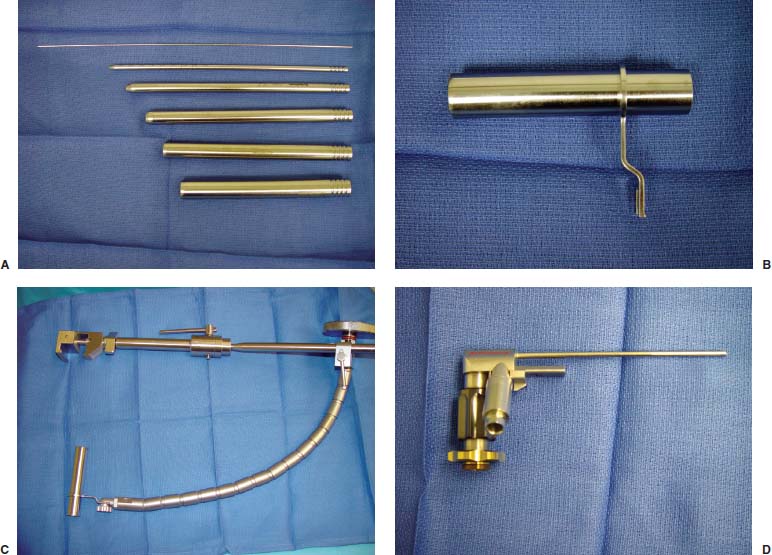
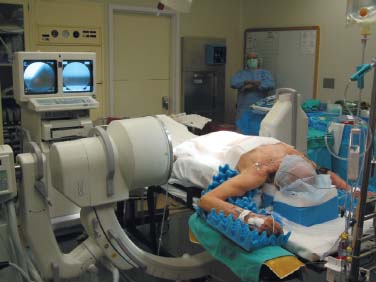
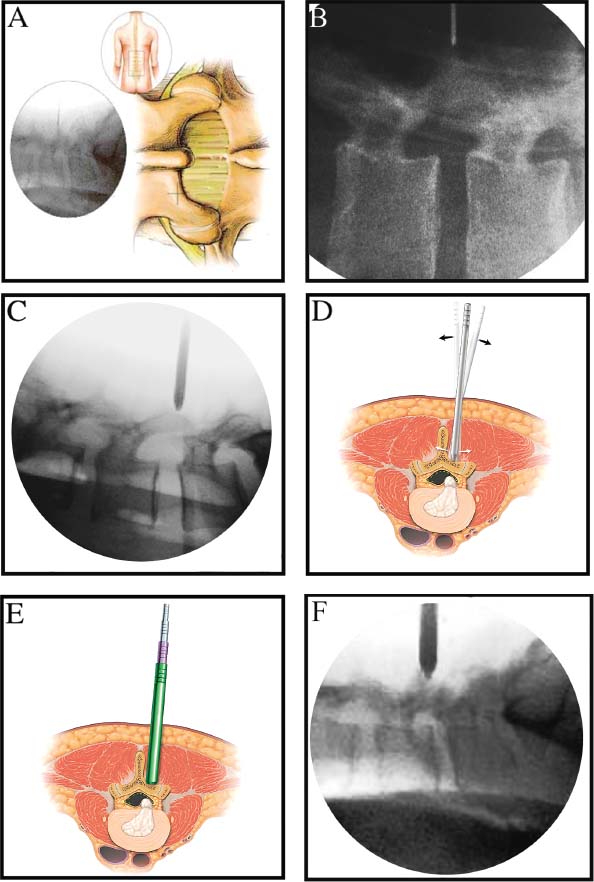
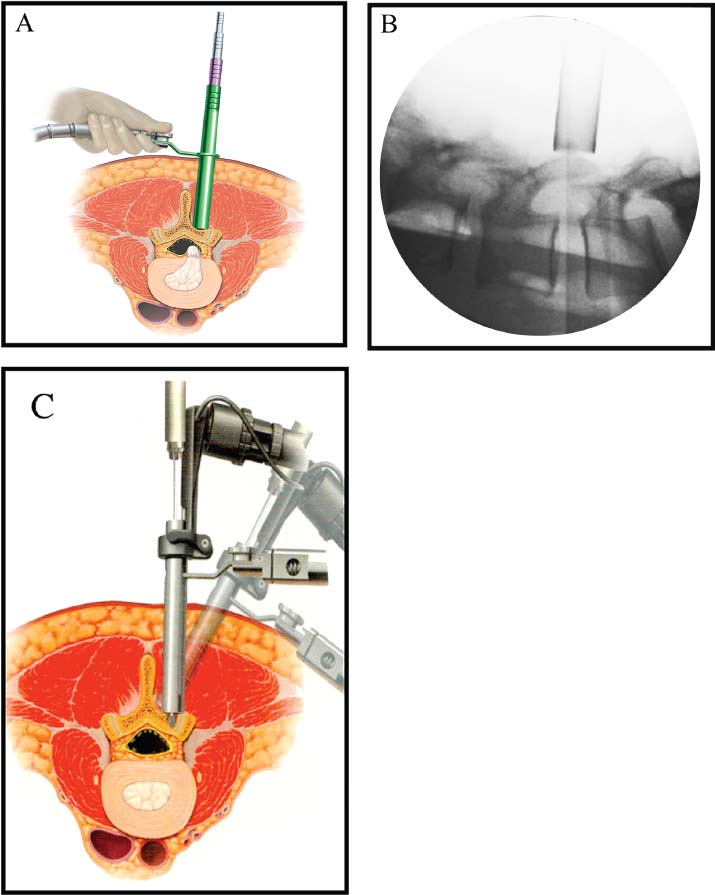
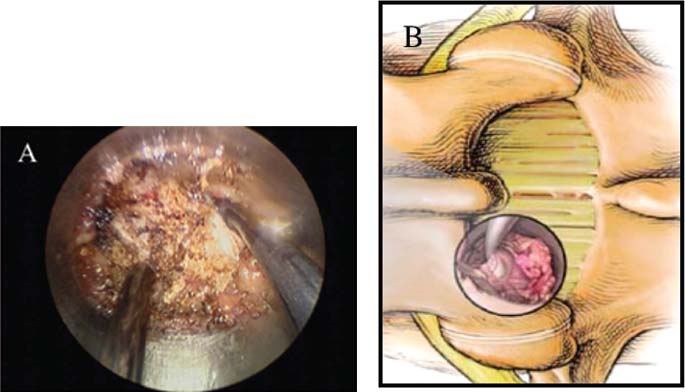
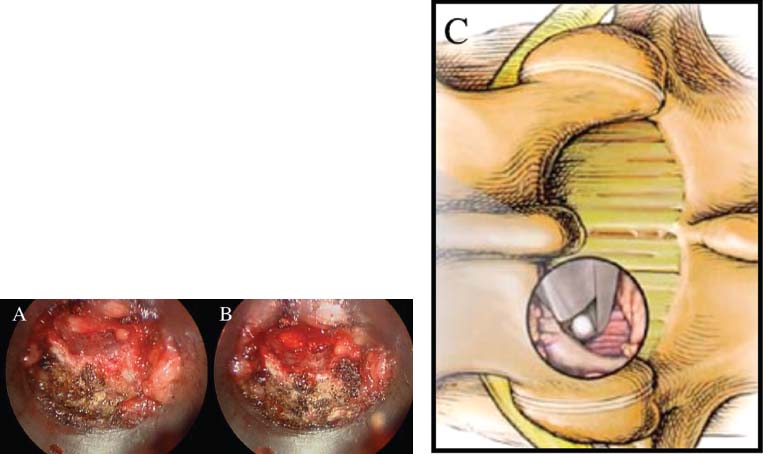
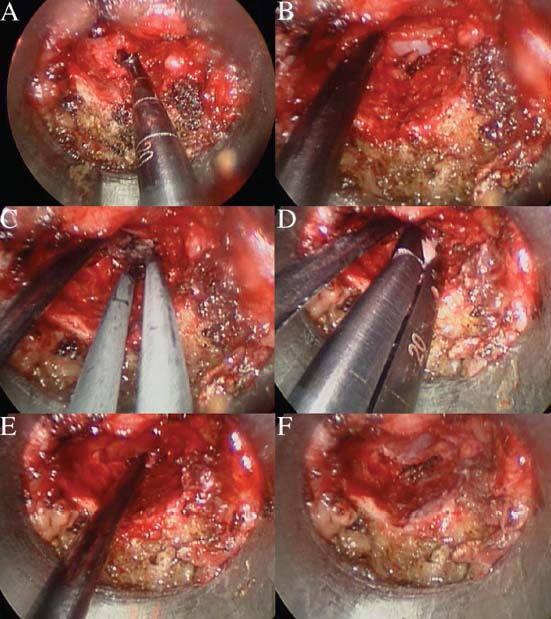
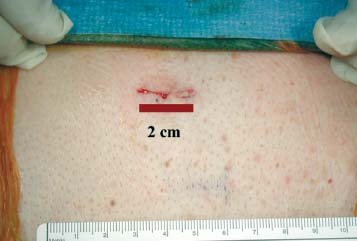
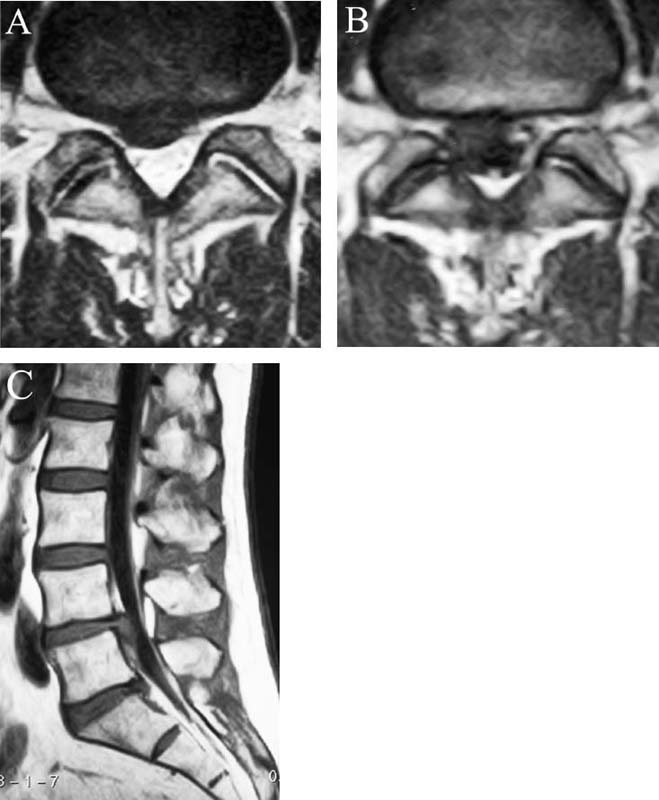
Lumbar radiculopathy is one of the most common problems encountered by the practicing spine surgeon. The source of the pathology is often entrapment of an exiting nerve root at the level of the neural foramen or disk space.1–4 In such cases, surgical intervention should be aimed at decompression of the exiting nerve root; however, iatrogenic injury to the surrounding structures is common and, in some cases, may precipitate further degenerative change. Lumbar diskectomy and foraminotomy are the most common approaches to lesions of this region.5,6 Although this approach is unilateral and limits exposure only to the affected level, the approach results in injury to the midline muscular and ligamentous structures. Furthermore, the pathology is encountered laterally as opposed to medially. We will describe in this chapter an endoscopy-based modification of the microdiskectomy/foraminotomy technique that preserves the midline structures, provides a lateral approach to the neural foramen, and results in minimal muscular injury.7–9 Indications Patients in our series complained of predominantly unilateral radicular symptoms, although in cases of large, central disks bilateral symptoms may be present. Standard preoperative imaging should start with anteroposterior (AP), lateral, flexion, and extension views of the lumbar spine. Patients with abnormal motion or greater than grade I spondylolisthesis should be considered for lumbar fusion. Further imaging should include an MRI of the lumbar spine. Patients with a history of previous surgery should also undergo MRI with and without gadolinium to distinguish recurrent disk herniation from scarring of the nerve root. In cases where the anatomy is unclear, lumbar myelography with postmyelography CT scanning remains the gold standard, albeit invasive, study. Myelography can be particularly useful in the assessment of pathology involving the neural foramen. Imaging should reveal disk herniation with impingement upon the adjacent nerve root as it traverses the neural foramen. In cases of large, rostrally displaced disk fragments, the nerve root can be compressed as it passes beneath the pedicle to exit through the neural foramen. Far lateral disk herniations may entrap the exiting nerve root within the foramen. In cases of degenerative facet disease, the adjacent nerve root may be compressed dorsally by hypertrophy of the facet complex as the nerve root crosses the medial aspect of the neural foramen to exit below the inferior pedicle. Patients with a history of multiple recurrences should be considered for lumbar fusion. Patients should have a complete medical work-up prior to surgery and meet the criteria for general anesthesia. The majority of our patients are treated as outpatients. Age is not a significant criterion; the senior author (RF) has performed the procedure successfully on patients in their 90s. Depending on their level of independence in the community and their home situation, elderly patients have been kept in the hospital overnight for observation. Obese patients in particular benefit from the use of the tubular retraction system (see later). Using standard microdiskectomy techniques, obese patients require an incision two to four times the standard length to provide adequate exposure. Using our technique, the same incision can be used for obese and nonobese patients. Surgical Equipment/Operating Room Setup A tubular retractor system is required. The only commercially available system currently is the MetRx system (Medtronic Sofamor Danek, Memphis, TN). This system provides both the dilators, retractors, and clamps required for the procedure as well as extra-long, antiglare coated instruments (Fig. 20–1A–C). Endoscopic diskectomy provides better visualization of the surgical anatomy over that provided by conventional microscopy. As such, a 30-degree angled endoscope and video tower are required (Fig. 20–1D). The coupler provided with the MetRx kit allows for the use of a variety of different endoscopes with either the 16 or 18 mm working channel. C-arm fluoroscopy is mandatory. FIGURE 20–1 Surgical equipment. (A) Dilators. (B) An 18 mm working channel. (C) Table clamp with flexible arm. (D) A 30-degree angled endoscope. The patient is transported to the operating room while awake. General endotracheal tube anesthesia is administered while the patient is supine on a hospital stretcher. Perioperative antibiosis consists of a first-generation cephalosporin or vancomycin/clindamycin in penicillinallergic patients. Intraoperative EMG-SSEP monitoring is generally not used. A Foley catheter is not inserted because operative time is ~45 to 60 minutes per level. Once the patient has been released by anesthesia for positioning, he or she is turned from the supine to the prone position onto an awaiting Wilson frame. The patient should be positioned such that the level of interest is centered on the apex of the frame. This position results in distraction of the laminae and facets at this level, aiding in exposure of the nerve root and foramen. For obese patients and in cases where a Wilson frame is unavailable, chest and pelvic rolls may also be used. Care should be taken to ensure that the patient’s neck is in a neutral position and that the eyes are free of any pressure. Pressure points should be examined and adequately padded. FIGURE 20–2 Room setup for a microendoscopic case. The patient is positioned prone on a Wilson frame. The C-arm is brought in from the contralateral side. The video tower is placed directly across from the patient. The C-arm monitor is placed at the foot of the operating table. Surgical Technique Once the patient has been properly positioned, the fluoroscope is wheeled into place. To avoid later confusion, a true lateral C-arm image must be obtained, adjusting the patient and C-arm as needed. It is also important to study a preoperative AP image of the spine to confirm the number of lumbar vertebrae because this can be quite difficult intraoperatively. Based on this review, the sacrum is used as the starting point for counting. Once the desired disk space has been identified, a long Steinmann pin can be placed along the patient’s flank, directly overlying the disk space. This should cross the more dorsally located facet complex. With this as a guide, a point 1.5 cm off the midline should then be marked on the patient’s skin ipsilateral to the desired approach. If done correctly, this point should lie superficial to the facet complex at the previously identified disk space. The skin should then be prepped and draped in the standard sterile fashion. The C-arm should be draped sterilely into the field. As opposed to the standard microdiskectomy technique, the C-arm is used throughout the procedure, and as such, the surgeons and OR staff should wear safety aprons throughout the case. The operative site is then infiltrated with local anesthetic containing epinephrine. The assistant can simultaneously secure the clamp and snake retractor to the operative table. The clamp and retractor should be placed at approximately the level of the hip, contralateral to the operative site. The endoscopy tower and C-arm base are similarly placed directly across from the surgeon. If a microscope is to be used in lieu of endoscopy, the microscope base can be brought in from the ipsilateral side to avoid the C-arm base. It is usually easiest to position the C-arm monitor at the foot of the operating table (Fig. 20–2). An 11-blade scalpel is used to make a small stab incision in the skin as previously marked. The stainless steel guide pin provided with the tubular retractors should then be passed through the stab incision and soft tissues onto the underlying bone. Fluoroscopy should be used to confirm the location of the guide pin. The safest approach is to direct the guide pin perpendicular to the entry point. This will often result in contact with the transverse process rather than the facet complex. It is preferable to be lateral to the facet complex rather than medial, where there is risk of entering the interlaminar space and injuring the contents of the spinal canal. The guide pin can then be directed medially to dock on the facet complex and centered on the disk space (Fig. 20–3A,B). A second stab wound can be made if the first proves to be inadequate. Once the appropriate trajectory has been found, the incision can be lengthened to 2 cm. The first dilator is then passed over the guide pin. Fluoroscopy is used to confirm that the dilator is docked on the facet complex. To avoid entering the spinal canal, the guide pin is removed, and the first dilator is left in place. The remaining dilators are then passed sequentially. Fluoroscopy is used to ensure maintenance of the desired trajectory. The dilators should be passed with a twisting motion to split the dorsal lumbar fascia. The underlying musculature is split along its fibers rather than torn. It is important to confirm that the dilators are resting against bone, minimizing the amount of tissue that needs to be removed later (Fig. 20–3B-F). Again under fluoroscopic guidance, the working channel is passed over the final dilator and angled slightly medially. The working space can be dilated to 16 or 18 mm for endoscopic applications and up to 22 mm for use with the microscope. We find 18 mm to be ideal for lumbar applications. FIGURE 20–3 (A) The cross marks the target for the guide pin on the facet complex. (B) Guide pin docked on the facet complex over the disk space. (C) First dilator passed over guide pin. (D) The dilators can be arced to ensure ideal placement. (E) Serial dilation of the soft tissues. (F) C-arm image prior to passage of working channel. The endoscope is then attached to the working channel (Fig. 20–4A). A radiopaque sucker is then passed into the working channel, and the location of the working channel is confirmed on fluoroscopy (Fig. 20–4B). Care should be taken to attach the endoscope as high up on the working channel as possible to avoid damage to the endoscope when using monopolar cautery. Monopolar cautery is then used to divide the residual muscular attachments to the underlying bone. It is important to ensure that cautery is used only when working on bone. It is safest to begin work laterally on the facet complex and then work medially, taking care to identify the interlaminar space. The muscle should be detached completely along the circumference of the tube prior to attempting removal of the muscle. Failure to do so can result in excessive bleeding and pulling more muscle into the field beneath the edges of the working channel. The “cut” setting on the cautery appears to be more effective than the “coagulation” setting during initial exposure. Once the muscle has been cleared, a straight current can be used to remove any residual soft tissue and to identify the interlaminar space and the inferior edge of the lamina. The working channel should be directed medially to identify the junction of the spinous process and lamina (Fig. 20–4C). It is usually necessary to use cautery to remove additional tissue. A straight curette should then be used to detach the ligamentum flavum medially from the overlying lamina (Fig. 20–5). It is safest to define this plane medially and then to work laterally. A curved curette can be passed under the lamina to detach the ligamentum flavum from the undersurface of the lamina. Fluoroscopy should be used to confirm the location under the lamina dorsal to the desired disk space. Once an adequate plane has been established, 3 and 4 mm Kerrison punches should be used to perform a laminotomy. For the purposes of a foraminotomy or diskectomy, work should be aimed laterally. An angled curette is useful to define the plane and to prevent injury to the underlying dura and nerve roots. In general, the laminotomy should extend the length of the neural foramen, from pedicle to pedicle. At the junction of the lamina and facet complex it is usually necessary to use a high-speed drill to perform a medial facetectomy (Fig. 20–6). The facetectomy should continue until an angled curette passes easily into the neural foramen. Again, fluoroscopy is used to confirm the extent of the laminotomy and foraminotomy. It can be helpful when working laterally to reposition the working channel laterally. Bony bleeding is easily addressed using bone wax. In most cases, the bony opening exposes the ligament superficial to the shoulder and lateral margin of the nerve root as it passes along the foramen. With the bony opening completed, attention is then turned toward removal of the ligamentum flavum. A Penfield number 4 is used to make an opening in the ligament along the rostral-most aspect of the laminotomy (the ligamentum is often thinnest at this point). If another opening has been made elsewhere, it can be used. A curved curette is then used to establish a plane between the dura and ligament and can be used to detach the ligament from the bone along the edges of the laminotomy (Fig. 20–7A). Right-angle rather than angled punches are useful for removal of the ligamentum. The ligament should be resected along the lateral border of the nerve root. In cases of disk herniation, the nerve root may be distorted, and it may be necessary carefully to resect more of the medial facet to visualize the shoulder of the nerve root adequately (Fig. 20–7B). In cases of foraminal disease only, a ball-tip probe should be passed into the foramen to confirm adequacy of decompression. A spatula or blunt hook can be used to explore the ventral aspect of the dura for an occult disk herniation. Acute disk herniations are handled in a fashion similar to standard microdiskectomy. A Penfield number 4 is used to explore the axilla and shoulder of the nerve root. If the disk is found to herniate through the axilla and prevent mobilization of the nerve root medially, the axilla should be decompressed first. A combination nerve root retractor/ sucker is used to retract the thecal sac medially (Fig. 20–7C). It is often necessary at this point to position the working channel laterally to retract the thecal sac medially. A suction retractor can then be used to protect the nerve root. Epidural veins are coagulated as necessary, the anulus is incised, and the disk fragment is removed (Fig. 20–7D). Once the axilla has been decompressed, the retractors should be positioned lateral to the shoulder of the nerve root, and the nerve root and shoulder pulled medially. Further diskectomy should then be performed in the standard fashion (Fig. 20–7E). Because a 30-degree endoscope is used for the procedure, the endoscope can be directed medially to help with decompression of more medially located disks, a maneuver not possible with the operating microscope (Fig. 20–7F). After completion of the diskectomy, the wound is irrigated with copious amounts of antibiotic saline. Hemostasis should be confirmed, and, if necessary, a small piece of thrombin-soaked Gelfoam may be left behind. If the nerve root appears swollen, Gelfoam soaked in 40 mg methylprednisolone acetate (Depo-Medrol, Pharmacia & Upjohn Co., Kalamazoo, MI) may also be used. The snake retractor is then loosened, and the working channel with attached endoscope is slowly removed. Muscle bleeders tamponaded by the retractor are thus easily identified and coagulated. The wound should again be irrigated. The dorsal lumbar fascia is then closed, the subcutaneous tissues are approximated, and the skin is closed with interrupted subcuticular stitches. The wound is dressed with cyanoacrylate (Fig. 20–8). The patient is then turned over to the anesthesia team. FIGURE 20–4 (A) The working channel is passed over the final dilator and secured to the table clamp. (B) Ideal placement of working channel over the disk space. (C) The endoscope is attached and angled medially. FIGURE 20–6 (A) Starting medially, a punch is used to perform a hemilaminotomy. (B) The hemilaminotomy extends laterally. A partial medial facetectomy is also performed with the aid of a high-speed drill. (C) Artist’s representation. Note that the ligamentum flavum is kept intact to protect the underlying dura and a hemilaminectomy with Karrison punch. FIGURE 20–7 (A) An angled curette is used to establish a plane between the dura and the ligamentum flavum. Kerrison punches are then used to resect the ligament. (B) The exposed nerve root distorted by the ventrally placed disk is then visualized. (C) A suction retractor is used to retract the nerve root and the thecal sac medially. Bipolar cautery is used to control epidural bleeding. (D) The anulus is divided with a scalpel, and the disk fragment is removed with a pituitary rongeur. (E) The disk space is explored for further disk fragments. (F) With the herniated disk removed, the nerve and its relation to the thecal sac are clearly visualized. Because our technique allows for a lateral approach to the disk space, microendoscopy is particularly useful in the management of recurrent disk herniation. In this situation, care is again taken to localize the facet complex from lateral to medial. Soft tissue resection begins laterally, and curettage is used medially. The edge of the neural foramen is defined with a curette, and a medial facetectomy is performed with the high-speed drill and Kerrison punches. The majority of the scar remains attached to the midline. Once the lateral edge of the nerve root is identified, the surgeon continues as with the standard procedure. FIGURE 20–8 Standard microendoscopic diskectomy incision. The wound is dressed with cyanoacrylate. Patients are discharged with a 2-week supply of oral narcotics (oxycodone/acetaminophen or hydrocodone/ acetaminophen every 4 to 6 hours as needed) and stool softeners. We have also found that the addition of baclofen 10 mg PO tid for 30 days is quite effective in the management of postoperative muscular spasm and pain. Patients are seen in follow-up by our nurse practitioner at 7 to 10 days for a wound check and pain medication adjustment as needed. Six weeks of physical therapy commences 1 week postoperatively. Most patients are off narcotics by the fourth postoperative week. Patients are again seen in follow-up at 6 weeks and discharged from our clinic if no further issues remain. Potential Complications and Avoidance Vascular and intra-abdominal injury is a potential complication of the microendoscopic diskectomy procedure during localization and diskectomy. During localization it is important to use fluoroscopy to confirm the depth of the guide pin. The tip of the guide pin should be kept dorsal to the transverse process. The goal is to dock on the facet complex firmly. During the diskectomy portion of the procedure, fluoroscopy can be used to confirm the depth of the instruments within the disk, ensuring that the ventral aspect of the anulus fibrosus is not violated, risking injury to the vascular structures of the retroperitoneum and bowel. In a previously reported series, the incidence of durotomy was ~3 to 5%.10 During localization, care should be taken to ensure that the guide pin is firmly docked on the facet complex. Directing the guide pin perpendicular to the skin and not medially protects against entering the interlaminar space, which is especially important in recurrent disk herniations, when a laminar defect is present. Removal of the guide pin after passage of the first dilator prevents migration of the pin during passage of the larger dilators. Establishment of the plane between the lamina and the ligamentum flavum medially prior to the laminotomy helps to prevent accidental durotomy. If a small durotomy does occur, we have been successful in using a small piece of Gelfoam and 24 hours of supine bed rest. Larger durotomies have been repaired primarily with 48 to 72 hours of lumbar drainage. Delayed pseudomeningocele has been encountered in only one patient.10 Acute radiculopathy has been encountered in patients with large disk herniations, requiring aggressive mobilization of the nerve root. In these patients, a rapid methylprednisolone taper has proven useful. Although quite painful, the symptoms usually resolve within 7 to 10 days. Persistence of symptoms at the 6-week follow-up, despite physical therapy and a steroid taper, are managed with a repeat MRI. In the absence of a gross recurrence, epidural steroid injections can be attempted for pain control. If a small-to-moderately sized disk herniation is obvious, repeat exploration, and decompression can be attempted. Case Illustration A 60-year-old female presented with a 6-month history of right-sided sciatica, radiating to the dorsum of her right foot. There were no sensory or motor deficits detected on physical examination. Imaging revealed a large rightsided disk herniation at the L4–L5 level (Fig. 20–9). Bed rest and physical therapy failed to result in lasting symptomatic improvement. The patient underwent right-sided L4–L5 microendoscopic diskectomy. There were no complications. The patient was seen at 1 week for a wound check and at 4 weeks for postoperative evaluation. Except for a few episodes of mild sciatica associated with heavy activity, the patient’s symptoms were completely resolved. REFERENCES 7. Foley KT, Smith MM. Microendoscopic discectomy. Tech Neurosurg. 1997;3:301–307.
20

Lumbar Microendoscopic Laminoforaminotomy and Diskectomy

< div class='tao-gold-member'>
Lumbar Microendoscopic Laminoforaminotomy and Diskectomy
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








