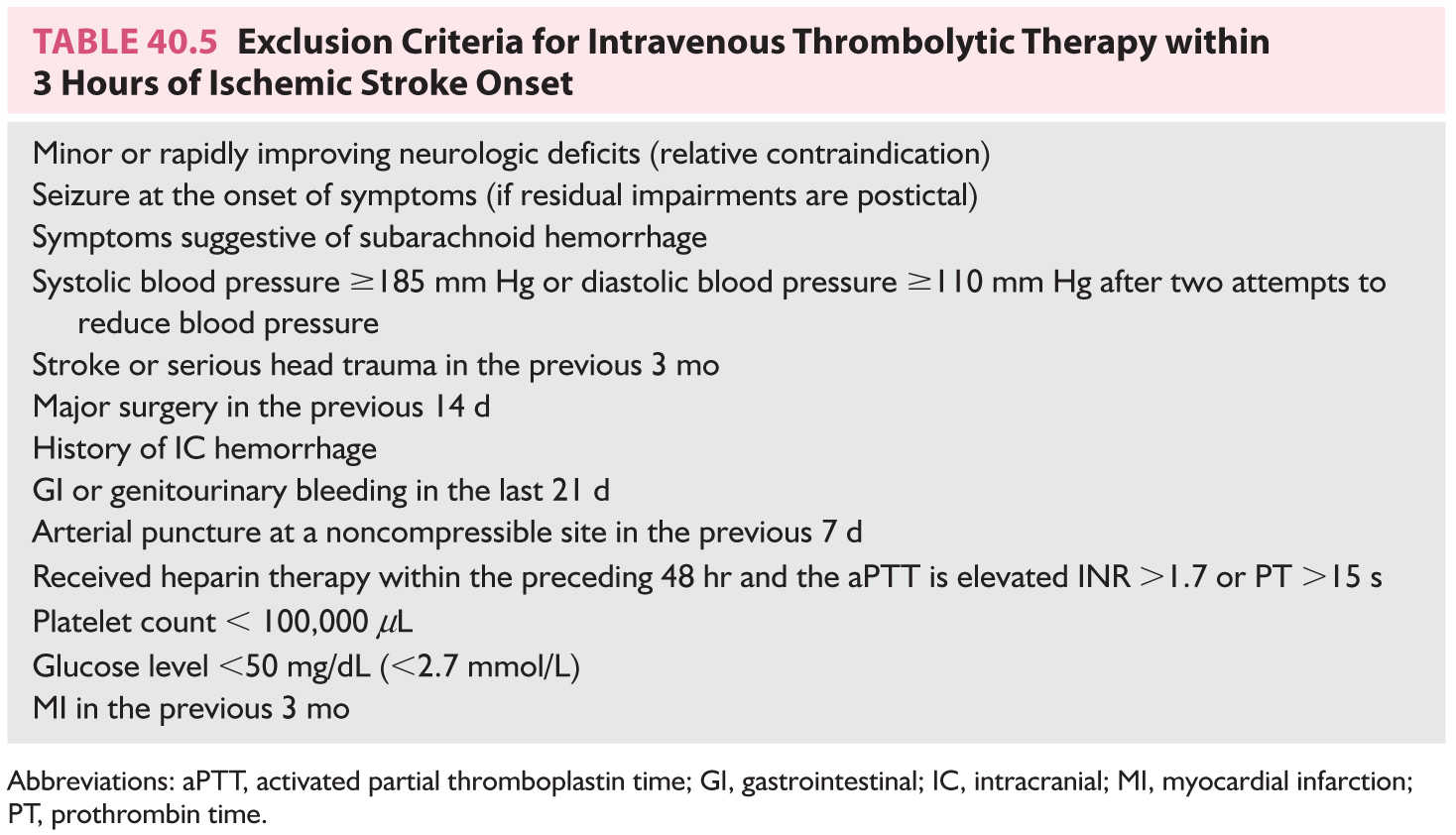
(a) Thrombolytics. Thrombolytic therapy has been a major milestone in the management of acute ischemic stroke. In June 1996, the US FDA approved the use of IV tPA (Alteplase) for ischemic stroke within 3 hours of symptom onset. IV tPA initiated within 3 hours of symptom presentation is a first-line treatment for acute ischemic stroke in selected patients (Table 40.5). Patients are given 0.9 mg/kg/dose (maximum 90 mg dose); 10% of the total dose is administered as a loading dose over 1 minute with the remainder administered over 60 minutes. In the NINDS rt-PA Stroke Trial, favorable outcome was achieved in 31% to 50% compared with 20% to 38% of patients given placebo. Overall, for every 100 patients with acute ischemic stroke treated with IV tPA in the 0- to 3-hour window, 32 patients are benefitted and 3 patients are harmed. The major risk of treatment is symptomatic intracerebral hemorrhage that occurred in 6.4% of patients treated with IV tPA compared to 0.6% of patients given placebo in the NINDS rt-PA Stroke Trial. Predictors of favorable outcome associated with thrombolytic therapy include treatment within 90 minutes, normal baseline CT scan, milder baseline stroke severity, no history of diabetes mellitus, normal pretreatment blood glucose level, and normal pretreatment blood pressure. Predictors of less favorable outcome and/or cerebral hemorrhage include increasing age, hypoattenuation with mass effect or hypoattenuation in ≥one-third of the MCA territory, diabetes mellitus, pretreatment blood glucose >11 mmol/L, hypertension before, during, and after treatment or requiring postrandomization treatment, severe pretreatment neurologic deficit.
Data by the investigators of the ECASS 3 trial show that IV thrombolytic therapy is also beneficial when initiated within 3 to 4.5 hours of onset of acute ischemic stroke. The ECASS 3 study had strict exclusion criteria such as age over 80 years, combination of previous stroke and diabetes mellitus, oral anticoagulant therapy (regardless of INR values), an NIHSS score of >25, and evidence of major infarct on CT scan with compromise of >one-third of the MCA territory.
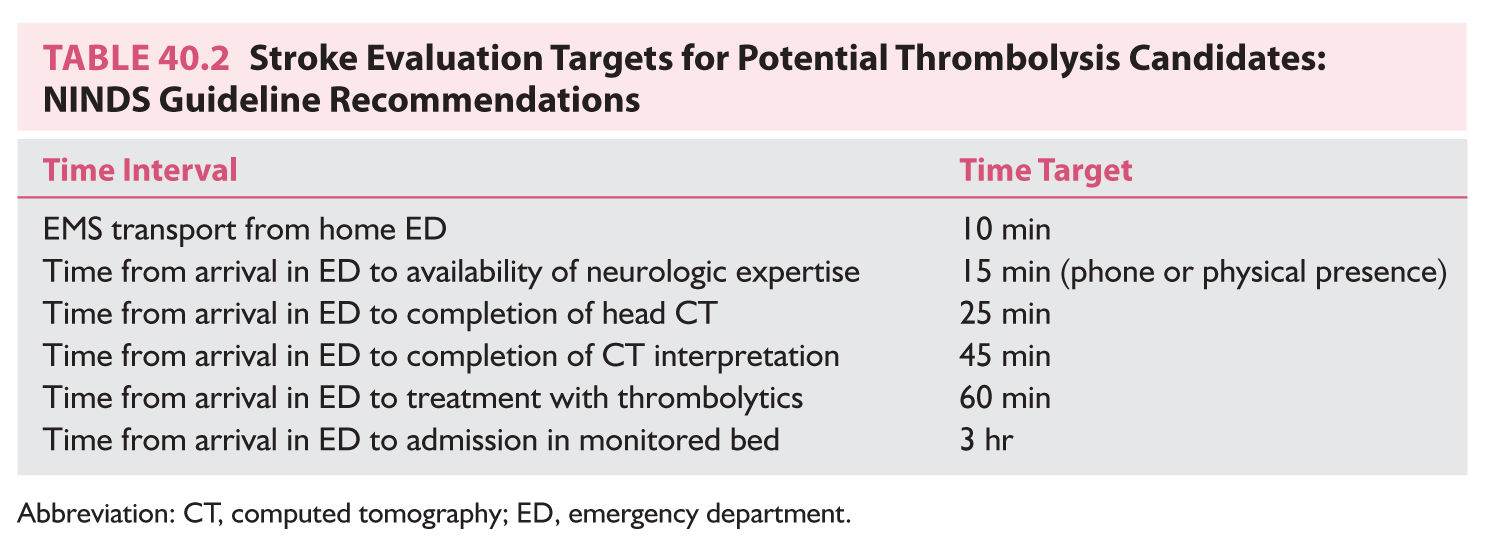
NINDS time target recommendations for potential thrombolysis candidates are as follows: Door to door: 10 minutes, Access to neurologic expertise: 15 minutes, Door to CT completion: 25 minutes, Door to CT interpretation: 45 minutes, Door to treatment: 60 minutes, Door to monitored bed: 3 hours.
If IC hemorrhage occurs after thrombolytic therapy, stop tPA if still infusing, obtain emergent nonenhanced head CT, check CBC, PT (INR), aPTT, fibrinogen, type, and screen. Transfuse 10 units of cryoprecipitate, 6 to 8 units platelets, 2 units fresh frozen plasma (FFP) every 6 hours; hematology and neurosurgery should be consulted.
If orolingual edema occurs, administer IV solumedrol, Benadryl, and an H2 blocker; hold ACE inhibitor. Awake fiberoptic intubation may be necessary if symptoms progress.
(b) Endovascular treatment. Certain patients, deemed unsuitable for IV thrombolytic therapy, may be candidates for mechanical thrombectomy that is recommended over intra-arterial thrombolysis. Patients deemed suitable for IV thrombolysis should still receive IV rtPA even if endovascular treatment is being considered. Inclusion criteria include prestroke modified Rankin Scale (mRS) score 0 to 1, IV tPA administered within 4.5 hours, age 18 years or older, NIHSS ≥6, occlusion of ICA terminus or proximal MCA (M1), and Alberta Stroke Program Early CT Score (ASPECTS) ≥6, and groin puncture can be achieved within 6 hours of symptom onset. Initial imaging should consist of nonenhanced CT head and noninvasive IC vascular study (CT angiogram). Benefit of perfusion imaging is uncertain and may delay time to groin puncture and reperfusion (Fig. 40.6).
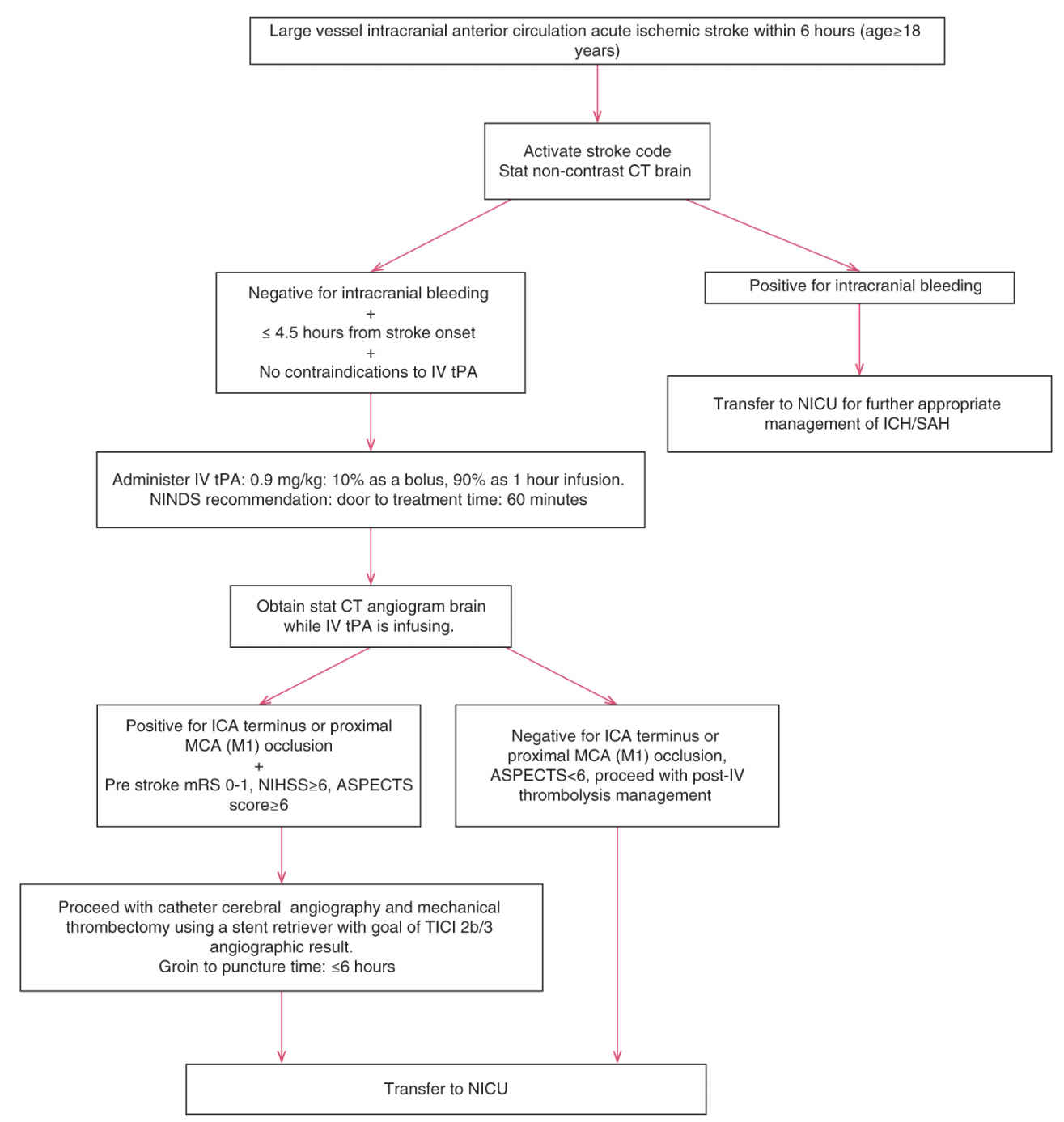
FIGURE 40.6 ASPECTS, Alberta stroke program early CT score; CT, computed tomography; ICA, internal carotid artery; MCA, middle cerebral artery.
Endovascular treatment within 6 hours may also be reasonable for carefully selected patients with occlusion of the M2 or M3 portions of the MCA, anterior, or posterior circulation. Conscious sedation is recommended over general anesthesia. The usefulness of mechanical thrombectomy devices other than stent retrievers is not well established; stent retrievers are therefore indicated in preference to other devices. The goal of thrombectomy should be a TICI 2b/3 angiographic result as soon as possible and within 6 hours in order to maximize functional outcome. Additional use of IA tPA to endovascular therapy may be reasonable if completed within 6 hours of symptom onset. The use of a proximal balloon guide catheter or a large-bore distal access catheter in conjunction with a stent retriever may be beneficial. Although benefits remain uncertain, endovascular treatment may be used in patients younger than 18 years with large-vessel occlusion if initiated within 6 hours, those with prestroke mRS >1, ASPECTS score <6, and NIHSS <6 with occlusion of ICA or proximal MCA. At the time of thrombectomy, angioplasty and stenting of proximal cervical ICA stenosis or occlusion may be considered but utility is unknown.
(c) Anticoagulants. Randomized trials of UFH, low-molecular-weight heparin, or heparinoids for the treatment of acute arterial ischemic stroke showed no proven benefits in the reduction of stroke-related mortality, stroke-related morbidity, early stroke recurrence, or stroke prognosis, except in cases of cerebral venous thrombosis.
2. Surgical therapy.
a. Carotid endarterectomy (CEA). Approximately, 15% of ischemic strokes are caused by EC ICA stenosis. In addition to the degree of carotid artery stenosis, plaque structure has been postulated as a critical factor in defining stroke risk. Various features of plaque morphology have been used to identify symptomatic risk. Inflammation is also important in carotid artery plaques. Clinicopathologic correlates of examined surgical carotid plaque specimens show that ulceration is more prevalent among symptomatic patients, and that thrombus formation is more common in cases with ulcerated plaques. Ulceration is described as an observable disruption of the intima, exposing underlying atheromatous plaque. Atherosclerotic plaques prone to rupture consist of an inflammatory process within the cap, angiogenesis, and intraplaque hemorrhage with gradual thinning of the fibrous cap, loss of integrity, and ulceration. Plaque hemorrhage specifically has been associated with ischemic neurologic deficits; the origin remains unclear but is thought to occur secondary to fissures within the cap, angiogenesis within the atherosclerotic plaque, and/or a perivascular inflammatory infiltrate. Precise determination of vessel stenosis using noninvasive studies has significant potential in the routine clinical assessment of ischemic cerebrovascular disease. Carotid ultrasonography is commonly used, but MRI is now a promising tool, accurately identifying ulcerated plaque cap or a large necrotic core and quantifying intraplaque hemorrhage. Fluorodeoxyglucose positron emission tomography (PET) CT scanning is also a promising tool for assessing carotid atherosclerotic plaques but is expensive, exposes patients to radiation, and not widely available.
Results from landmark prospective studies comparing best medical therapy with CEA provide compelling evidence of the benefit of CEA performed by experienced surgeons in improving the chance of stroke-free survival in high-risk symptomatic patients. Timely surgical intervention in selected patients with hemispheric TIAs, amaurosis fugax, or completed nondisabling ICA territory ischemic strokes within the previous 6 months, and with 70% to 99% diameter reducing ICA stenosis, can significantly reduce the risk for recurrent cerebral ischemia or death. With low surgical risk, CEA also provides modest benefit in symptomatic patients with ICA stenosis of 50% to 69%, especially among men with hemispheric ischemia who are not diabetic. The procedure’s benefit is greatest if done within the first 2 weeks from the ischemic event. There is no evidence that CEA provides any benefit over medical therapy if the degree of stenosis is <50%.
Controversy still surrounds the selection of asymptomatic patients (60% to 99% stenosis) for CEA. Based on combined data from the Asymptomatic Carotid Atherosclerosis Study and the Asymptomatic Carotid Surgery Trial Collaborators, the 5-year risk of stroke in these patients randomized to best medical therapy was around 12%, falling to approximately 6% with CEA. This modest benefit favoring CEA assumes that operative complications are below 3%. Postoperatively, patients should be monitored closely for development of hyperperfusion syndrome and IC hemorrhage.
It is not known when CEA can be performed safely after thrombolysis; some studies have reported no major complications when CEA was performed a median of 8 days with close monitoring of blood pressure in the pre-, peri-, and postoperative period.
b. Carotid angioplasty and stenting (CAS). Preliminary data of CAS suggest that CAS may have comparable efficacy to CEA. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) showed no significant difference in the composite of any stroke, MI, or death between symptomatic and asymptomatic patients receiving carotid artery stenting or CEA. In contrast to CREST, other studies have reported an excess risk of new ischemic lesions in patients who underwent CAS. Thus, the relative merit of CEA versus CAS remains a matter of intense debate.
c. EC/IC bypass surgery. An early randomized study of medical therapy versus EC/IC bypass surgery failed to show a benefit for surgery. The measurement of oxygen extraction fraction (OEF) by PET has allowed investigators to identify particular high-risk patients who might benefit from EC/IC bypass. However, preliminary data showed no benefit in the reduction of ipsilateral ischemic stroke in patients with symptomatic ICA occlusion and increased OEF on PET.
d. Decompressive surgery. In some circumstances of malignant cerebral edema (malignant MCA infarction), early (<48 hours) decompressive surgery with hemicraniectomy and durotomy may be lifesaving (Fig. 40.7A–F). A fronto-temporo-parietal decompressive hemicraniectomy and durotomy should be at least 12 cm in diameter with extension to the floor of the middle cranial fossa with preservation of the superficial temporal artery and facial nerve branches. Mortality and functional outcome are improved in patients 18 to 60 years old with NIHSS ≥15, decreased level of consciousness, intact pupil reactivity, and infarct of at least 50% MCA territory (![]() Video 40.2). For patients 61 to 82 years old, decompressive hemicraniectomy increases survival but has no impact on functional outcome of mRS 0 to 2, but does decrease severe disability (mRS 3–4). A pooled analysis of the three major decompressive hemicraniectomy trials, Decimal, Destiny, and Hamlet, was published in the Lancet in 2007 looking at primary outcome measure of mRS ≤4 in patients 18 to 60 years old, <45 hours from symptom onset, NIHSS >15, infarct volume >145 cm³, and CT with at least 50% infarction of the MCA territory. Although decompressive hemicraniectomy improved survival, 78% versus 28% (NNT 2), it also resulted in a 15-fold increase in patients with mRS of 4 at 1 year compared to the conservative group (75% surgical vs 24% conservative, NNT 2). In cases of cerebellar infarction with mass effect, when fourth ventricular compression and hydrocephalus are the primary concerns, some neurosurgeons prefer to perform a ventriculostomy. This procedure may be associated with a risk of upward cerebellar herniation through the free edge of the tentorial incisura (Fig. 40.8). For this reason, other neurosurgeons favor posterior fossa decompressive surgery for such patients.
Video 40.2). For patients 61 to 82 years old, decompressive hemicraniectomy increases survival but has no impact on functional outcome of mRS 0 to 2, but does decrease severe disability (mRS 3–4). A pooled analysis of the three major decompressive hemicraniectomy trials, Decimal, Destiny, and Hamlet, was published in the Lancet in 2007 looking at primary outcome measure of mRS ≤4 in patients 18 to 60 years old, <45 hours from symptom onset, NIHSS >15, infarct volume >145 cm³, and CT with at least 50% infarction of the MCA territory. Although decompressive hemicraniectomy improved survival, 78% versus 28% (NNT 2), it also resulted in a 15-fold increase in patients with mRS of 4 at 1 year compared to the conservative group (75% surgical vs 24% conservative, NNT 2). In cases of cerebellar infarction with mass effect, when fourth ventricular compression and hydrocephalus are the primary concerns, some neurosurgeons prefer to perform a ventriculostomy. This procedure may be associated with a risk of upward cerebellar herniation through the free edge of the tentorial incisura (Fig. 40.8). For this reason, other neurosurgeons favor posterior fossa decompressive surgery for such patients.
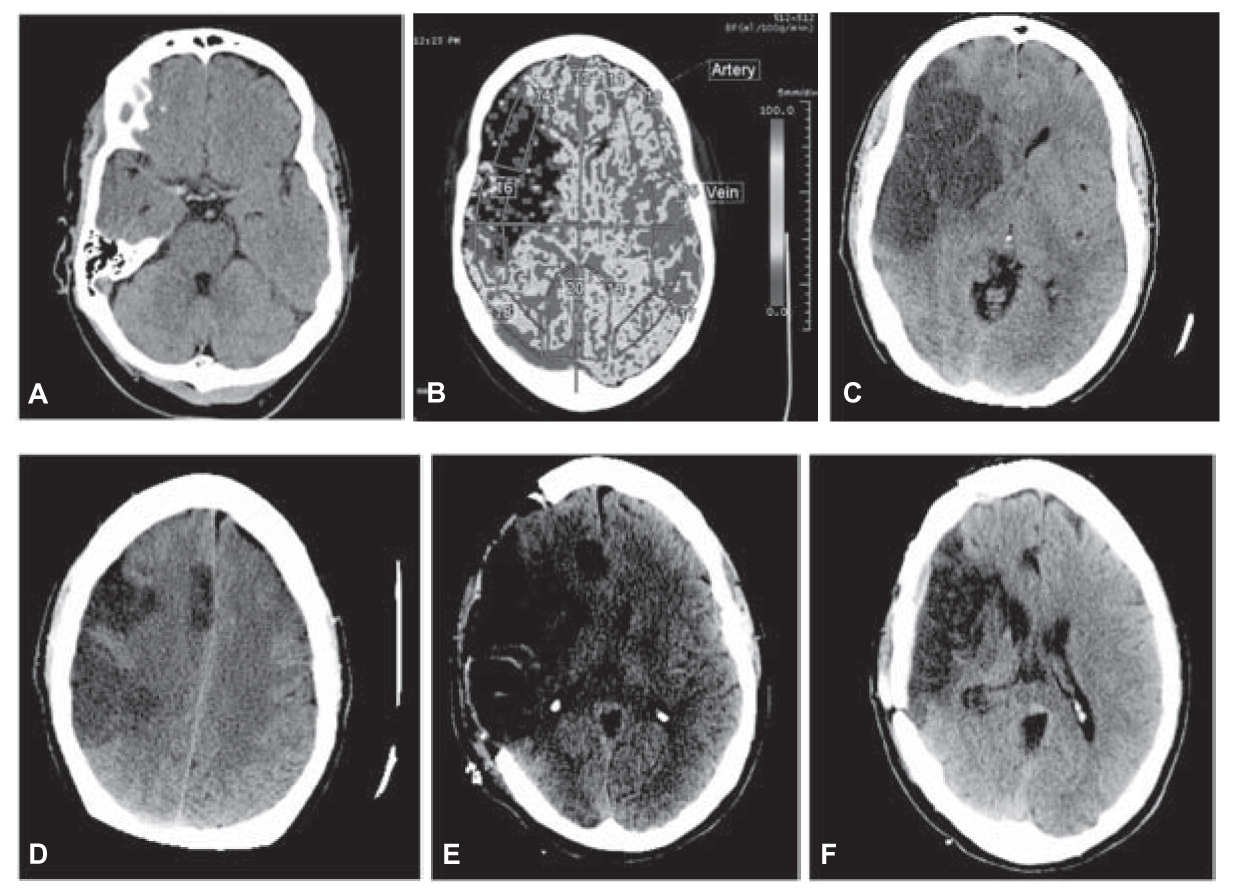
FIGURE 40.7 A: Noncontrast CT of the brain shows hyperdensity in the expected location of the right MCA suggestive of thrombus. There was also loss of the normal gray–white differentiation involving the right frontal and temporal lobes in the territory of the MCA. B: CT perfusion demonstrates decreased CBF. There was also decreased cerebral blood volume with associated increased mean transit time (not shown in this composite) in the right frontal and temporal lobes compatible with infarct. C: Large area of ischemic change involving the distribution of the right MCA. There is mass effect with midline shift to the left approximately 1.1 cm. There is no evidence of hemorrhagic transformation. D: Large area of ischemic change involving the distribution of the right MCA as well as a smaller focus involving the distal right ACA territory. E: Postoperative changes from a large right hemicraniectomy and duraplasty. A previously noted subfalcine herniation to the left has decreased from 8 to 4 mm. F: Postsurgical change of recent large right convexity cranioplasty. There is abnormal low density and focal volume loss involving the cortex and subcortical white matter of the right cerebral hemisphere, predominately in the right MCA distribution including the right basil ganglia as well as portions of the right ACA territory. These findings are consistent with encephalomalacia at the sites of chronic infarct. There is ex vacuo dilatation of the frontal horn of the right lateral ventricle. ACA, anterior cerebral artery; CBF, cerebral blood flow; CT, computed tomography; MCA, middle cerebral artery. (See color plates.)
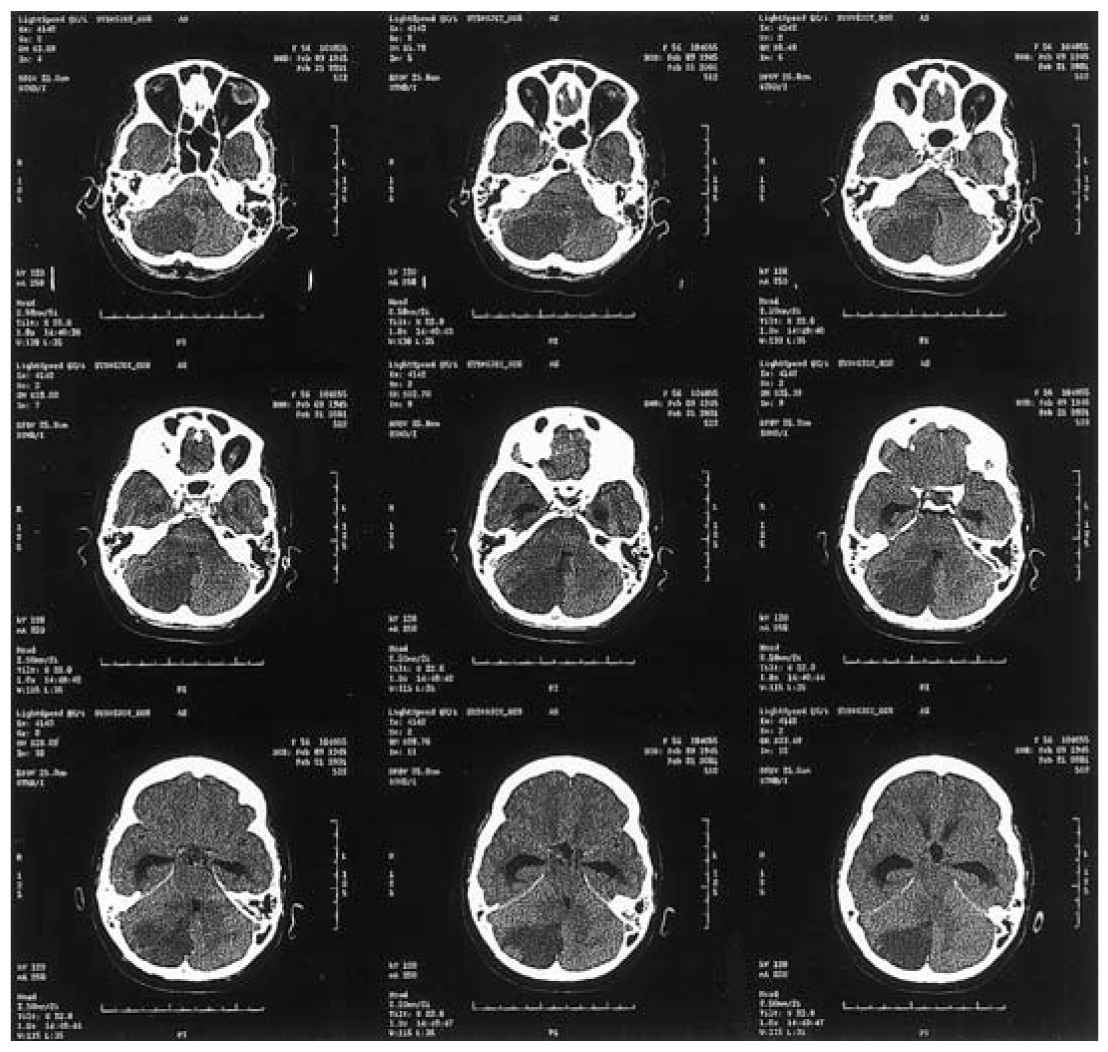
FIGURE 40.8 Axial unenhanced CT demonstrates a large area of low attenuation involving the inferior and posterior aspects of the right cerebellar hemisphere (territory of the posterior–inferior cerebellar artery) causing effacement of the brainstem cisterns and compression of the fourth ventricle, causing acute obstructive hydrocephalus. The patient required a right occipital craniectomy secondary to her large edematous cerebellar infarction. CT, computed tomography.
• Ischemic stroke can be caused by large-artery atherosclerotic disease, small-artery disease (lacunes), cardioembolism, hemodynamic (watershed) infarction, non-atherosclerotic vasculopathies, hypercoagulable disorders, or cryptogenic or infarction of undetermined causation.
• Prevention of ischemic stroke entails control of modifiable risk factors (arterial hypertension, diabetes mellitus, dyslipidemia, cigarette smoking, obesity, excessive alcohol intake), pharmacologic therapy, and surgical intervention.
• Antithrombotic agents (antiplatelet agents or anticoagulants) and thrombolytic agents are the mainstays of medical therapy for acute ischemic stroke.
• IV tPA initiated up to 4.5 hours of symptom onset is a first-line treatment for acute ischemic stroke in selected patients.
• Endovascular mechanical thrombectomy should be pursued for patients with an occlusion of the ICA terminus or proximal MCA (M1) with groin puncture achieved within 6 hours of symptom onset. Patients deemed suitable for IV thrombolysis should still receive IV tPA even if endovascular treatment will be pursued.
• Endovascular treatment within 6 hours is also reasonable for patients with occlusion of the M2 or M3 portions of the MCA, anterior, or posterior circulation.
• CEA performed by experienced surgeons improves survival in high-risk symptomatic patients. The benefit of carotid artery angioplasty and stenting over CEA remains uncertain.
• In patients with malignant cerebral edema, early (<48 hours) decompressive surgery with hemicraniectomy and durotomy may be lifesaving.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree