Modified from Cook RJ, Sackett DL. The number needed to treat: A clinically useful measure of treatment effect. BMJ 1995;310:452–454. Based on the results of Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke and coronary heart disease. Part 2: Short-term reductions in blood pressure. Lancet 1990;335:827–838.
As shown in the far right column of Table 5-1, these investigators propose the use of the measure number needed to treat (NNT), calculated as the inverse of the absolute risk reduction, because it “conveys both statistical and clinical significance to the doctor” and “can be used to extrapolate published findings to a patient at an arbitrary specified baseline risk” (Cook & Sackett, 1995).
The need for using absolute risk, or the NNT, is well demonstrated in Figure 5-1 (Lever & Ramsay, 1995). Figure 5-1A shows the quite similar reductions in relative risk for stroke in six major trials in the elderly and in the earlier Medical Research Council trial of younger hypertensives. Figure 5-1B shows the same data in absolute terms, clearly portraying the progressively greater benefit of therapy with increasing pretherapy risk, as reflected in the rates in the placebo groups.
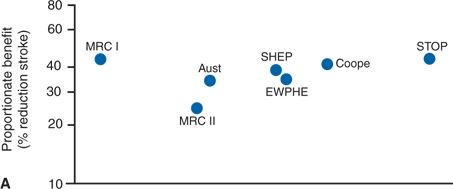
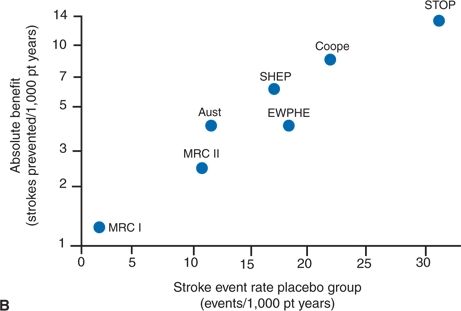
FIGURE 5.1 Comparison of (A) proportionate (relative) and (B) absolute benefit from reduction in the incidence of stroke in six trials in the elderly and in one other trial (Medical Research Council I [MRC-I]) having a similar design but in which the absolute stroke risk was much lower. Event rates are for fatal and nonfatal stroke combined. Aust, Australian study; EWPHE, European Working Party on High BP in the Elderly trial; Cooper, Cooper and Warrender; SHEP, Systolic Hypertension in the Elderly Program; STOP, Swedish Trial in Old Patients with Hypertension. (Modified from Lever AF, Ramsay LE. Treatment of hypertension in the elderly. J Hypertens 1995;13:571–579.)
The use of NNTs based on absolute risk reduction is clearly more accurate than the portrayal of relative risks. The NNT must be related to the duration of the trial. This is best done by using the hazard difference, expressed as mortality per unit of patient-time (Lubsen et al., 2000). However, in most recent reports, results are presented as survival curves, showing differences in outcomes that change over time, using the Kaplan-Meier life table methods for estimating the proportion of patients who experience an event by time since randomization (Pocock et al., 2002). When properly constructed, i.e., showing both the number of subjects remaining in the trial over time and a display of statistical uncertainty, such survival curves portray RCT results very well.
Admixture of Drugs: To achieve the preset goal of therapy, e.g., BP below 140/90, most trials comparing a drug versus placebo (as examined in this chapter) or one drug versus another (as examined in Chapter 7) must add additional drugs to the study drug. In some trials, 80% or more of the patients end up on two or more. What is ascribed to only the study drug may represent the effect of many others (Mancia et al., 2014).
Reliance on Conventional Clinic-Based BP Measurements
In all RCTs, outcomes are related to changes in conventional clinic-based measurement of BP, which (as detailed in Chapter 2) can both overestimate out-of-office BP due to white-coat hypertension and underestimate out-of-office BP due to masked hypertension. Drug treatment can convert sustained hypertension (i.e., high BP both in and out of the office) to a form of masked (i.e., partially treated) hypertension, by lowering clinic BP more than ambulatory BP (Franklin et al., 2013). While daytime ambulatory BPs and nocturnal BPs are superior to conventional office BPs as predictors of target organ damage and clinical outcomes (O’Brien et al., 2013), few RCTs have included ambulatory BP monitoring and no RCT has tested whether treatment of masked HTN improves outcomes.
Solutions to the Problems of Trials
Ambulatory BP monitoring needs to become a standard part of future RCTs. Those who perform and report RCTs must follow established guidelines such as the Consolidated Standards of Reporting Trials (CONSORT) (Schulz et al., 2010). However, clinicians themselves must be prepared to assess the validity of trial data, since in the words of Montori (Montori et al., 2004),
Science is often not objective. Emotional investment in particular ideas and personal interest in academic success may lead investigators to overemphasize the importance of their findings and the quality of their work. Even more serious conflicts arise when for-profit organizations, including pharmaceutical companies, provide funds for research and consulting, conduct data management and analyses, and write reports on behalf of the investigators.
Montori et al. (2004) provide this set of guides for clinicians to avoid being misled by biased presentation and interpretation of trial data:
- Read the “Methods and Results” sections. Remember that the “Discussion” section often offers inferences that differ from those a dispassionate reader would draw.
- Read abstracts and comments in objective secondary publications such as the ACP Journal Club, Evidence-Based Medicine, UpToDate, and The Medical Letter.
- Beware of faulty comparators. A weak comparator is often chosen in comparative trials, perhaps the most egregious being the β-blocker atenolol (Carlberg et al., 2004).
- Beware of composite end points; as noted previously, all-cause mortality can hardly be fudged.
- Beware of small treatment effects, particularly when the data are reported as differences in relative risks. If the 95% confidence interval (CI) crosses the midline, beware.
- Beware of subgroup analyses. A number of provisos should be met to ensure that apparent differences in subgroup responses are real, particularly that only a small number of hypotheses were tested that were specified before the results became available.
Meanwhile, students and practitioners need to take better advantage of available sources of evidence-based clinical information (Zwolsman et al., 2012). The Cochrane Library is now the most prolific provider, but more and more sources are available, many at no cost.
Problems with Meta-Analyses and Systematic Reviews
Meta-analyses and systematic reviews of multiple RCTs are often the highest level of evidence used by expert panels whether formulating clinical practice guidelines, formulary composition, payment schedules, or textbook content (Thompson & Higgins, 2005). Unfortunately, biases may affect them as well. As Kicinski (2013) notes,
When some study outcomes are more likely to be published than others, the literature that is available to doctors, scientists, and policy makers provides misleading information. It is clear that statistically significant results supporting the hypothesis of the researcher often have a greater chance of being published and fully reported. One consequence of underreporting is that it influences the sample of studies that is available for a meta-analysis. When publication bias occurs, the validity of the meta-analysis is uncertain.
To reduce underreporting of negative trials, since 2005, the International Committee of Medical Journal Editors has required that all clinical trials be registered prospectively in the public domain (www.clinicaltrials.gov) as a condition for publication, and, since 2007, the U.S. Food and Drug Administration (FDA) has required the registration of all trial results (Kicinski, 2013).
An even bigger issue perhaps is an author’s personal bias in setting the criteria as to which trials to include and which to exclude from meta-analysis. If there are only a small number of trials on a given topic, restrictive inclusion criteria can eliminate a single trial and sway the overall conclusion about the putative treatment effect as being positive or negative.
Even under the best of conditions, meta-analyses and systemic reviews of RCTs may not be able to provide adequate information about long-term outcomes of chronic diseases such as hypertension, since almost all RCTs are of relatively short-term duration.
Problems with Guidelines
The most authoritative recommendations on how to best manage hypertension are the guidelines issued by national, or international, expert committees.
However, there are problems with current guidelines, including these:
- There are increasing numbers of hypertension guidelines, and their recommendations differ.
- They are too long-winded to be used when needed, although shorter “Practice Guidelines” are now being provided and software applications develop to incorporate point-of-care recommendations into electronic health records (Vandvik et al., 2013).
- They fail to take patients’ beliefs and abilities into account (Vandvik et al., 2013).
- The participants in guideline committees may be too narrow in outlook, may be beholden to commercial interests, or may not include the most critical observers.
Realizing these issues, the Institute of Medicine (Institute of Medicine, 2011a) issued the following standards for improving the trustworthiness of clinical practice guidelines:
- Establish transparency in the guideline development process.
- Manage conflicts of interest among panel members.
- Establish committees that are multidisciplinary and include affected patients and representatives of patient advocacy groups.
- Utilize systemic reviews to evaluate the evidence.
- Delineate the evidence foundation and grade the strength of the evidence for each recommendation (see below).
- Articulate recommendations unambiguously.
- Submit the guideline recommendations for external review by the full spectrum of stakeholders including scientific experts, clinical experts, specialty societies, federal government agencies, patients, and public representatives.
- Update the recommendations continually as new evidence comes to light.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system has become an international standard for grading the quality of the evidence and the corresponding strength of the recommendation (Guyatt et al., 2011).
Using the criteria shown in Table 5-2, the quality of the evidence for any specific recommendation is rated as:
- High—Further research is very unlikely to change our confidence in the estimate of effect.
- Moderate—Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
- Low—Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
- Very low—Any estimate of effect is very uncertain.
This rating system is quite reproducible (Mustafa et al., 2013).
Then, the strength of the recommendation is rated as:
- Strong For—The panel is highly confident that desirable consequences outweigh undesirable consequences.
- Strong Against—The panel is highly confident that the undesirable consequences outweigh the desirable consequences.
- Weak For—The panel is less confident that the desirable consequences outweigh the undesirable consequences.
- Weak Against—The panel is less confident that the undesirable consequences outweigh the desirable consequences.
TABLE 5-2 GRADE System for Assessing Quality of Evidence
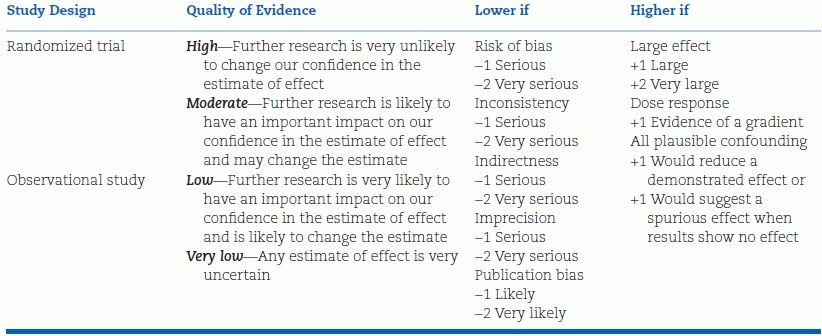
Adapted from Guyatt G, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings Tables. J Clin Epidemiol 2013;64:383–394.
Table 5-3 shows the somewhat different grading systems developed on one hand by the National Heart Lung and Blood Institute (NHLBI) and on the other by the American College of Cardiology (ACC)/American Heart Association (AHA) (Eckel et al., 2013).
TABLE 5-3 American College of Cardiology (ACC)/American Heart Association (AHA) and National Heart Lung and Blood Institute (NHLBI) Grading Systems for Evidence Quality and Strength of Recommendations
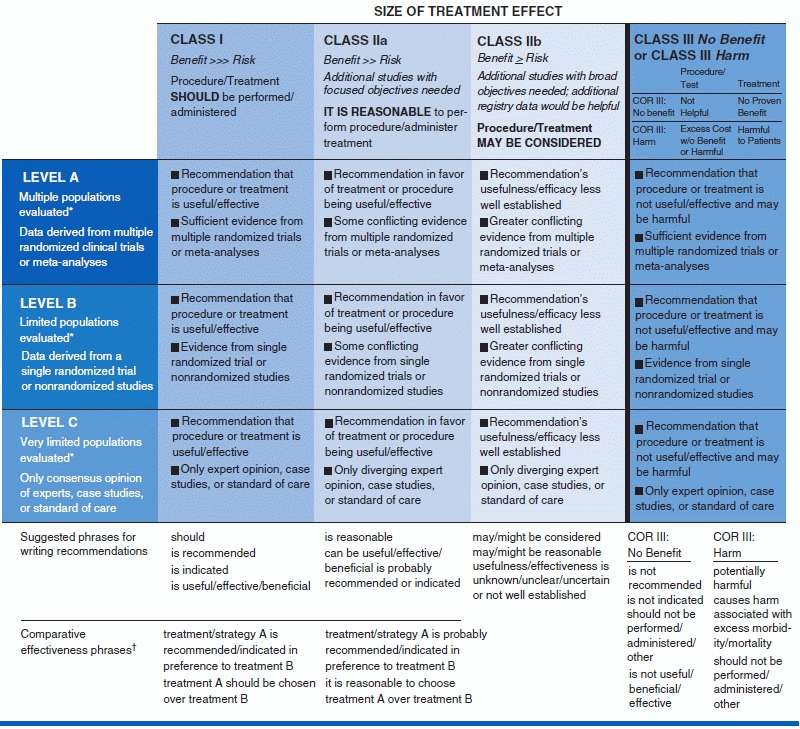
From Eckel RH, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013. doi: 10.1161/01.cir.0000437740.48606.d1
Despite problems with trials, meta-analyses, and guidelines, we must use them to determine the most effective way to manage hypertension. The following will examine the evidence that lowering BP with drugs provides benefit, starting with the most severe degree of hypertension and ending with prehypertension.
As will become obvious, the evidence of benefit becomes progressively more difficult to document as the baseline level of BP and the overall degree of risk decrease. Investigators seldom live long enough or have funds enough to obtain “hard” outcome data on patients with minimally elevated BP or little cardiovascular risk.
Trial Results
Trials in Malignant Hypertension
The benefits of drug therapy in malignant hypertension were easy to demonstrate in view of its predictable, relatively brief, and almost uniformly fatal course in untreated patients. Starting in 1958, a number of studies appeared showing a significant effect of medical therapy in reducing mortality in malignant hypertension (see Chapter 8).
Trials in Less Severe Hypertension
Demonstrating that therapy made a difference in nonmalignant, primary hypertension took a great deal longer. However, during the late 1950s and early 1960s, reports began to appear that suggested that therapy of nonmalignant hypertension was helpful (Hodge et al., 1961; Hood et al., 1963; Leishman, 1959). The first placebo-controlled, albeit small, study by Hamilton et al. (1964) showed a marked decrease in complications over a 2- to 6-year interval for 26 effectively treated patients as compared to 31 untreated patients.
Veterans Administration Cooperative Study
The first definitive proof of the protection provided by antihypertensive therapy in nonmalignant hypertension came from the Veterans Administration Cooperative Study begun in 1963. The value of therapy in the 73 men with diastolic BPs of 115 to 129 mm Hg given hydrochlorothiazide, reserpine, and hydralazine versus the 70 men given placebo became obvious after less than 1.5 years, with a reduction in deaths from four to zero and, in major complications, from twenty-three to two (Veterans Administration Cooperative Study Group on Antihypertensive Agents, 1967).
Along with the men with diastolic BPs of 115 to 129 mm Hg, another 380 with diastolic BPs between 90 and 114 mm Hg also were assigned randomly to either placebo or active therapy. It took a longer time—up to 5.5 years, with an average of 3.3 years—to demonstrate a statistically clear advantage of therapy in this group (Veterans Administration Cooperative Study Group on Antihypertensive Agents, 1970). A total of 19 of the placebo group, but only eight of the treated group, died of hypertensive complications, and serious morbidity occurred more often among the placebo group. Overall, major complications occurred in 29% of the placebo group and 12% of the treated group.
The promising results of the Veterans Administration study prompted the initiation of a number of additional controlled trials of therapy of hypertension. Data from trials completed before 1995, primarily with diuretics and β-blockers, are separated from those completed since 1995, primarily with angiotensin-converting enzyme inhibitors (ACEIs), calcium channel blockers (CCBs), and angiotensin II receptor blockers (ARBs).
Trials Before 1995
The 21 trials listed in Table 5-4 included a total of 56,078 patients followed up for an average of 5 years (Psaty et al., 1997; 2003). In all these trials, the primary drugs were either β-blockers or diuretics; in trials done before the mid-1980s, almost all used higher doses of diuretic. It should be noted that the entry BP criterion for all of the trials before the Systolic Hypertension in the Elderly Program Pilot Study (SHEP-P) in 1989 was the diastolic level, reflecting the greater emphasis placed, until recently, on diastolic rather than systolic blood pressure (SBP) as the major determinant of risk.
TABLE 5-4 Randomized Placebo-Controlled Trials of Antihypertensive Drug Treatment Published Before 1995
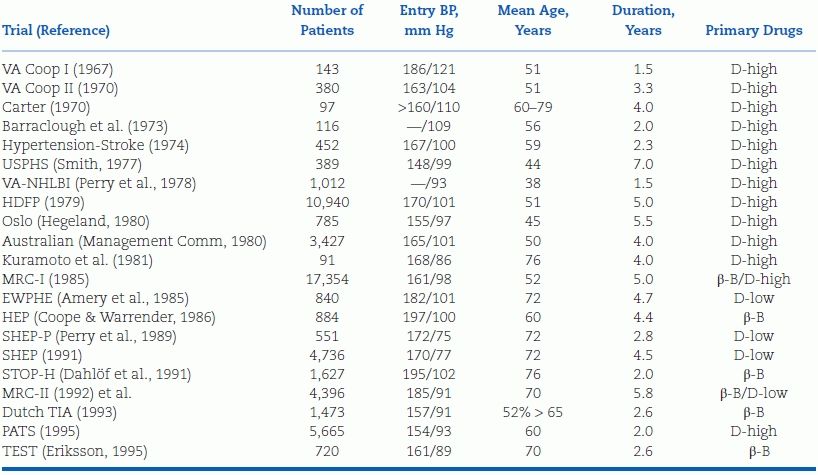
β-B, beta-blocker; BP, blood pressure; D-high, diuretic dose ≥50 mg hydrochlorothiazide; D-low, diuretic dose <50 mg hydrochlorothiazide; EWPHE, European Working Party on Hypertension in the Elderly; HDFP, Hypertension Detection and Follow-up Program; MRC, Medical Research Council; NHLBI, National Heart, Lung, and Blood Institute; PATS, Poststroke Antihypertensive Treatment; SHEP, Systolic Hypertension in the Elderly Program; SHEP-P, SHEP Pilot Study; STOP-H, Swedish Trial in Old Patients with Hypertension; TEST, Tenormin after Stroke and TIA; USPHS, U.S. Public Health Service; VA, Veterans Administration.
The trials published before 1985 mainly involved younger patients; those in the early 1990s enrolled elderly hypertensives with either combined hypertension or isolated systolic hypertension (ISH), who will be examined separately.
Separation of the Data by Doses
Psaty et al. (1997) separated the nine trials that involved high doses of diuretic (equivalent to 50 mg or more of hydrochlorothiazide) from the four that involved lower doses (equivalent to 12.5 to 25.0 mg hydrochlorothiazide) and the four that used a β-blocker as the primary drug (Fig. 5-2). The Hypertension Detection and Follow-up Program study was considered separately, as it was not placebo controlled: Half of the patients were more intensively treated (stepped care); the other half were less intensively treated (referred care).
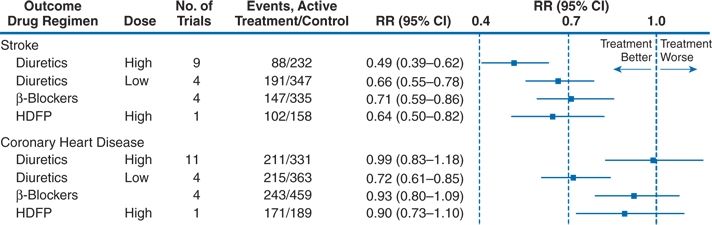
FIGURE 5-2 Meta-analysis of randomized, placebo-controlled clinical trials in hypertension according to first-line treatment strategy. For these comparisons, the numbers of participants randomized to active therapy and placebo were 7,758 and 12,075 for high-dose diuretic therapy; 4,305 and 5,116 for low-dose diuretic therapy; and 6,736 and 12,147 for β-blocker therapy. HDFP, Hypertension Detection and Follow-up Program; RR, relative risk; CI, confidence interval. (Adapted from Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997;277:739–745.)
The separation of the data by doses clearly reveals the lack of protection from CHD by high doses of diuretic and β-blockers, whereas all therapies had a significant impact on stroke. The later four studies with lower doses of diuretic showed excellent protection against CHD.
Conclusion
Based on these early trials—primarily in middle-aged patients with combined systolic and diastolic hypertension—the evidence was clear: Reductions in BP of 10 to 12 mm Hg systolic and 5 to 6 mm Hg diastolic for a few years conferred relative reductions of 38% for stroke and 16% for CHD (Collins & MacMahon, 1994).
Placebo-Controlled Trials After 1995
After 1995, a new series of trials have been completed, and more started, to determine the effects of the newer antihypertensive agents—ACEIs, ARBs, and CCBs—and to broaden the patient population to those with associated conditions including coronary disease, diabetes, and renal insufficiency (Table 5-5).
TABLE 5-5 Randomized Placebo-Controlled Trials of Antihypertensive Drug Therapy Published After 1995
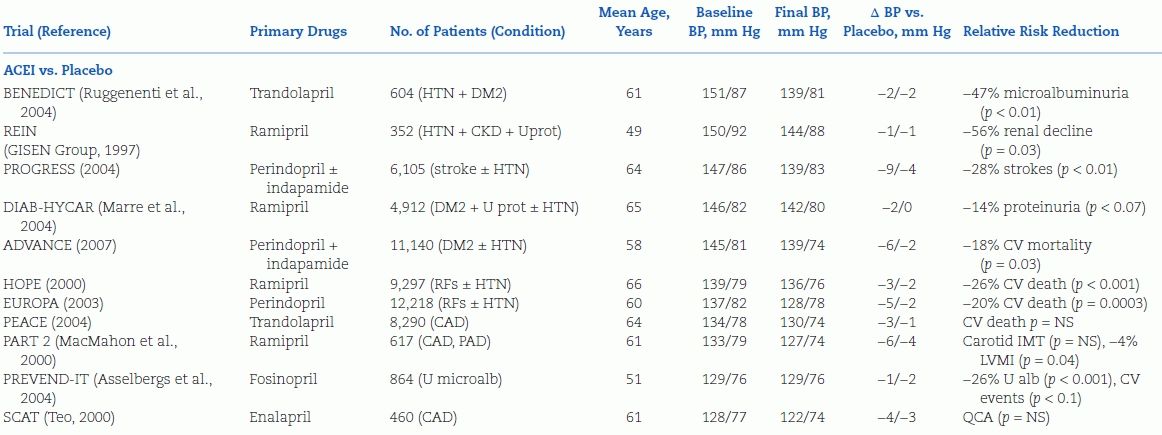
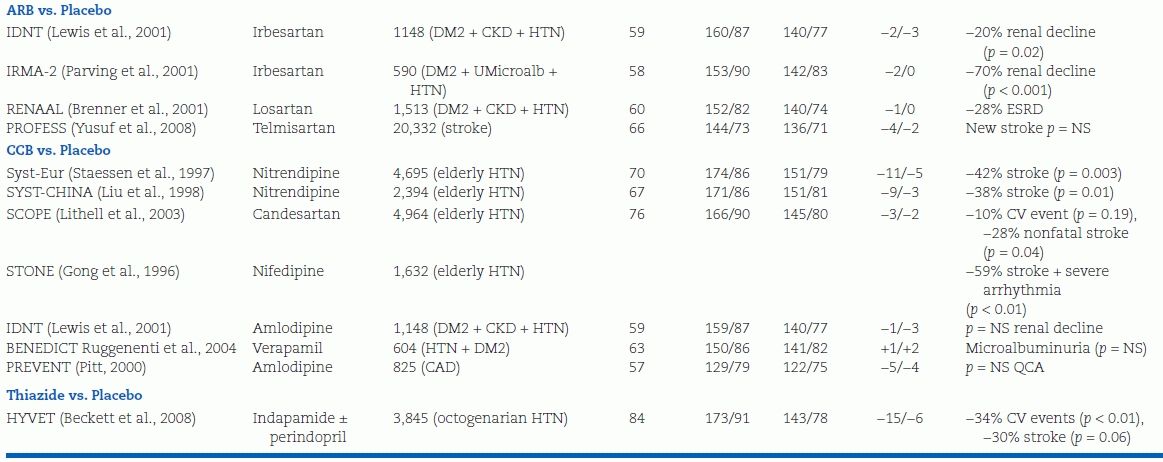
DM2, type 2 diabetes mellitus; HTN, hypertension; U prot, proteinuria; U mircoalb, urinary microalbuminuria; RFs, risk factors for atherosclerotic cardiovascular disease; CV, cardiovascular; QCA, quantitative coronary angiography; IMT, intimal–medial thickness.
Figure 5-3 is a 2003 overview of data from 31 RCTs showing the relation between odds ratios for cardiovascular events and differences in SBP (Staessen et al., 2003). The figure portrays data from 15 of the 21 placebo-controlled trials published before 1995 that are listed in Table 5-2, the others being too small or too short to be included. Most of the placebo-controlled trials published before 2003 are included. In addition, data from some of the comparative trials to be covered in Chapter 7 are included, since the purpose of the graph is to show the degree of protection with varying differences of SBP. In some of the comparative trials, lesser SBP reduction was seen with the “experimental” drug, with resultant increases in cardiovascular events.
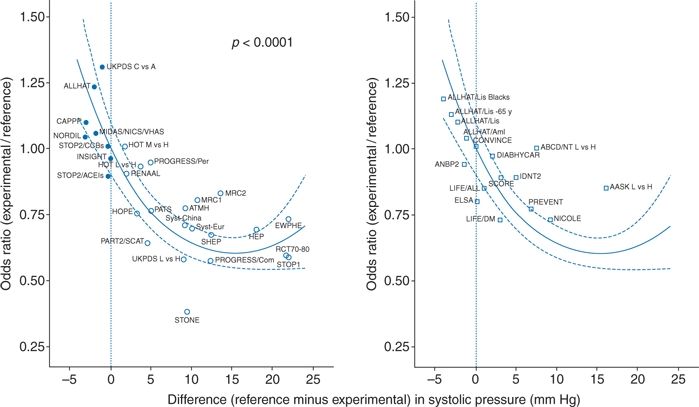
FIGURE 5-3 Relationship between odds ratio for cardiovascular events and corresponding differences in SBP in trials published before (left) and after (right) 2000. Odds ratios were calculated for experimental versus reference treatment. BP differences were obtained by subtracting achieved levels of experimental groups from those in reference groups. Negative values indicate tighter BP on control than on reference treatment. The regression lines were plotted with 95% CI and were weighted for the inverse of the variance of the individual odds ratios. (Modified from Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: A quantitative overview updated until 1 March 2003. J Hypertens 2003;21:1055–1076.)
The message of Figure 5-3 is clear: The degree of BP reduction is the primary determinant of cardiovascular protection, not the type of drug that provided the reduction in BP. As discussed later in the chapter, subsequent meta-regression analyses after 2003 by the Blood Pressure Lowering Treatment Trialists’ Collaboration all have confirmed this conclusion: A few mm Hg additional reduction in SBP is accompanied by large additional reductions in cardiovascular (CV) disability and death.
The only apparent exception, to be described in more detail in Chapter 7, is that β-blocker–based therapy has not been as protective against stroke as other drugs, despite equal reduction in BP (Carlberg et al., 2004).
In placebo-controlled trials of ACEIs, ARBs, and CCBs, published before 2003, the only apparent difference is lesser protection against heart failure by CCBs (Turnbull, 2003) (Fig. 5-4). Subsequent trials with the CCB amlodipine have shown better protective effects with this agent (Wang et al., 2007).
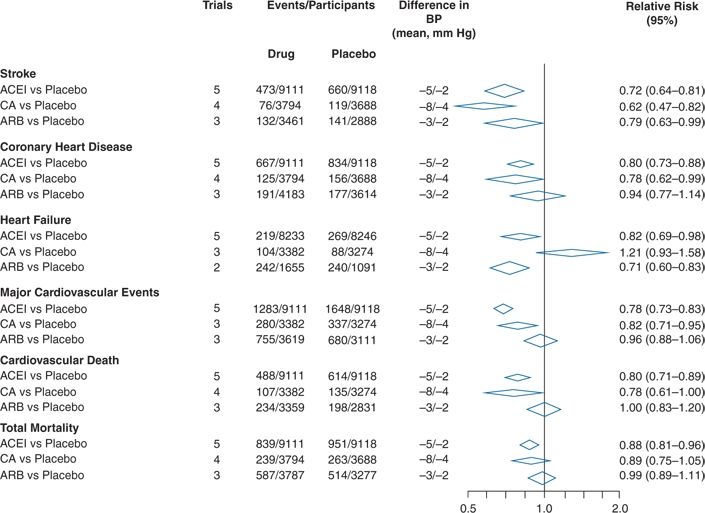
FIGURE 5-4 Comparisons of the effects of therapy based on ACEI, angiotensin-converting enzyme inhibitor; CA, calcium antagonist; ARB, angiotensin II receptor blocker; and all versus placebo on cardiovascular events and mortality. (Modified from Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527–1535.)
Trial Results: Special Populations
Trials in the Elderly with Isolated Systolic Hypertension (ISH)
Although both the earlier and the later trials listed in Tables 5-2 and 5-3 include some elderly patients with ISH, defined in most of these trials as a SBP 160 mm Hg or higher with a diastolic BP below 95 mm Hg, the fact that such patients make up the largest portion of hypertensive patients now and will do so, to an even greater degree, in the future as our population ages, justifies a closer, separate look at the data on their therapy. Staessen et al. (2000) have provided a meta-analysis of these trials, which are listed in Table 5-6. The 2008 Hypertension in the Very Elderly Trial (HYVET) is discussed separately.
TABLE 5-6 Randomized Placebo-Controlled Trials of Antihypertensive Drug Treatment in Elderly Patients with Isolated Systolic Hypertension above 160 mm Hga
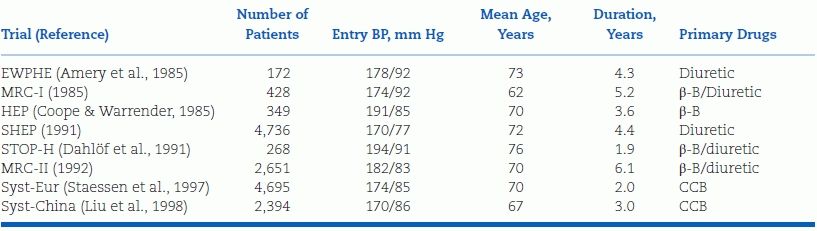
aDiagnosis of systolic hypertension based on SBP above 160 and diastolic BP below 95 mm Hg in all trials except SHEP, which required a diastolic BP of ≤90 mm Hg.
β-B, beta-blocker; CCB, calcium channel blocker; EWPHE, European Working Party on Hypertension in the Elderly; MRC, Medical Research Council; SHEP, Systolic Hypertension in the Elderly Program; STOP-H, Swedish Trial in Old Patients with Hypertension; Syst-China, Systolic Hypertension in China trial; Syst-Eur, Systolic Hypertension in Europe trial.
Figure 5-5 summarizes the data from these eight trials of 15,693 elderly patients with ISH. The average BP at entry was 174/83 mm Hg, and the mean fall in BP over the median 3.8-year follow-up was 10/4 mm Hg. Therapy significantly reduced all-cause and cardiovascular mortality by 13% and 18%, respectively, but had an even greater impact on morbidity: Coronary events were reduced by 23% and strokes by 30%.
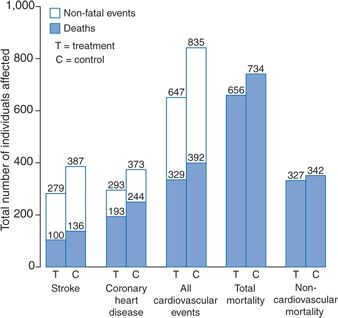
FIGURE 5-5 Summarized results in 15,693 older patients with ISH above 160 mm Hg enrolled in eight trials of antihypertensive drug treatment. BP at entry averaged 174/83 mm Hg. During follow-up (median, 3.8 years), the mean difference in BP between the treated and control patients was 10.4 mm Hg systolic and 4.1 mm Hg diastolic.(Modified from Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: Meta-analysis of outcome trials. Lancet 2000;355:865–872.)
In these trials, the absolute benefits of active therapy were greater in men, older patients, and those with prior cardiovascular complications, reflecting the higher initial risk status of such patients. To prevent one major cardiovascular event, the numbers of patients that needed to be treated for 5 years were 18 men versus 38 women, 19 patients who were 79 years or older versus 39 patients who were 60 to 69 years old, and 16 of those with prior cardiovascular complications versus 37 of those without (Staessen et al., 2000). Moreover, in a 15-year follow-up of a portion of the participants in the SHEP trial, a persistent reduction in fatal plus nonfatal cardiovascular events was found among the original drug-treated group compared to the placebo group, 58% versus 79%, despite the eventual use of antihypertensive therapy in 65% of the placebo group, compared to 72% of the active group (Sutton-Tyrrell et al., 2003).
As impressive as these data are, they must be recognized as covering only the higher range (stage 2) of ISH, i.e., systolics of 160 mm Hg or higher, which has uniformly been the criterion for entry into the trials shown in Table 5-4 and Figure 5-5. Most ISH is between 140 and 159 mm Hg, and most premature cardiovascular events occur in patients in that range rather than in those with higher SBP (Chaudhry et al., 2004). As of now, there still are no placebo-controlled RCTs documenting the benefit for those with stage 1 ISH. Yet, when meta-regression analyses include RCTs with active comparator groups, it becomes clear that small additional reductions in SBP (as little as a few mm Hg) between groups are accompanied by sizeable reductions in CV events for patients younger and older than age 65 (Turnbull et al., 2008a).
HYVET Trial in Those Over Age 80
Data are now available on the effect of therapy for patients over the age of 80 years, percentage-wise the fastest growing demographic group (Beckett et al., 2008). The HYVET included 3,845 hypertensives over age 80 with a sustained SBP of 160 mm Hg or higher. Their mean seated BP was 173/91 mm Hg. Half were assigned to placebo and the other half to active therapy, starting with the diuretic indapamide and adding the ACEI perindopril, if needed, to achieve the goal of 150 mm Hg. With the average additional decrease in BP of 15/6 mm Hg over placebo, the actively treated half achieved significantly greater protection against stroke, heart failure, and all-cause mortality after a median follow-up of only 1.8 years, causing the trial to be stopped prematurely by the data and safety monitoring board.
These impressive results are in keeping with the observation that older patients derive greater absolute benefit from any reduction in BP than do younger patients. As shown by Wang et al. (2005), the relative slopes of decreasing events with therapy are similar in the younger, older, and very old, but since the older start at higher degrees of risk, they achieve a greater absolute benefit (Fig. 5-6). Wang et al. (2005) further show that the lowering of systolic pressure is the critical element of therapy, regardless of the magnitude of the fall in diastolic pressure.
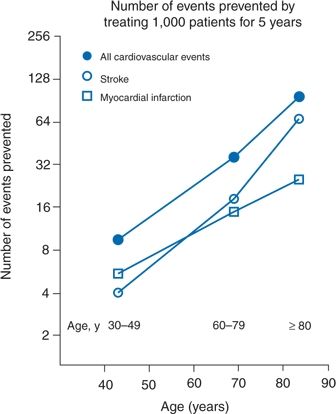
FIGURE 5-6 Absolute benefits in the prevention of fatal and nonfatal cardiovascular events, stroke, and MI in three age groups. Symbols represent the number of events that can be prevented by treating 1,000 patients for 5 years. (Modified from Wang JG, Staessen JA, Franklin SS, et al. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension 2005;45:907–913.)
The results of all published antihypertensive RCTs on major cardiovascular events in patients under age 65, and those 65 years and older (not including HYVET), show similar risk reductions (Table 5-7) (Turnbull et al., 2008a). Thus, age per se is not a defining issue: Patients at any age with a reasonable life expectancy deserve antihypertensive drug therapy if their SBP is 160 mm Hg or higher.
TABLE 5-7 Mean Differences in BP Between Randomized Groups in Younger and Older Adults
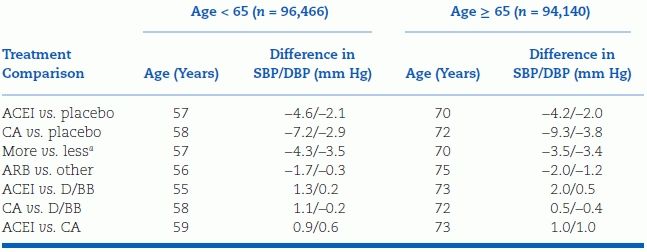
aMore vs. less intensive BP-lowering regimen.
SBP/DBP, systolic/diastolic blood pressure; ACEI, angiotensin-converting enzyme inhibitor; CA, calcium antagonist; ARB, angiotensin receptor blocker; D/BB, diuretic or β-blocker. From Blood Pressure Lowering Treatment Trialists’ collaboration. BMJ 2008;336:1121.
The data in Table 5-7 cover RCTs with either an ACEI or CCB versus placebo. The trials with an ARB were not placebo controlled.
Trials in Women
Meta-analysis of 31 RCTs by the Blood Pressure Lowering Treatment Trialists’ Collaboration of 31 RCTs found that all major classes of antihypertensives produce similar BP reductions in men and women and no evidence for gender-based differences in the CV protection afforded (Turnbull et al., 2008b).
Trials in Black Hypertensives
Non-Hispanic black patients have been underrepresented in most hypertension RCTs with the exception of ALLHAT and AASK. BP in black hypertensives responds less to monotherapy with renin-inhibiting drugs than it does in white hypertensives, and, in the ALLHAT trial, black hypertensives on the ACEI lisinopril had more heart failure and strokes than those on the diuretic chlorthalidone, which caused a larger fall in BP (Wright et al., 2005). In the long-term follow-up of the AASK cohort, the group originally randomized to the more stringent BP goal of <130/80 had slower progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD) than the group originally randomized to the less stringent BP goal of <140/90 mm Hg but only in those with proteinuria (Appel et al., 2010).
Trials in Diabetic Patients
Fifteen RCTs of antihypertensive therapy in patients with type 2 diabetes mellitus or impaired fasting glucose are summarized in Table 5-8 (Bangalore et al., 2011). The two largest trials deserved special mention. In the Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) trial (see Tables 5-5 and 5-8), the relative risk of CV death fell by 18% when BP was reduced from 144/81 mm Hg to 139/79 mm Hg with a fixed combination of perindopril and indapamide versus placebo (Patel et al., 2007). In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, the risk of stroke fell by 50%, while the risk of coronary events was unchanged when SBP was reduced to 119 mm Hg rather than to 133 mm Hg (Cushman et al., 2010). The added benefit of more intensive BP reduction on stroke but not myocardial infarction (MI) in patients with diabetes is supported by two separate meta-analyses, the first of which is shown in Figure 5-7, (Bangalore et al., 2011; McBrien et al., 2012) but not by a more restrictive Cochrane review, which found no added benefit of intensive BP lowering on stroke or MI (Arguedas et al., 2013). Post hoc analysis of the diabetic subgroup (9,603 patients) in ONTARGET showed a progressive reduction in the risk of stroke but not MI with progressively lower achieved SBP down to a value of 111 mm Hg (Redon et al., 2012).
TABLE 5-8 Randomized Controlled Trials of Antihypertensive Drug Therapy in Patients with Type 2 Diabetes or Impaired Fasting Glucose
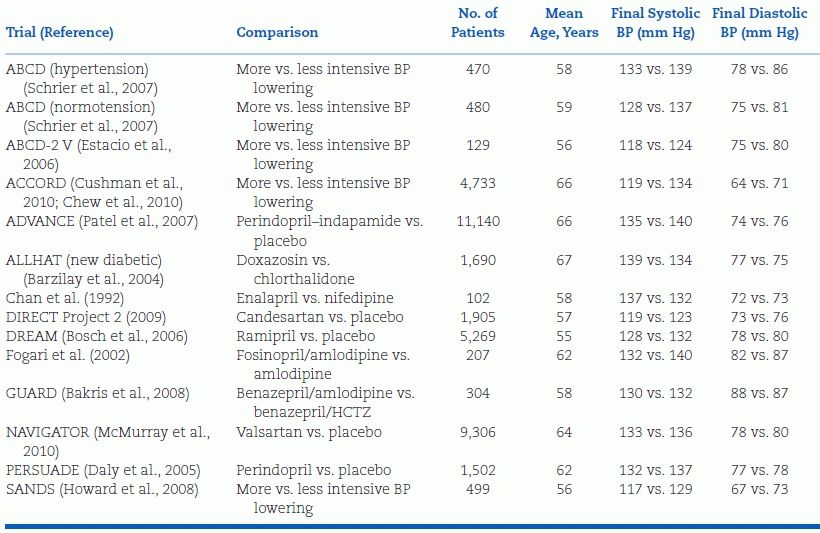
BP, blood pressure; ABCD, Appropriate Blood Pressure Control in Diabetes; ABCD-2 V, ABCD-2 Valsartan; ACCORD, Action to Control Cardiovascular Risk in Diabetes; ADVANCE, Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation; ALLHAT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; DIRECT, Diabetic Retinopathy Candesartan Trials; DREAM, Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication; GUARD, Gauging Albuminuria Reduction with Lotrel in Diabetic Patients with Hypertension; NAVIGATOR, Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research; PERSUADE, Perindopril Substudy in Coronary Artery Disease and Diabetes; and SANDS, Stop Atherosclerosis in Native Diabetics Study. Adapted from Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: Observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation 2011;123:2799–2810.
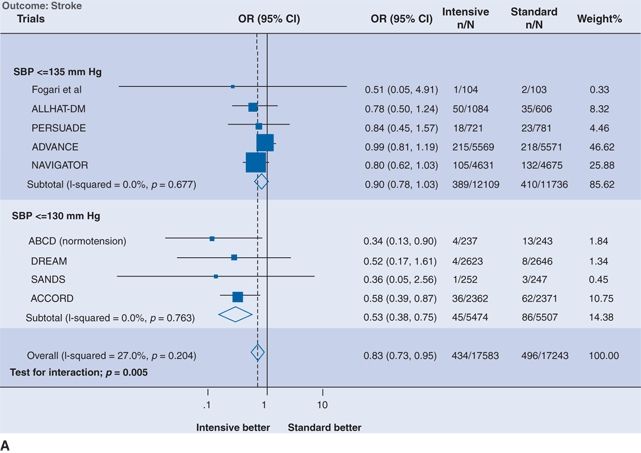
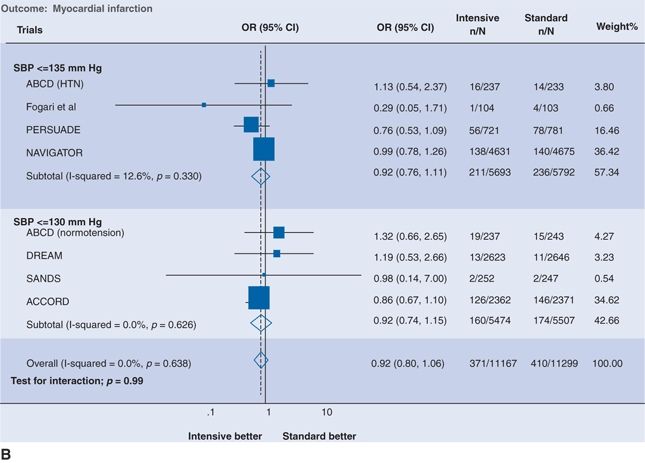
FIGURE 5.7 Intensive versus standard BP control and (A) stroke and (B) MI. Results are further stratified by achieved systolic pressure in the intensive group. The size of the data marker represents the weight of each trial. OR indicates odds ratio; CI, confidence interval; SBP, systolic blood pressure; ALLHAT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; PERSUADE, Perindopril Substudy in Coronary Artery Disease and Diabetes; ADVANCE, Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation; NAVIGATOR, Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research; ABCD, Appropriate Blood Pressure Control in Diabetes; DREAM, Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication; SANDS, Stop Atherosclerosis in Native Diabetics Study; and ACCORD, Action to Control Cardiovascular Risk in Diabetes. (From Bangalore S, Kumar S, Lobach I, et al. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: Observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation 2011;123:2799–2810, p. 9)
Trials in Patients with Chronic Kidney Disease
In patients with CKD, the goals of antihypertensive therapy are to reduce the risks of both ESRD and CV events. In two trials of patients with diabetic nephropathy (IDNT and RENAAL), those treated with an ARB had slowed progression of renal damage (Brenner et al., 2001; Lewis et al., 2001)—a conclusion that has been confirmed by a recent meta-analysis (Khan et al., 2013). The achieved BPs in the active treatment groups of IDNT and RENAAL were 140/74–77, with small additional reductions in BP from addition of an ARB being associated with 20% to 28% reduction in the rate of renal decline or incident ESRD (see Table 5-5). Available data on nondiabetic CKD are limited with a recent meta-analysis of only 2,272 participants of 3 trials (REIN, AASK, and MDRD) showing suggestive but inclusive evidence that a BP target of <130/80 mm Hg might afford greater renal protection than a BP target of <140/90 mm Hg but only in the subset of patients with proteinuria (300 to 1,000 mg of protein per 24 hours) (Upadhyay et al., 2011).
A recent meta-analysis of 152,290 participants of 26 RCTs compared the CV benefits of antihypertensive therapy in patients with eGFR above or below 60 mL/min/1.73 m2; mean baseline BP ranged from 141/82 to 170/104 mm Hg, while mean achieved BP ranged from 135/78 mm Hg to 151/81 mm Hg (Table 5-9) (Ninomiya et al., 2013). Patients with CKD derived the same relative CV risk reduction as non-CKD patients but greater reduction in absolute risk. The benefit was tied to BP reduction rather than drug class, even when comparing ACEIs and CCBs (Fig. 5-8).
TABLE 5-9 Mean Baseline Characteristics and Follow-Up Differences in BP Between Randomized Groups According to Different eGFR
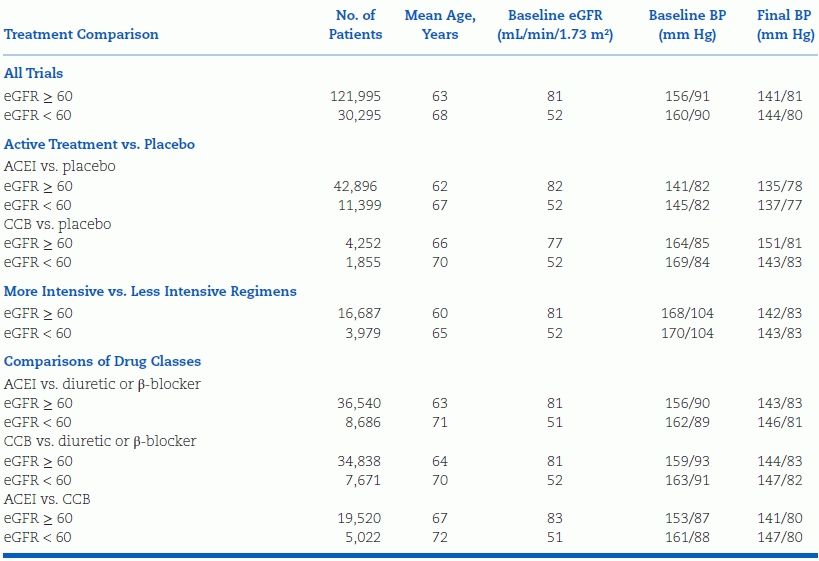
ACEI, angiotensin-converting enzyme inhibitor. Adapted from Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: Meta-analysis of randomized controlled trials. BMJ 2013;347:15680. doi: 10.1136/bmj.55680
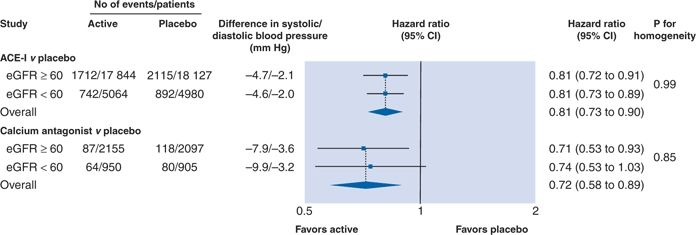
FIGURE 5-8 Effects of angiotensin-converting enzyme inhibitor or calcium antagonist–based regimens versus placebo for risk of major cardiovascular events according to kidney function status. P value for homogeneity indicates consistency of effect of treatment regimen among subgroup. Overall mean difference in systolic and diastolic BP during follow-up in actively treated/first listed regimens versus control/second listed regimens, calculated by weighting difference observed in each contributing trial by number of patients in trial. Negative values indicate lower mean systolic and diastolic BP during follow-up in actively treated/first listed groups than in control/second listed groups. (From Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: Meta-analysis of randomized controlled trials. BMJ 2013;347:15680. doi: 10.1136/bmj.55680)
Trials in Cardiac Patients
In addition to the documentation that coronary artery disease (CAD) morbidity and mortality have been significantly prevented by low-dose diuretics, CCBs, ACEIs, and ARBs reviewed earlier in this chapter (Figs. 5-2 and 5-4), additional RCTs have examined the effect of antihypertensive agents for secondary prevention of CAD.
Angina and Coronary Disease
Nitrates, β-blockers, and CCBs had been used for many years on the basis of efficacy in reducing symptoms with little or no hard outcome data. When given to patients with high CV risk profiles, whether hypertensive or normotensive, addition of an ACEI reduced major CV events more than placebo (with ramipril) in the Heart Outcomes Protection Evaluation (HOPE) study (Yusuf et al., 2000) or (with perindopril) in the European Trial on Reduction of Cardiovascular Events with Perindopril in Stable Coronary Artery Disease (EUROPA) study (Fox, 2003) but not (with trandolapril) in the Prevention of Events with Angiotensin-Converting Enzyme Inhibitor (PEACE) trial (Braunwald et al., 2004), in which most patients with stable coronary disease were on background statin therapy and other CV reduction therapies. The CAMELOT trial (Nissen et al., 2004) showed that amlodipine, but not enalapril, further protected patients with CAD even when they were normotensive. It is unclear whether the added benefit with amlodipine seen in this rather small study was due to the drug’s antiischemic rather than antihypertensive effect.
Congestive Heart Failure
In ALLHAT, the ACEI was somewhat less effective than the diuretic chlorthalidone in primary prevention of heart failure, but this may be an artifact of the design in which preexisting diuretic therapy was withdrawn in the ACEI arm (ALLHAT Officers and Coordinators, 2002). Multiple trials, a few placebo controlled and mostly of short duration, have shown reduction in hospitalizations and mortality in patients with chronic heart failure with diuretics, β-blockers, ACEIs, ARBs, aldosterone antagonists, and, in blacks, a combination of hydralazine and nitrate. However, none of these trials were hypertensive treatment trials as medication was not titrated to lower BP. In the Irbesartan in Heart Failure with Preserved Systolic Function (I-PRESERVE), the ARB did not reduce CV events more than placebo (Massie et al., 2008). In the recent TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) trial, results presented at the 2013 AHA Scientific Sessions (Pfeffer and on behalf of the TOPCAT Investigators, 2013) indicate that spironolactone did not reduce the primary outcome of cardiovascular death, heart failure hospitalization, or surviving a cardiac arrest in patients with heart failure and preserved ejection fraction; however, spironolactone did reduce the major burden faced by these patients—the risk of repeated hospitalizations for heart failure.
Trials in Patients with Stroke
In the Perindopril Protection Against Recurrent Stroke Study (PROGRESS) trial of patients who had survived ischemic or hemorrhagic stroke, outpatient SBP reduction by 12 mm Hg to a value of 135 mm Hg with an ACEI/diuretic (perindopril/indapamide) combination reduced the relative risk of recurrent ischemic stroke by 36% and recurrent hemorrhagic stroke by 76% more than placebo; however, a smaller reduction in BP of only 5 mm Hg with perindopril monotherapy showed no stroke protection (PROGRESS Collaborative Group, 2001). Similarly, in the subsequent Prevention Regimen for Effectively Avoiding Second Strokes (PROFESS) trial of patients with ischemic stroke, no statistical benefit was found when SBP was reduced by only 4 mm Hg with ARB (telmisartan) monotherapy versus placebo (Yusuf et al., 2008).
Along with antihypertensive therapy, reduction of blood cholesterol with statins has provided another 21% reduction in stroke incidence in high-risk patients (Heart Protection Study, Collins et al., 2004), a 48% reduction in patients with high C-reactive protein levels and LDL cholesterol < 130 mg/dL (Ridker et al., 2008), and a 27% reduction in hypertensives (Sever et al., 2003). Statin therapy also is effective in secondary stroke prevention (Amarenco et al., 2006).
Cognitive Function
Strong observational data suggest that antihypertensive therapy reduces the risk of dementia, but the data from RCTs still are inconclusive (Gorelick et al., 2012). The only specific drug that has been shown in an RCT to prevent dementia is the CCB nitrendipine in the Syst-Eur trial (Gorelick et al., 2012).
An Overview of the Benefits of Therapy
Despite all of the preceding evidence that treatment of hypertension reduces cardiovascular disease, the overall role of antihypertensive therapy in the impressive falls in coronary and stroke mortality seen in most developed societies over the past 40 years turns out to be rather small. Recall from Chapter 1 that the best available evidence gives the treatment of hypertension only 3% and population-wide lowering of BP 9.5% of the credit for the 62% decline in men and the 45% decline in women in coronary mortality in England and Wales between 1981 and 2000 (Unal et al., 2004).
The reasons for this limited role are multiple, including
- Suboptimal hypertension control rates in the population despite gradual improvement
- Inability to provide effective preventive therapy before the inexorable progress of hypertension-related complications
- Inadequate attention to concomitant risk factors, leaving a large residual of risk even among those treated
These issues and others are addressed in the remainder of this chapter and in Chapter 7, but first we will examine one of the more attractive aspects of treating hypertension, namely, its cost-effectiveness.
Cost-Effectiveness of Treating Hypertension
The treatment of hypertension is among the most cost-effective measures now available for preventing avoidable death (Stason, 2009). Using various mathematical modeling techniques and Markov decision analyses, most recent estimates find that treatment of hypertension provides additional quality-adjusted life-years (QALYs) for a far lower cost than treatment of dyslipidemia or diabetes. In patients with diabetes, the message is clear: Control of hypertension actually has a net cost savings, whereas control of hyperglycemia and control of hypercholesterolemia—though cost-effective (i.e., <$50,000 per QALY)—still entail a net-positive cost (CDC Diabetes Cost-Effectiveness Group, 2002). The cost savings from fewer hospitalizations and less long-term care for stroke, coronary events, and microvascular complications of diabetes outweigh the costs of office visits and drugs to manage hypertension.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








