Lamotrigine
Torbjörn Tomson
Linda J. Stephen
Martin J. Brodie
Introduction
Lamotrigine, a phenyltriazine derivative, has been available globally as an antiepileptic drug (AED) for more than 15 years. The drug was initially synthesized as one of a sequence of folic acid antagonists following the suggestion that antifolate drugs may have antiepileptic properties. Although this theory was subsequently discredited, lamotrigine was discovered to have multiple mechanisms of action that may account for its broad spectrum of activity.62 Following regulatory studies during the 1980s and 1990s, lamotrigine became available as an adjunctive agent for the treatment of partial and primary and secondary generalized seizures in adults. More recently, it has been used in children, in patients with the Lennox-Gastaut syndrome (LGS), and as monotherapy in newly diagnosed epilepsy.121
Chemical structure, formulations, and methods for determination in body fluids
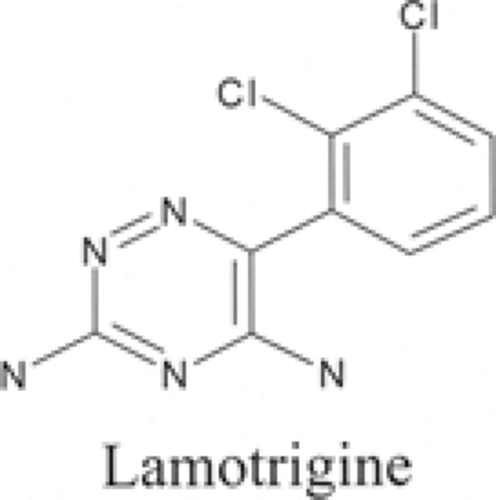
Lamotrigine (3,5-diamino-6-[2,3-dichlorophenyl]-1,2,4-tria-zine) (Fig. 1) is synthesized by reacting thionyl chloride with 2,3-dichlorobenzoic acid to make an acid chloride derivative, which is converted to the corresponding ketonitrile in the presence of cuprous cyanide.34 When the ketonitrile is condensed with aminoguanidine under strongly acidic conditions, the resulting amidinohydrazone compound is cyclized in basic conditions to produce lamotrigine. The drug is poorly soluble in water and ethanol, and has a molecular weight of 256.09 and a pKa of 5.5.
Lamotrigine is available as Lamictal (GlaxoSmithKline) in 25-, 50-, 100-, and 200-mg tablets, and as dispersible chewable tablets in 2-, 5-, 25-, and 100-mg doses.66 No difference has been shown in bioequivalency between the two formulations. A number of generic preparations are available. The drug can be measured in serum or plasma by high-performance liquid chromatography27,39 and by immunofluorometric assay.102
Pharmacology
Seizure Models
The anticonvulsant activity of lamotrigine has been demonstrated in models of different seizure types. Positive results have been obtained with maximal electroshock (MES) and pentylenetetrazole-induced tonic seizures, which are thought to be models of partial and generalized tonic–clonic seizures.82,120 The drug abolished hind limb extension in the MES model at an oral mean effective dose (ED50) of 2.6 mg/kg in the mouse and 1.9 mg/kg in the rat.81 The ED50 was smaller and the therapeutic index and duration of action greater than those of phenyt-oin, carbamazepine, sodium valproate and diazepam. Similar ED50 values were obtained in maximal seizure tests with pic-rotoxin and bicuculline but, like phenytoin and in contrast to ethosuximide and valproate, lamotrigine had no effect on leptazol threshold64 or on clonus latency after leptazol,81 suggesting lack of efficacy against absence seizures. The drug was ineffective in the genetic absence epilepsy rat from Strasbourg, although it did not aggravate spike-and-wave discharges, unlike some other antiepileptic drugs.33 Lamotrigine yielded positive results in the lethargic mouse model of absence epilepsy.57 As with valproate and ethosuximide, lamotrigine inhibited visually evoked afterdischarges in the rat, which may also be interpreted to suggest efficacy against absence seizures.65 Lamo-trigine delayed the development of electrical kindling and modified seizures, but failed to prevent subclinical afterdischarges during the kindling process in the rat.89
Mechanisms of Action
Lamotrigine acts by use- and voltage-dependent blockade of neuronal voltage-activated sodium (Na+) channels124 in a similar way to carbamazepine and phenytoin.67
Waldmeier et al. found that lamotrigine attenuated veratrine-induced glutamate, γ-aminobutyric acid (GABA), and dopamine release in rat brain slices, but much less potently inhibited electrical stimulation–induced GABA and dopamine release.118 The drug also inhibited electrical stimulation–induced release of serotonin, acetylcholine and, to a lesser extent, noradrenaline. Leach et al. reported that lamotrigine inhibited veratrine-induced glutamate and aspartate release, but less potently inhibited GABA and acetylcholine release.68
Lamotrigine also has effects on other ion channels.117,119 In rodents, the drug modulated transient potassium outward currents in CA1 pyramidal cells,52 and inhibited cortical and striatal voltage-activated calcium currents.111 Some studies have suggested that lamotrigine may have selective neuroprotective effects. Hippocampal neuronal loss and optic nerve axonopathy were reduced by the drug in rats.31 Lamotrigine was also neuroprotective in gerbil107 and pig29 models of global ischaemia.
Clinical pharmacokinetics
Lamotrigine is rapidly absorbed after oral administration. The peak plasma concentration is achieved in 1 to 3 hours, and increases linearly with dosage (Table 1).28 Bioavailability is almost 100%. The weight-normalized volume of distribution after intravenous administration and the weight-normalized apparent volume of distribution after oral administration vary between 0.9 to 1.5 L/kg.28 Protein binding is approximately 55%.43 Salivary concentrations appear to be similar to free plasma concentrations.114
Table 1 Pharmacokinetic properties of lamotrigine | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Lamotrigine undergoes linear pharmacokinetics, with an elimination half-life varying between 24 and 35 hours.28
The drug is metabolized primarily by hepatic glucuronidation catalyzed by a number of different isoforms of UDP-glucuronosyltransferase converting lamotrigine to N-2- and N-5-glucuronides (80% and 10%, respectively) and other minor N-oxide metabolites.28 The metabolites are almost entirely excreted in the urine, with 94% of radiolabeled lamotrigine being recovered in this way and 2% being found in the faeces.35 There is conflicting evidence as to whether the drug induces its own metabolism.58 As would be expected, the drug passes freely into breast milk.90
The drug is metabolized primarily by hepatic glucuronidation catalyzed by a number of different isoforms of UDP-glucuronosyltransferase converting lamotrigine to N-2- and N-5-glucuronides (80% and 10%, respectively) and other minor N-oxide metabolites.28 The metabolites are almost entirely excreted in the urine, with 94% of radiolabeled lamotrigine being recovered in this way and 2% being found in the faeces.35 There is conflicting evidence as to whether the drug induces its own metabolism.58 As would be expected, the drug passes freely into breast milk.90
Lamotrigine’s clearance is reduced in patients with unconjugated hyperbilirubinemia (Gilbert syndrome96) and in severe hepatic cirrhosis,74 but not in end-stage renal failure.42 There is little difference in lamotrigine pharmacokinetics in the elderly compared with younger adults.58 In children, oral clearance and volume of distribution are greater, but half life is similar to corresponding values in adults.25 Limited data available for different races suggests that clearance may be lower in Asians.34
Concentration–Effect Relationships
A variety of reports have been published regarding the relationship between lamotrigine dose and circulating plasma concentration. Of six large scale clinical trials, only two demonstrated a significant relationship between plasma concentration and efficacy.70,104 Loiseau et al. found lamotrigine concentrations ranged from 0.3 to 4.9 mg/L on 150 to 300 mg/day.70 Schapel et al. documented concentrations ranging from 0.66 to 4.65 mg/L in patients taking 150 or 300 mg daily.104 Of the four studies that found no clinically statistical relationship,60,80,99,110 Jawad et al. reported that 75 to 400 mg lamotrigine produced trough plasma concentrations of between 1.5 and 2.5 mg/L.60 Using 200 to 400 mg lamotrigine daily, Smith et al. achieved mean plasma levels of 2.2 to 2.4 mg/L.110 Messenheimer et al. achieved mean steady-state concentrations of 1.6 mg/L on 200 mg/day and 2.9 mg/L on 400 mg/day.80 Concentrations of 3.8 mg/L on 200 mg and 2.06 mg/L on 100 mg were reported by Reunanen et al. when la-motrigine was used as monotherapy.99
Kilpatrick et al.63 found widely varying lamotrigine doses in patients who were seizure-free (median dose 200 mg, range 25–850 mg; median concentration 3.8 mg/L, range 1.4–8.7 mg/L) and in those reporting side effects (median dose 300 mg, range 100–900 mg; median concentration 4.0 mg/L, range 0.4–18.5 mg/L). These results are partly at odds with those from Hirsch et al.,55 who reported a significant relationship between increasing lamotrigine concentrations and toxicity. With concentrations of less than 0.5 mg/L, 7% of patients reported side-effects; with 5 to 10 mg/L, 14%; with 15 to 20 mg/L, 34%; and with greater than 20 mg/L, 59%. Increasing efficacy occurred with concentrations up to and exceeding 20 mg/L. A target range of 1.5 to 10 mg/L was recommended. Another study retrospectively examined the relationship between plasma lamo-trigine concentrations and dosage and concluded that a therapeutic range of 3 to 14 mg/L was realistic, although hardly precise.85
Chong and Dupuis argued that, although the majority of studies did not demonstrate a clear relationship between lamotrigine concentration and pharmacologic response, many of these were methodologically flawed.26 They concluded that clinical end-points rather than plasma concentrations remain the most important guide for lamotrigine therapy. Certain special populations exist, however, in which obtaining a circulating concentration may be useful. These include patients in whom poor drug adherence is suspected and those with renal or hepatic failure.55 Because lamotrigine concentrations are decreased markedly during pregnancy,93,94 and dose adjustments are frequently needed under these circumstances to control seizures,115 therapeutic drug monitoring has benefit in pregnant women with poor seizure control or symptoms of drug toxicity. The ethinylestradiol component of oral contraceptives can reduce circulating lamotrigine concentration by up to 50%.98,101 Women taking the combined oral contraceptive pill could, therefore, also be candidates for lamotrigine measurement.
Clinical efficacy
The clinical effects of lamotrigine have been investigated in a wide range of seizure and epilepsy types in children and adults, in previously untreated patients, as well as in those with refractory epilepsy.
Partial Seizures and Localization-Related Syndromes
A series of randomized, double-blind, placebo-controlled studies have assessed the effectiveness of lamotrigine as add-on therapy in patients with refractory partial epilepsy. Three studies in adults77,80,104 and one in children36 met the Class I criteria in the American Academy of Neurology assessment of new AEDs.46 In the largest, 191 patients taking enzyme-inducing AEDs were randomized to placebo, or lamotrigine 300 mg/day or 500 mg/day.75 The responder rate (that proportion of patients with at least 50% seizure reduction from baseline) was 18% on placebo, 20% on lamotrigine 300 mg/day, and 34% on lamotrigine 500 mg/day. In a second study, 88 adult patients taking enzyme-inducing drugs were randomized in a cross-over design to placebo or lamotrigine 400 mg/day.80 The
responder rate with lamotrigine was 20% compared with 0% with placebo. The third study included only 41 patients on enzyme-inducers and or valproic acid randomized in a cross-over design to placebo or lamotrigine 300 mg/day (150 mg/day if on valproic acid).104 The responder rate on lamotrigine was 22%, and 0% on placebo.
responder rate with lamotrigine was 20% compared with 0% with placebo. The third study included only 41 patients on enzyme-inducers and or valproic acid randomized in a cross-over design to placebo or lamotrigine 300 mg/day (150 mg/day if on valproic acid).104 The responder rate on lamotrigine was 22%, and 0% on placebo.
In a meta-analysis, Ramaratnam et al.97 included eight additional randomized trials—two cross-over and six parallel studies.10,14,60,70,99,103,106,110 A total of 1,243 patients (1,044 adults and 199 children) were included in this analysis of la-motrigine add-on for drug-resistant partial epilepsy. The overall odds ratio versus placebo for 50% or greater reduction in seizure frequency was 2.71 (95% confidence interval [CI] 1.87–3.91), demonstrating the efficacy of lamotrigine in this patient population. The odds ratio for treatment withdrawal in the same meta-analysis was 1.12 (95%; CI,0.78–1.61), indicating that patients were not more likely to discontinue lamotrigine than placebo.97 One of these studies evaluated lamotrigine as add-on treatment in children with refractory partial epilepsy.36 In this double-blind parallel group study, 199 children aged 2 to 16 years were randomized to placebo or lamotrigine (2.7–12.9 mg/kg/day). The responder rate was 45% with lamotri-gine and 25% with placebo, whereas the discontinuation rates were similar in the two groups, 5% and 6%, respectively.
One randomized double-blind study assessed lamotrigine as monotherapy in adults and adolescents with refractory partial epilepsy.50 Overall, 156 patients with different baseline medications were randomized to receive lamotrigine 500 mg/day or a low dose of valproic acid (1,000 mg/day). Baseline medication was gradually withdrawn, aiming at monotherapy with lamo-trigine or valproic acid. More patients on lamotrigine (56%) compared to valproic acid (20%) were successfully maintained on monotherapy. Although this regulatory trial demonstrated the effect of lamotrigine as monotherapy in refractory partial epilepsy, it is difficult to translate the results into clinical practice.
Lamotrigine has also been assessed as monotherapy in new-onset partial epilepsy in adults. Three randomized double-blind parallel group studies met American Academy of Neurology Class I criteria.45 The first study18 randomized adult patients with partial seizures (n = 146) or primary generalized tonic–clonic seizures (n = 122) to lamotrigine (initial target dose 150 mg/day) or carbamazepine (initial target dose 600 mg/day). Of patients with partial seizures, 22% on lamotrigine and 31% on carbamazepine remained seizure-free during the last 40 weeks of treatment, compared to 35% and 37%, respectively, during the last 24 weeks. Retention was presented for partial and generalized seizure patients together. More lamotrigine (65%) than carbamazepine (51%) recipients completed the study. This difference was due to fewer withdrawals because of adverse events on lamotrigine.
Steiner et al.112 randomized 90 patients with partial and 91 with primary generalized tonic–clonic seizures to lamotri-gine (modal dose 150 mg/day) or phenytoin (modal dose 300 mg/day). Of those with partial seizures only randomized to lamotrigine, 16% remained on treatment and were seizure-free during the last 40 weeks of the study, compared with 22% on carbamazepine. Corresponding figures for the last 24 weeks were 41% and 48%, respectively. These differences were not statistically significant, nor was there a significant difference between the drugs in time to discontinuation from the trial.
In a third study, 150 elderly (65 years and older) patients with newly diagnosed epilepsy were randomized in a 2:1 ratio to lamotrigine (median dose 100 mg/day) or carbamazepine (median dose 400 mg/day).17 Although a proportion was classified as having idiopathic generalized epilepsy, the vast majority of patients had partial seizures. Significantly more patients continued on treatment with lamotrigine than on carbamazepine, 71% versus 42%. This was largely explained by a higher dropout rate due to adverse events on carbamazepine (42%) compared with lamotrigine (18%).
Lamotrigine has been assessed in an additional comparative study of new-onset partial epilepsy in older people.100 In this double-blind, parallel group study, 593 patients aged 60 years or older were randomized to gabapentin (target dose 1,500 mg/day), lamotrigine (target dose 150 mg/day), or carbamazepine (standard tablets, target dose 600 mg/day). Although epilepsy was of new onset, 43% of the patients were already taking AEDs, which were tapered to zero during titration of the study medication. The primary outcome measure was retention in the trial for 12 months. Early termination, largely due to adverse events, was significantly more common with carbamazepine (65%) than with lamotrigine (44%) or gabapentin (51%). Seizure-free rates at 12 months were similar across treatment groups.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree