2
Laboratory Investigations
LUMBAR PUNCTURE
INDICATIONS
Lumbar puncture is indicated for the following purposes:
1. Diagnosis of meningitis and other infective or inflammatory disorders, subarachnoid hemorrhage, hepatic encephalopathy, meningeal malignancies, paraneoplastic disorders, or suspected abnormalities of intracranial pressure.
2. Assessment of the response to therapy in meningitis and other infective or inflammatory disorders.
3. Administration of intrathecal medications or radiologic contrast media.
4. Rarely, to reduce cerebrospinal fluid (CSF) pressure.
CONTRAINDICATIONS
1. Suspected intracranial mass lesion. In this situation, performing a lumbar puncture can hasten incipient transtentorial herniation.
2. Local infection overlying the site of puncture. Under this circumstance, cervical or cisternal puncture should be performed instead.
3. Coagulopathy. Clotting-factor deficiencies and thrombocytopenia (platelet count below 50,000/μL or rapidly falling) should be corrected before lumbar puncture is undertaken to reduce the risk of hemorrhage.
4. Suspected spinal cord mass lesion. In the case of complete spinal block, only a small quantity of CSF should be removed because fluid removal can produce a pressure differential above and below the block, which can increase the degree of spinal cord compression.
PREPARATION
A. Personnel
With a cooperative patient, lumbar puncture can generally be performed by a single person. An assistant can be helpful in positioning the patient and handling CSF samples, especially if the patient is uncooperative or frightened.
B. Equipment and Supplies
The following items, which are usually included in preassem-bled lumbar puncture trays, are required. All must be sterile.
1. Gloves.
2. Iodine-containing solution for sterilizing the skin.
3. Sponges.
4. Drapes.
5. Lidocaine (1%).
6. Syringe (5 mL).
7. Needles (22- and 25-gauge).
8. Spinal needles (preferably 22-gauge) with stylets.
9. Three-way stopcock.
10. Manometer.
11. Collection tubes.
12. Adhesive bandage.
C. Positioning
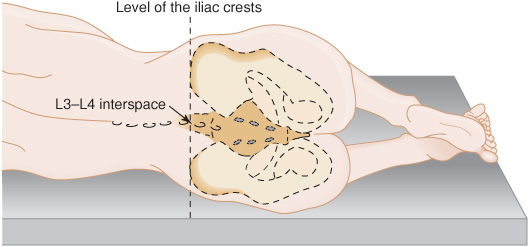
Lumbar puncture is usually performed with the patient in the lateral decubitus position (Figure 2-1), lying at the edge of the bed and facing away from the person performing the procedure. The patient’s lumbar spine should be maximally flexed to open the intervertebral spaces. The spine should be parallel to the surface of the bed, and the hips and shoulders should be aligned in the vertical plane.
Figure 2-1. Lateral decubitus position for lumbar puncture.
Occasionally it is desirable to perform lumbar puncture with the patient seated. In this case, the patient is seated on the side of the bed, bent over a pillow that rests on a bedside table, while the physician reaches over the bed from the opposite side to perform the procedure.
D. Site of Puncture
The usual practice is to enter the L3-L4 or L4-L5 vertebral interspace, because the spinal cord (conus medullaris) terminates at approximately the L1-L2 level in adults. Thus the procedure is performed without danger of puncturing the cord. The L3-L4 interspace is located at the level of the posterior iliac crests.
PROCEDURE
1. If a comparison between blood and CSF glucose levels is planned, venous blood is drawn for glucose determination. Ideally, blood and CSF glucose levels should be measured in samples obtained simultaneously after the patient has fasted for at least 4 hours.
2. The necessary equipment and supplies are placed within easy reach.
3. Sterile gloves are worn by the person performing the procedure.
4. A wide area surrounding the interspace to be entered is sterilized, using iodine-containing solution applied to sponges; the solution is then wiped off with clean sponges.
5. The area surrounding the sterile field may be draped.
6. The skin overlying the puncture site is anesthetized using lidocaine, a 5-mL syringe, and a 25-gauge needle. A 22-gauge needle is then substituted to anesthetize the underlying tissues.
7. With the stylet in place, the spinal needle is inserted at the midpoint of the chosen interspace. The needle should be parallel to the surface of the bed and angled slightly cephalad, or toward the umbilicus. The bevel of the needle should face upward, toward the face of the person performing the procedure.
8. The needle is advanced slowly until a pop, from penetration of the ligamentum flavum, is felt. The stylet is withdrawn to determine whether the CSF space has been entered, which is indicated by flow of CSF through the needle. If no CSF appears, the stylet is replaced and the needle advanced a short distance; this is continued until CSF is obtained. If at some point the needle cannot be advanced, it is likely that bone has been encountered. The needle is withdrawn partway, maintained parallel to the surface of the bed, and advanced again at a slightly different angle.
9. When CSF is obtained, the stylet is reinserted. The patient is asked to straighten his or her legs, and the stopcock and manometer are attached to the needle. The stopcock is turned to allow CSF to enter the manometer to measure the opening pressure. The pressure should fluctuate with the phases of respiration.
10. The stopcock is turned to allow the CSF to be collected, and the appearance (clarity and color) of the fluid is noted. The amount obtained and the number of tubes required varies, depending on the tests to be performed. Typically, 1 to 2 mL is collected in each of five tubes for cell count, glucose and protein determination, Venereal Disease Research Laboratory (VDRL) test for syphilis, Gram stain, and cultures. Additional specimens may be collected for other tests, such as cryptococcal antigen, other fungal and bacterial antibody studies, polymerase chain reaction for herpes simplex virus and other viruses, oligoclonal bands, glutamine, and cytologic study. If the CSF appears to contain blood, additional fluid should be obtained so that the cell count can be repeated on the specimen in the last tube collected. Cytologic studies, if desired, require at least 10 mL of CSF.
11. The stopcock and manometer are replaced to record a closing pressure.
12. The needle is withdrawn and an adhesive bandage is applied over the puncture site.
13. It has been customary to have the patient lie prone or supine for 1 or 2 hours after the procedure to reduce the risk of post–lumbar puncture headache. Current evidence suggests this is unnecessary.
COMPLICATIONS
A. Unsuccessful Tap
A variety of conditions, including marked obesity, degenerative disease of the spine, previous spinal surgery, recent lumbar puncture, and dehydration, can make it difficult to perform lumbar puncture in the conventional manner. When puncture in the lateral decubitus position is impossible, the procedure should be attempted with the patient in a sitting position. If the tap is again unsuccessful, alternative methods include lumbar puncture by an oblique approach or guided by fluoroscopy; lateral cervical puncture; or cisternal puncture. These procedures should be undertaken by a neurologist, neurosurgeon, or neuroradiologist experienced in performing them.
B. Arterial or Venous Puncture
If the needle enters a blood vessel rather than the spinal subarachnoid space, the needle should be withdrawn and a new needle should be used to attempt the tap at a different level. Patients who have coagulopathy or are receiving aspirin or anticoagulants should be observed with particular care for signs of spinal cord compression (Chapter 9) from spinal subdural or epidural hematoma.
C. Post–Lumbar Puncture Headache
A mild headache, worse in the upright position but relieved by recumbency, is not uncommon after lumbar puncture and will usually resolve spontaneously over hours to days. The frequency of this complication is directly related to the size of the spinal needle, but not to the volume of fluid removed. Vigorous hydration or keeping the patient in bed for 1 or 2 hours after the procedure does not reduce the likelihood of headache. The headache usually responds to nonsteroidal anti-inflammatory drugs or caffeine (Chapter 6). Severe and protracted headache can be treated by an autologous blood clot patch, which should be applied by experienced personnel. The use of an atraumatic spinal needle has been shown to reduce the incidence of post–lumbar puncture headache.
ANALYSIS OF RESULTS
A. Appearance
The clarity and color of the CSF should be observed as it leaves the spinal needle, and any changes in the appearance of fluid during the course of the procedure should be noted. CSF is normally clear and colorless. It may appear cloudy or turbid with white blood cell counts that exceed approximately 200/μL, but counts as low as about 50/μL can be detected by holding the tube up to direct sunlight and observing the light-scattering (Tyndall) effect of suspended cells. Color can be imparted to the CSF by hemoglobin (pink), bilirubin (yellow), or, rarely, melanin (black).
B. Pressure
With the patient in the lateral decubitus position, CSF pressure in the lumbar region does not normally exceed 180 to 200 mm water in adults. In children, the 90th percentile for opening pressure is 280 mm water. When lumbar puncture is performed with patients in the sitting position, patients should assume a lateral decubitus posture before CSF pressure is measured. Increased CSF pressure may result from obesity, agitation, or increased intra-abdominal pressure related to position; the latter factor may be eliminated by having the patient extend the legs and straighten the back once the CSF space has been entered and before the opening pressure is recorded. Pathologic conditions associated with the increased CSF pressure include intracranial mass lesions, meningoencephalitis, subarachnoid hemorrhage, and pseudotumor cerebri.
C. Microscopic Examination
This may be performed either by the person who performed the lumbar puncture or by a technician at the clinical laboratory; it always includes a cell count and differential. Gram stain for bacteria, acid-fast stain for mycobacteria, and cytologic examination for tumor cells may also be indicated. The CSF normally contains up to five mononuclear leukocytes (lymphocytes or monocytes) per microliter, no polymorphonuclear cells, and no erythrocytes. Erythrocytes may be present, however, if the lumbar puncture is traumatic (see next section). Normal CSF is sterile, so that in the absence of central nervous system (CNS) infection, no organisms should be observed with the various stains listed above.
D. Bloody CSF
If the lumbar puncture yields bloody CSF, it is crucial to distinguish between CNS hemorrhage and a traumatic tap. The fluid should be watched as it leaves the spinal needle to determine whether the blood clears, which suggests a traumatic tap. This can be established with greater accuracy by comparing cell counts in the first and last tubes of CSF obtained; a marked decrease in the number of red cells supports a traumatic cause.
The specimen should also be centrifuged promptly and the supernatant examined. With a traumatic lumbar puncture, the supernatant is colorless. In contrast, after CNS hemorrhage, enzymatic degradation of hemoglobin to bilirubin in situ renders the supernatant yellow (xanthochromic). Xanthochromia may be subtle, however. Visual inspection requires comparison with a colorless standard (a tube of water) and is best assessed by spectrophotometric quantitation of bilirubin.
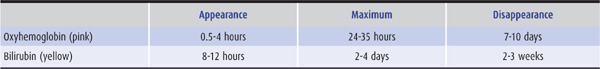
The time course of changes in CSF color after subarachnoid hemorrhage is outlined in Table 2-1. Blood in the CSF after a traumatic lumbar puncture usually clears within 24 hours; blood is usually present after subarachnoid hemorrhage for at least 6 days. In addition, blood related to traumatic puncture does not clot, whereas clotting may occur with subarachnoid hemorrhage. Crenation (shriveling) of red blood cells is of no diagnostic value. In addition to breakdown of hemoglobin from red blood cells, other causes of CSF xanthochromia include jaundice with serum bilirubin levels above 4 to 6 mg/dL, CSF protein concentrations greater than 150 mg/dL, and, rarely, the presence of carotene pigments.
Table 2-1. Pigmentation of the CSF after Subarachnoid Hemorrhage.
White blood cells seen in the CSF early after subarachnoid hemorrhage or with traumatic lumbar puncture result from leakage of circulating whole blood. If the hematocrit and peripheral white blood cell count are within normal limits, there is approximately one white blood cell for each 1,000 red blood cells. If the peripheral white cell count is elevated, a proportionate increase in this ratio should be expected. In addition, every 1,000 red blood cells present in the CSF will increase the CSF protein concentration by approximately 1 mg/dL.
PROCEDURE NOTES
Whenever a lumbar puncture is performed, a note describing the procedure should be recorded in the patient’s chart. This note should provide the following information:
2. Name of person or persons performing the procedure.
3. Indication.
4. Position of patient.
5. Anesthetic used.
6. Interspace entered.
7. Opening pressure.
8. Appearance of CSF, including changes in appearance during the course of the procedure.
9. Amount of fluid removed.
10. Closing pressure.
11. Tests ordered; for example: Tube #1 (1 mL), cell count; tube #2 (1 mL), glucose and protein levels; tube #3 (1 mL), microbiologic stains; tube #4 (1 mL), bacterial, fungal, and mycobacterial cultures.
12. Results of any studies, such as microbiologic stains, performed by the operator.
13. Complications, if any.
ELECTROPHYSIOLOGIC STUDIES
ELECTROENCEPHALOGRAPHY
The electrical activity of the brain can be recorded noninvasively from electrodes placed on the scalp. Electroencephalography (EEG) is easy to perform, is relatively inexpensive, and is helpful in several different clinical contexts.
EVALUATION OF SUSPECTED EPILEPSY
EEG is useful in evaluating patients with suspected epilepsy. The presence of electrographic seizure activity (abnormal, rhythmic electrocerebral activity of abrupt onset and termination and showing an evolving pattern) during a behavioral disturbance that could represent a seizure, but about which there is clinical uncertainty, establishes the diagnosis beyond doubt. Because seizures occur unpredictably, it is often not possible to obtain an EEG during a seizure. Despite that, the EEG findings may be abnormal interictally (at times when the patient is not experiencing clinical attacks) and are therefore still useful for diagnostic purposes. The interictal presence of epileptiform activity (abnormal paroxysmal activity containing some spike discharges) is of particular help in this regard. Such activity is occasionally encountered in patients who have never had a seizure, but its prevalence is greater in patients with epilepsy than in normal subjects. Epileptiform activity in the EEG of a patient with an episodic behavioral disturbance that could be a manifestation of seizures on clinical grounds markedly increases the likelihood that the attacks are indeed epileptic, thus providing support for the clinical diagnosis.
CLASSIFICATION OF SEIZURE DISORDERS
In known epileptic patients, the EEG findings may help in classifying the seizure disorder and thus in selecting appropriate anticonvulsant medication. For example, in patients with the typical absences of petit mal epilepsy (Chapter 12), the EEG is characterized both ictally and interictally by episodic generalized spike-and-wave activity (Figure 12-3). In contrast, in patients with episodes of impaired external awareness caused by complex partial seizures, the EEG may be normal or show focal epileptiform discharges interictally. During the seizures, there may be abnormal rhythmic activity of variable frequency with a localized or generalized distribution, or, in some instances, there may be no electrographic correlates. The presence of a focal or lateralized epileptogenic source is of particular importance if surgical treatment is under consideration.
ASSESSMENT & PROGNOSIS OF SEIZURES
The EEG findings may provide a guide to prognosis and have been used to follow the course of seizure disorders. A normal EEG implies a more favorable prognosis for seizure control, whereas an abnormal background or profuse epileptiform activity implies a poor prognosis. The EEG findings do not, however, provide a reliable guide to the subsequent development of seizures in patients with head injuries, stroke, or brain tumors. Some physicians have used the EEG findings to determine whether anticonvulsant medication can be discontinued in patients who have been free of seizures for several years. Although patients are more likely to be weaned successfully if the EEG is normal, the findings provide only a general guide, and patients can have further seizures, despite a normal EEG, after withdrawal of anticonvulsant medication. Conversely, they may have no further seizures despite a continuing EEG disturbance.
MANAGEMENT OF STATUS EPILEPTICUS
The EEG is of little help in managing tonic–clonic status epilepticus unless patients have received neuromuscular blocking agents and are in a coma induced by medication. In this case, the electrophysiologic findings are useful in indicating the level of anesthesia and determining whether the seizures are continuing. The status itself is characterized by repeated electrographic seizures or continuous epileptiform (spike-and-wave) activity. Nonconvulsive status may follow control of convulsive status. In patients with nonconvulsive status epilepticus, the EEG findings provide the only means of making the diagnosis with confidence and in distinguishing the two main types. In absence status epilepticus, continuous spike-and-wave activity is seen, whereas repetitive electrographic seizures are found in complex partial status.
DIAGNOSIS OF NEUROLOGIC DISORDERS
Certain neurologic disorders produce characteristic but nonspecific abnormalities in the EEG. Their presence is helpful in suggesting, establishing, or supporting the diagnosis. In patients presenting with an acute disturbance of cerebral function, for example, the presence of repetitive slow-wave complexes over one or both temporal lobes suggests a diagnosis of herpes simplex encephalitis. Similarly, the presence of periodic complexes in a patient with an acute dementing disorder suggests a diagnosis of Creutzfeldt-Jakob disease or subacute sclerosing panencephalitis.
EVALUATION OF ALTERED CONSCIOUSNESS
The EEG tends to become slower as consciousness is depressed, but the findings depend at least in part on the etiology of the clinical disorder. Findings such as the presence of electrographic seizure activity can suggest diagnostic possibilities (eg, nonconvulsive status epilepticus) that might otherwise be overlooked. Serial records permit the prognosis and course of the disorder to be followed. The EEG response to external stimulation is an important diagnostic and prognostic guide: Electrocerebral responsiveness implies a lighter level of coma. Electrocerebral silence in a technically adequate record implies neocortical death in the absence of hypothermia or drug overdose. In some patients who appear to be comatose, consciousness is, in fact, preserved. Although there is quadriplegia and a supra-nuclear paralysis of the facial and bulbar muscles, the EEG is usually normal and helps in indicating the diagnosis of locked-in syndrome.
EVOKED POTENTIALS
The spinal or cerebral potentials evoked by noninvasive stimulation of specific afferent pathways are an important means of monitoring the functional integrity of these pathways. They do not, however, indicate the nature of any lesion that may involve these pathways. The responses are very small compared with the background EEG activity (noise), which has no relationship to the time of stimulation. The responses to a number of stimuli are therefore recorded and averaged with a computer to eliminate the random noise.
TYPES OF EVOKED POTENTIALS
A. Visual
Monocular visual stimulation with a checkerboard pattern is used to elicit visual evoked potentials, which are recorded from the midoccipital region of the scalp. The most clinically relevant component is the P100 response, a positive peak with a latency of approximately 100 ms. The presence and latency of the response are noted. Although its amplitude can also be measured, alterations in amplitude are far less helpful in recognizing pathology.
B. Auditory
Monaural stimulation with repetitive clicks is used to elicit brainstem auditory evoked potentials, which are recorded at the vertex of the scalp. A series of potentials are evoked in the first 10 ms after the auditory stimulus; these represent the sequential activation of various structures in the subcortical auditory pathway. For clinical purposes, attention is directed at the presence, latency, and interpeak intervals of the first five positive potentials recorded at the vertex.
C. Somatosensory
Electrical stimulation of a peripheral nerve is used to elicit the somatosensory evoked potentials, which are recorded over the scalp and spine. The configuration and latency of the responses depend on the nerve that is stimulated.
INDICATIONS FOR USE
Evoked potential studies are useful in several clinical contexts.
A. Detection of Lesions in Multiple Sclerosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree