Localization of Lesions in the Autonomic Nervous System
Organization of the Autonomic Nervous System
This section considers only the general anatomic principles of the autonomic nervous system (ANS) that should assist the examiner in determining whether significant autonomic dysfunction is present. For more detailed information, readers are referred to corresponding chapters on this textbook, and standard neuroanatomy textbooks [33,52,84,100,101,117].
The ANS is commonly divided into two major components: (a) sympathetic and (b) parasympathetic, each usually made of preganglionic and postganglionic neurons (Fig. 21.1). These two major components provide a dual autonomic innervation to every organ in the body, which have in general opposite effects. Most authors now consider the enteric nervous system (ENS), also referred to as the gut brain, to be a third component of the ANS.
The sympathetic and parasympathetic components have two neuronal populations interposed between the central nervous system and the effector organs. The ANS is bidirectionally connected with a central autonomic network (CAN), which includes the medial prefrontal cortex, insular cortex, central nucleus of the amygdala, hypothalamus, periaqueductal gray region, parabrachial/Kölliker-Fuse nuclear complex, nucleus ambiguous, and nucleus tractus solitarius. This extensively interconnected network controls sympathetic and parasympathetic, neuroendocrine, and respiratory motor neurons [14,77,104].
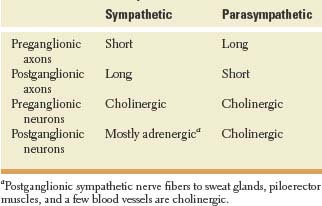
The ANS consists of both afferent and efferent fibers. Basic morphologic and physiologic differences between the sympathetic and parasympathetic systems are shown on Table 21.1. Sensory afferent signals that drive autonomic activity originate from viscera (visceral sensory), body surface (somatic sensory), and external environment (special sensory). The perikarya of visceral afferent axons are located in dorsal root ganglia.
A two-neuron pathway conveys motor impulses to the autonomic effectors. The perikaryon of the first visceral efferent neuron resides inside the central nervous system (brainstem or spinal cord); its axon (preganglionic) synapses on a ganglion or plexus of neurons. The perikaryon of the second visceral efferent neuron resides outside the central nervous system in a ganglion or plexus of neurons; its axon (postganglionic) synapses on an autonomic effector.
Sympathetic Nervous System
The majority of the sympathetic preganglionic neurons (or primary efferent neurons) are located in the intermediolateral gray cell column (lamina VII or intermediate zone) of spinal cord levels T1 to L2 (or L3). These neurons are organized somatotopically; rostral neurons supply the head and neck, while those in more caudal segments supply the heart, lungs, abdominal viscera, and pelvic viscera in that order. Their axons project onto a postganglionic neuron (or secondary efferent neuron) with its cell body in one of the 22 to 23 pairs of paravertebral sympathetic ganglia (3 cervical, 10–12 thoracic, 4 lumbar, 4–5 sacral) located on either side of the vertebral column or in related prevertebral sympathetic ganglia (celiac, superior mesenteric, and inferior mesenteric). Preganglionic nerve fibers reach the ganglionic neurons via the ventral spinal roots and the myelinated white rami communicantes (14 pairs). Once preganglionic sympathetic fibers reach the chain ganglion, they may synapse with the first paravertebral ganglion they encounter at the level of entry, they may ascend or descend to synapse on neighboring ganglia forming a sympathetic chain, or they may bypass the paravertebral ganglia, joining with other preganglionic fibers to terminate in one of the prevertebral ganglia.
The paravertebral ganglia in the neck fuse to form three ganglia: superior cervical ganglion, middle cervical ganglion, and inferior cervical ganglion. Sometimes, the inferior cervical ganglion (or lower two cervical ganglia) fuses with the first thoracic (sometimes the second thoracic ganglia) sympathetic chain ganglion to form the cervicothoracic or stellate ganglion. At the coccygeal region, the caudal-most ganglia of the right and left paravertebral chains fuse to form the unpaired ganglion impar.
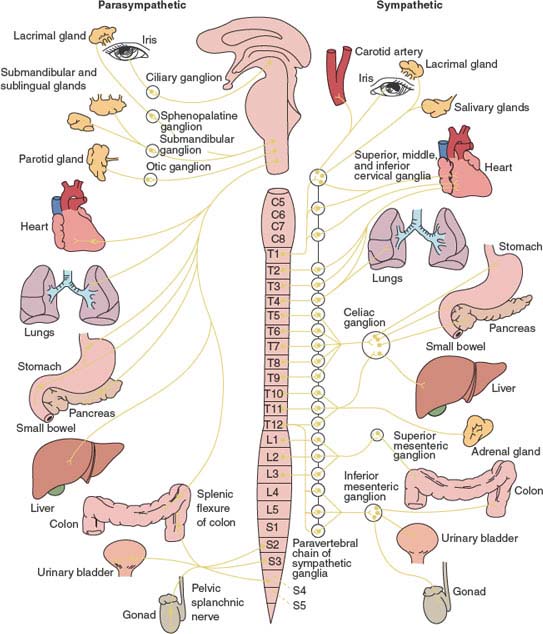
FIG. 21.1. Diagram of the autonomic nervous system (ANS). The parasympathetic division (left) arises from CN III, CN VII, CN IX, and CN X and from spinal cord segments S2 to S4. The sympathetic division (right) arises from spinal cored segments T1 to L2.
From a paravertebral ganglion, postganglionic fibers are distributed as branches to the spinal nerves through the gray rami communicantes (31 pairs), or cranial nerves (IX, X, XII), or they travel along the wall of a blood vessel, or proceed directly to their destination in the various organs including the heart, blood vessels, broncho-pulmonary system, sweat glands, smooth muscles within the gut and bladder, and sexual organs.
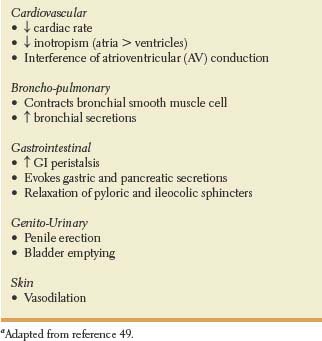
Some fibers pass through the splanchnic ganglia without synapsing, providing innervation to the adrenal medulla. Thus, the adrenal medulla is the only gland innervated by preganglionic (cholinergic) sympathetic nerve fibers, ending directly on modified neuronal cells that secrete adrenaline and noradrenaline into the blood stream. Table 21.2 illustrates the main effects of sympathetic stimulation [49].
TABLE 21.1 Basic Characteristics of Sympathetic and Parasympathetic Systes
Parasympathetic Nervous System
The parasympathetic autonomic nervous system has a cranial (brainstem) division and a sacral division. Parasympathetic preganglionic neurons of the cranial division are located within the nuclei of CN III (Eddinger-Westphal nucleus situated in the rostral midbrain at the level of the superior colliculus), CN VII (superior salivatory and lacrimal nuclei in the pontine tegmentum a), CN IX (inferior salivatory nucleus in the periventricular gray matter of the rostral medulla), and CN X (nucleus ambiguous in the reticular formation of the medulla, and dorsal motor nucleus of the vagus situated on the floor of the fourth ventricle where it forms the vagal trigone). The largest group of parasympathetic nerve fibers in the body is in the vagus nerves (CN X). Myelinated fibers of the cranial outflow synapse in peripheral ganglia located near or within the innervated organs: ciliary ganglion [CN III], submandibular and pterygopalatine ganglia [CN VII], otic ganglion [CN IX], and cardiac ganglion and plexuses [CN X]. Parasympathetic nerve fibers of CN III flow to the pupillary sphincters and ciliary muscles of the eyes. Parasympathetic fibers of CN VII flow to the lacrimal, nasal, and submandibular glands. Fibers from CN IX pass to the parotid gland. The vagus (CN X) supplies parasympathetic innervation to the heart, lungs, esophagus, stomach, small intestine, proximal half of the colon up to the splenic flexure, liver, gallbladder, pancreas and upper ureters [52,84,101].
TABLE 21.2 Main Effects of Diffuse Sympathetic Stimulationaa

The sacral parasympathetic division resides in the intermediolateral cell columns of the second to fourth segments of the sacral (S2–S4) spinal cord. Myelinated axons exit through the ventral roots forming the sacral plexus, and then enter the pelvic splanchnic nerves also called nervi erigentes. Myelinated efferent fibers of the sacral outflow synapse in the intramural ganglion and hypogastric (pelvic) plexuses. The sacral parasympathetic outflow distributes its fibers to the descending colon, rectum, lower portion of the ureters, bladder, and external genitalia [52,84,101]. The genital organs are innervated by three sets of peripheral nerves, namely the sacral parasympathetic (pelvic nerves or nervi erigentes), the thoracolumbar sympathetic (hypogastric and lumbar sympathetic chain), and the sacral somatic nerves (pudendal nerves). The pelvic nerves contain the axons of sacral parasympathetic preganglionic neurons located in the intermediolateral cell column of S2–S4. The hypogastric nerves contain the axons of the sympathetic preganglionic neurons located in the intermediolateral cell column of T11–L2 spinal segments. The pudendal nerves contain the axons of somatic motor neurons located in the ventral horn of S2–S4. Parasympathetic fibers play a major role in penile erection and micturition, while parasympathetic efferents in the pelvic nerves promote bladder emptying (see Chapter 5). The main effects of vagal (cholinergic) stimulation are show in Table 21.3.
Enteric Nervous System
The ENS, derived primarily from the vagal neural crest cells, consists of an extrinsic and an intrinsic component. The intrinsic component (500 million neurons) consists of two interconnected plexuses, the Meissner’s submucosal plexus, and the Auerbach’s myenteric plexus, within the gastrointestinal wall. In humans, the submucosal plexus of Meissner consists of three interconnected layers. The outer layer is also known as the outer submucosal plexus of Schadabasch’s or Henle’s plexus[15]. The extrinsic innervation of the ENS depends on preganglionic parasympathetic and sympathetic outputs that regulate peristalsis and secretion. The parasympathetic output arises in the dorsal motor nucleus of the vagus and in the sacral parasympathetic nucleus of the spinal cord. The sympathetic output originates in the prevertebral ganglia. The primary targets of the ENS are mucosal secretory cells, gastrointestinal neuroendocrine cells, gastrointestinal microvasculature, and immunomodulatory and inflammatory cells in the gut. The ENS influences these effector cells in the gut directly or indirectly through its actions on intermediate cells including neuroendocrine cells, cells of the immune system, and the interstitial cells of Cajal. The interstitial cells of Cajal are a network of small anastomosing cells located within the gut muscle layers, and an integral part of normal gastrointestinal motility and modulation of enteric neural activity [4,17,21,22,39,42,56,68,70,71,80,88]. Disorders of the enteric nervous system may produce motor, secretor, inflammatory, or immune dysfunction.
TABLE 21.3 Main Effects of Cholinergic Stimulationa a
CENTRAL AUTONOMIC NETWORK
Tightly interconnected neuroanatomical structures contribute to regulation of the autonomic circuits.
Medial Prefrontal Cortex. The medial prefrontal cortex is highly activated by stress and plays a key role in autonomic and affective responses. The medial prefrontal cortex is implicated in complex cognitive and emotional states and modulation of neuroendocrine and autonomic function [7,54,102,103]. In addition, the anterior cingulate cortex is implicated in modulating sympathetic nervous system tone [30,75].
Insular Cortex. The insular cortex plays an important role in the CAN, and is the most important cortical area involved in cardiovascular regulation. Insular cortex neurons have connections with the limbic system and lateral frontal cortical system, and project to the central nucleus of the amygdala and autonomic nuclei of the medulla oblongata [95]. The insula also has wide connections with temporal and parietal cortices, basal ganglia, thalamus, and olfactory cortex. Moreover, the anterior insula has connections to primary and supplementary motor cortices, ventroposterior medial nucleus of the thalamus, and nucleus tractus solitarius, all of them relevant to oropharyngeal swallowing [31]. A possible lateralization in autonomic activity in the insular cortex has been proposed. Some evidence exists for cortical lateralization in the regulation of cardiovascular functions. Patients with right insular cortex lesions seem to be more susceptible to develop cardio-autonomic dysfunction, while the left insular cortex is predominantly responsible for parasympathetic cardiovascular effects [18,29,59,77,83,104–105]. Strokes restricted to the insular cortex have been associated with arterial hypertension, cardiac arrhythmias, increased risk of myocardial injury, raised catecholamines levels, and an increased susceptibility to sudden death [6,11,20,27,44,106].
Central Nucleus of the Amygdala. The amygdala has a key role in regulating arousal and vigilance and responds to the acquisition and expression of conditional fear. It is particularly activated by stimuli such as a fearful face [24,51,115,116]. Moreover, the central nucleus of the amygdala is involved in cardiovascular regulation [94].
Hypothalamus. Anatomically, the hypothalamus is the highest level of integration of autonomic function. The hypothalamus controls the autonomic nervous system by means of the pituitary gland and by direct descending pathways. Connections to the pituitary enable the hypothalamus to influence activities of the endocrine glands. Descending sympathetic fibers from the hypothalamus are uncrossed, and by way of the lateral tegmentum of the brainstem and lateral medullary formation influence the craniosacral parasympathetic and thoracolumbar sympathetic outflows. In particular, the paraventricular nucleus of the hypothalamus has emerged as one of the most important control centers in the brain involved in cardiovascular regulation. This nucleus receives afferents from the insula, prefrontal cortex, amygdala, and other hypothalamic nuclei [100]. The lateral hypothalamus, in addition to the insula and the amygdala also play a key role in the autonomic control of the heart.
Periaqueductal Gray Region. The periaqueductal gray is divided into four regions, namely the dorsomedial, dorsolateral, lateral, and ventrolateral subdivisions. Chemical stimulation of the dorsolateral gray produces hypertension and tachycardia, while activation of the ventrolateral gray accounts for the opposite effect. Different sets of neurons located in different regions of the periaqueductal gray have been implicated in coordinated patterns of somatic and autonomic changes characteristics of defensive responses [12,19,40,41,110]. A major function of the periaqueductal gray is pain processing and modulation, particularly in descending pain inhibition [12,19,40,41,110].
Parabrachial Nuclear Complex. The parabrachial/Kölliker-Fuse nuclear complex located in the dorsolateral pontine tegmentum is divided into three well-defined regions: the medial parabrachial nucleus (corresponding to the classic “pneumotaxic center”), the lateral parabrachial nucleus, and the Kölliker-Fuse nucleus. This nuclear complex plays a key role in the sensory processing of gustatory and visceral information, pain modulation, and automatic control of respiration [13,46,48,67,118]. Moreover, vestibular input projecting to the nucleus tractus solitarius, dorsal motor nucleus of the vagus, and the parabrachial/Kölliker-Fuse nuclear complex, may account for the autonomic effects observed in cases of acute vestibular dysfunction [9,10].
Nucleus Ambiguous. The nucleus ambiguous, located in the ventrolateral part of the medullary tegmentum, extends rostrally to the dorsal aspect of the facial nucleus, and caudally to the level of the pyramidal decussation, and with the nucleus tractus solitarii, and the dorsal motor nucleus of the vagus participate in the control of automatic respiration [46]. Involvement of the nucleus ambiguous in the rostral part of the medulla oblongata is more likely to cause dysphagia than lesions involving the nucleus ambiguous in the middle-lower part of the medulla oblongata [58,61].
Nucleus Tractus Solitarius. The nucleus tractus solitarius in the dorsal medulla contains the medullary respiratory and lower brainstem hypnogenic neurons. It receives afferents from the cardiovascular and respiratory systems for autonomic control of cardiac rhythm, circulation, and respiration, and is believed to be of major importance in cardiovascular and respiratory regulation. Bilateral lesions in the nucleus tractus solitarius cause acute neurogenic hypertension [34].
Localization Principles. Autonomic dysfunction may be localized or generalized, primary or secondary, and is usually associated with underactivity (Table 21.4). However, severe paroxysmal sympathetic overactivity, including neurogenic pulmonary edema, may result from acute brain or spinal cord injuries [8,98].
A few examples of localized dysautonomias include Adie’s tonic pupil and Horner’s syndrome described in Chapter 8. Crocodile tears or Bogorad syndrome (gustatolacrimal syndrome) is a rare complication of facial paralysis with incomplete recovery and characterized by excessive lacrimation during food consumption. Gustatory sweating (Frey’s syndrome) is a potential complication of parotid surgery [36,86]. Primary hyperhidrosis, a common and troublesome condition, primarily involves the axillae as well as the palms and soles, and is characterized by an excessive and uncontrolled production of sweat by the sweat glands [92]. Reduced tear secretion is observed in many patients with acute pandysautonomias, multisystem atrophy (Shy Drager syndrome) and Riley-Day syndrome (Chapter 8). Finally, vasovagal syncope in the young, and carotid sinus hypersensitivity in the elderly represent examples of intermittent localized dysautonomias.
TABLE 21.4 Cardinal Signs of Autonomic Dysfunctionaa










