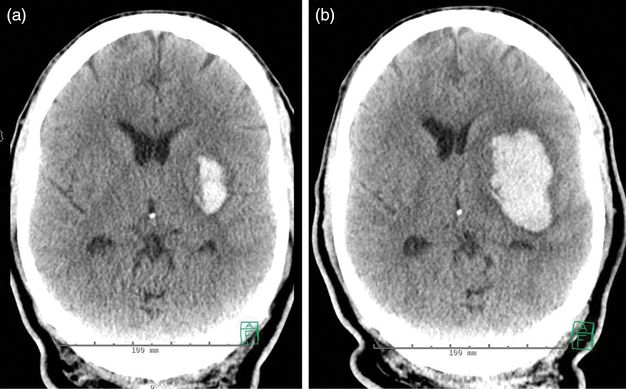
CT scan of the head demonstrating an acute intracranial hemorrhage in the medulla (a, axillary image), extending into the lower pons (b, sagittal image). The remainder of the brain parenchyma was normal.
Discussion
Immediate management
This case illustrates several issues that commonly arise in the immediate management of patients with a new neurological deficit consistent with a stroke. Immediate distinction between ischemic and hemorrhagic stroke is of utmost importance as treatment differs substantially. While certain clinical features are more typical for a hemorrhagic stroke – such as headache, nausea, vomiting, abrupt onset of symptoms with maximum of symptoms at onset – these are by no means sensitive and specific enough to establish the etiology. The diagnostic test of choice usually is non-contrasted CT scan of the head: this imaging modality is fast, readily available, and very sensitive in identification of an acute intracranial hemorrhage (ICH). In managing patients with ICH, one immediate goal of treatment should always be kept in mind: minimizing the risk of ongoing or expanding hemorrhage.
The arterial blood pressure is often elevated in patients with ICH, especially in patients who carry a diagnosis of arterial hypertension and in whom the ICH occurred due to hypertension. However, elevated blood pressure is also often noted in patients who are not known to have hypertension and for whom a different etiology of the ICH may be found. Controlling high blood pressure is one of the immediate treatment goals. High blood pressure in ICH has been shown to be linked to hematoma growth, as well as long-term negative impact on outcomes [1]. Therefore, the current guidelines of the American Heart Association (AHA) advise lowering the blood pressure at least to <180 mmHg for systolic blood pressure (SBP) or 130 mmHg for mean arterial pressure (MAP), with consideration of further modest reduction to a MAP of 110 mmHg or a target blood pressure of 160/90 mmHg.
Apart from blood pressure control, it is important to consider other factors that need immediate attention: a bleeding diathesis should always be excluded, and the basic vital functions need to be assessed. In this patient, difficulty with respiration due to bulbar musculature weakness and consequent poor airway protection were exacerbated by lying flat for the neuroimaging test. Potential inability to lie flat should always be considered in patients with neurological deficits involving the lower cranial nerves, especially when obtaining imaging studies that take longer to complete than a simple non-contrasted CT of the head – a magnetic resonance imaging (MRI) of the brain, for example. Some patients may even have to be intubated for the purpose of safely acquiring imaging studies. Early recognition of dysphagia in stroke patients is very important, as it is common and a major risk factor for aspiration pneumonia. Pneumonia contributes substantially to the morbidity amongst stroke patients. In order to guarantee appropriate monitoring of vital parameters in patients with ICH, the AHA guidelines recommend “initial monitoring and management of ICH patients in an intensive care unit with physician and nursing neuroscience intensive care expertise (Class I; Level of Evidence: B)” [2].
Diagnostic considerations
In a 47-year-old patient with a history of hypertension, the most likely differential diagnosis for an ICH is hypertensive etiology. While the brainstem, mainly the pons, is affected in 5–12% of spontaneous ICH, more common locations for hypertensive ICH are the putamen, the cerebellum, and the thalamus. The reason for these locations is that hypertensive ICHs occur preferentially in the areas of small penetrating arteries that branch off of the major intracranial arteries, often at 90 degree angles. If there is any suspicion that the ICH could be due to a reason other than hypertension – for example due to an unusual shape, location or extent of the ICH, presence of surrounding edema, or an atypical clinical constellation, vascular imaging should be considered. This patient’s ICH was located very low in the brainstem, in the medulla, which is not among the most typical locations for a hypertensive hemorrhage. Additionally, he presented at a relatively young age and was not known to carry a history of arterial hypertension. Therefore, a CT angiography of the head and neck vessels was obtained. This revealed dolichoectasia of the vertebrobasilar system, but no vascular malformations. Over the course of his hospitalization, the patient required four oral blood pressure lowering agents in order to reach a well-controlled state of normotension. This is a typical feature of patients presenting with hypertensive ICH. Often, blood pressure controlling medication regimens need to be continually adjusted in the outpatient setting.
Tip
Immediate management of an ICH includes blood pressure control and assessment of respiratory safety, especially if the level of consciousness or the cranial nerves are affected. Difficulty with respiration is often exacerbated during the recumbent position such as for imaging studies, and may not be as overt when positioned with the head of bed elevated at least 30 degrees as is generally recommended for patients at risk of aspiration.
Case 2. Hypertensive intracerebral hemorrhage
Case description
A 39-year-old manager of a grocery store experienced sudden onset of headache while sitting at her desk. Shortly thereafter, her staff noted that she appeared confused and unable to communicate coherently. She was taken to an emergency room, where her blood pressure was found to be 240/160 mmHg. On initial neurological examination, she was awake, appeared alert, but was unable to follow commands due to receptive aphasia. She was attempting to speak, but fluency of language was also disturbed. She had a left gaze preference, but was able to cross midline with her gaze to the right side under visual guidance. She would spontaneously move all extremities, but displayed a mild lag of activation of her right limbs and a drift of her right arm. Her sister, who was her closest relative and listed as emergency contact, reported that she had been diagnosed with arterial hypertension. However, she had not been taking medications in an effort to control her blood pressure by diet and physical exercise. A non-contrasted head CT was performed urgently (Figure 17.2), and showed a large left parietal lobar ICH with diffuse cerebral edema as evidenced by effacement of the cortical sulci, and the finding of pseudo-subarachnoid hemorrhage.
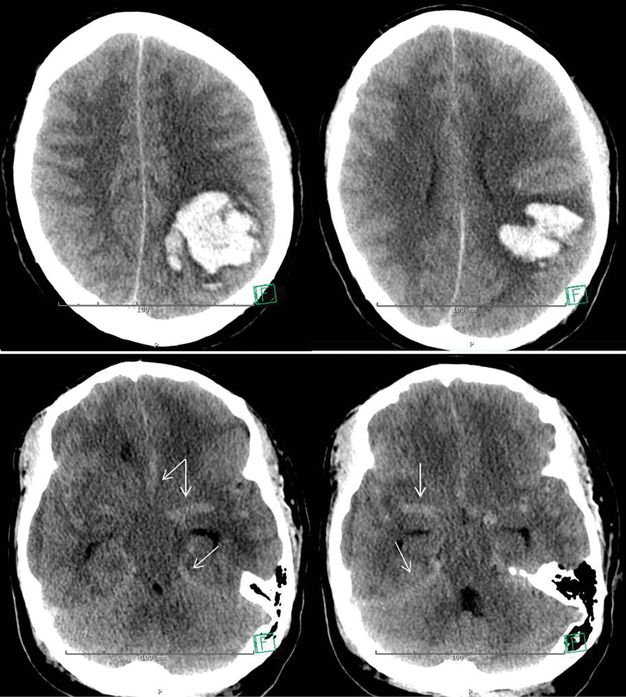
Non-contrast CT of the head, axial images. Upper panel: lobulated intraparenchymal hematoma in the left posterior parietal lobe. Diffuse effacement of the cortical sulci. Lower panel: increased attenuation within the basal cisterns and hyperdense appearance of the circle of Willis and the tentorium, consistent with pseudo-subarachnoid hemorrhage.
A continuous intravenous infusion of nicardipine was started to lower the blood pressure to an initial goal of SBP of 180 mmHg, as recommended in the AHA guidelines for management of ICH [2]. Coagulation parameters and platelet count were normal. Given the diffuse cerebral edema on head CT, the physician team discussed whether to evacuate the hematoma surgically or whether to first insert an intracranial pressure (ICP) monitor to both assess the intracranial pressure and guide the initial blood pressure management. However, while discussing treatment options with the patient’s sister, the patient proceeded to develop a marked right-sided hemiparesis on repeat neurological examination. Her blood pressure was 182/94 mmHg at that time. A CT of the head was repeated, showing the ICH stable in size. On return from the radiology suite, she abruptly deteriorated. She became obtunded, and the right pupil was found markedly dilated at 8 mm and nonreactive to light, as opposed to the left pupil which remained 3 mm in size and reactive to light. She was emergently intubated and taken to the operating room for hematoma evacuation. A postsurgical CT of the head is shown in Figure 17.3.
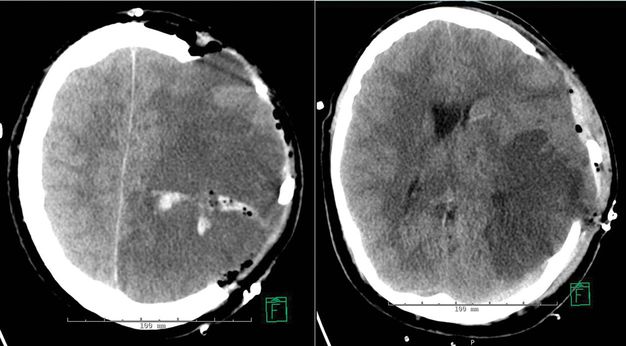
Non-contrast CT of the head, axial images, showing postsurgical changes after a left hemicraniectomy with residual left parietal hematoma, and large area of parenchymal hypodensity in the left cerebral hemisphere. There is marked protrusion of brain parenchyma through the skull defect.
Discussion
This patient presents in hypertensive crisis with an acute ICH. Immediate management consists of initiating blood pressure control in order to lower the risk of continued hematoma growth as well as the systemic effects of a hypertensive crisis. Prior to aggressively lowering the blood pressure, one must consider whether the ICP might be elevated. If that is the case, blood pressure lowering may lower the cerebral perfusion pressure and therefore be detrimental (by hypoperfusion of brain parenchyma) rather than beneficial. While this patient was awake and alert on initial presentation, her CT scan showed a large (48 mL) parietal hematoma with mass effect as evidenced by effacement of the cerebral sulci. Therefore, a discussion about surgical evacuation and ICP monitoring was initiated. However, deterioration in this patient happened abruptly and rapidly. The features of this deterioration, loss of consciousness and a dilated pupil, indicated an uncal herniation syndrome rather than cerebral hypoperfusion. Emergent hematoma evacuation became necessary.
This patient had a large ICH with profound mass effect, as shown in the upper panel in Figure 17.2. Even in the more caudad parts of the brain, the intracranial pressure was elevated. This is evidenced by the so-called pseudo-subarachnoid hemorrhage shown in Figure 17.2, lower panel. The subarachnoid spaces are narrowed by the elevation in ICP and cerebral edema, resulting in cerebrospinal fluid (CSF) displacement. CSF usually appears hypodense on CT scan, so that with its displacement, a larger proportion of relatively hyperdense structures – meninges and blood vessels – become visible, causing the increased attenuation on CT imaging [3]. This may be interpreted as the presence of subarachnoid hemorrhage, while its true meaning is the presence of marked cerebral edema.
One might ask: why was surgical evacuation not performed immediately upon the patient’s arrival? On arrival, this patient was awake with a good mental status, and without clinical signs of herniation. Therefore, possible treatment options were evaluated without anticipation of such a rapid deterioration. Based on currently available data, surgical ICH evacuation remains controversial. On the one hand, surgery may limit the mechanical compression of brain; on the other hand, there are surgical risks, especially when the progression of the hemorrhage has not ceased yet. The best-known study evaluating a possible benefit of surgical evacuation, the STICH trial, concluded that for patients with a superficial ICH (extending to 1 cm or closer to the cortical surface) there might be a benefit of surgery within 96 hours. This interpretation is based on a statistical trend towards a better outcome, but did not reach statistical significance (odds ratio 0.69; 95% confidence interval 0.47–1.01) [4]. However, one of the major critiques of that study was that young patients deemed at risk of herniation were likely not enrolled in the study. This pertained to our patient: given the large size of the ICH and the imaging findings indicative of mass effect throughout the brain parenchyma, the risks of increase of ICP and herniation were recognized, and therefore surgical options were discussed, however, not expecting such a drastic deterioration early on. The current AHA guidelines for treatment of ICH suggest considering evacuation by standard craniotomy for patients with lobar ICH >30 mL and within 1 cm of the surface (Class IIb; Level of Evidence: B), but also to have in mind that very early craniotomy may be harmful due to increased risk of recurrent bleeding [2].
The postoperative images (Figure 17.3) of this patient show the left hemisphere largely hypodense, reflecting a combination of cerebral edema and infarcted parenchyma. Furthermore, there is impressive protrusion of the brain parenchyma through the skull defect. Both findings point out under how much pressure the brain parenchyma was prior to the hemicraniectomy. Due to the rigidity of the skull, downward herniation of brain parenchyma is the only way for brain parenchyma to expand, prior to relieving pressure by opening dura and skull.
During her further hospital course, this young woman was diagnosed with renal artery stenosis, which explained her malignant arterial hypertension. She eventually was discharged to a rehabilitation facility, awake, aphasic, and densely hemiplegic on the right side, but able to participate in a rehabilitation program for her left hemispheric stroke syndrome.
Tip
This case highlights one of the most feared complications of ICH: early deterioration. In this situation, the deterioration was immediately life-threatening due to downward herniation, and the only available and life-saving treatment was emergent hemicraniectomy. While data on the benefit of surgical evacuation of ICH are overall still controversial, it should be considered for patients with large ICH who are deemed at risk of herniation.
Case 3. Coagulopathy-related ICH with expansion
Case description
This 51-year-old man developed sudden weakness of his right arm while at home watching TV. He was able to pick up the phone and notify emergency medical services, however with difficulty in speaking. On initial evaluation in the local emergency department, his blood pressure was 164/93 mmHg. He was found to be awake and alert, but had a right-sided facial droop, was severely dysarthric, had a flaccid plegia of his right arm, and a mild paresis of his right leg. His medical history included arterial hypertension, diabetes mellitus, heart failure, atrial fibrillation, and coronary artery disease. Due to his atrial fibrillation, he was anticoagulated with warfarin. Additionally, he had undergone a coronary catheter angiogram 2 months prior for angina pectoris. Coronary atherosclerosis and stenosis was found, and he had had inserted two drug-eluting coronary stents, for which he was now additionally taking aspirin and clopidogrel.
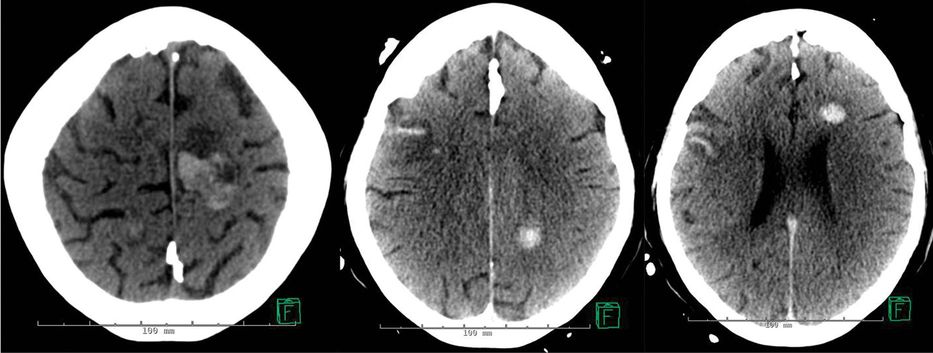
A CT of the brain showed a small hemorrhage in the left basal ganglia (Figure 17.4a). His platelet count was 265 000/µL, and his International Normalized Ratio (INR) was 2.6. He was given 10 mg of vitamin K intravenously, as well as a transfusion of 2 units of fresh frozen plasma (FFP). This reversal therapy was completed 2 hours after his arrival at the emergency room. Repeat testing of the coagulation parameters an hour later showed an INR of 1.8. Two more units of FFP were given, with the follow-up INR reported as 1.4, now 6 hours after initial presentation. However, just upon completion of the transfusions, the patient had a deterioration on his neurological examination. He developed global aphasia and right hemiplegia. A head CT was repeated urgently and showed an expansion of the ICH from 11 to 28 mL (Figure 17.4b).
Discussion
In managing patients with ICH, one immediate goal of treatment is to minimize the risk of ongoing or expanding hemorrhage. This patient had a risk factor for hemorrhage expansion that needed immediate attention: his medication-related coagulopathy. While many patients with ICH cannot provide a history due to their neurological deficits, this patient was able to provide a detailed treatment history of his anticoagulant and antiplatelet medications. Even if patients do not or cannot provide a history relevant to risk of hemorrhage or impaired coagulation, the basic laboratory parameters assessing hemostasis (platelet count, prothrombin time (PT), activated partial thromboplastin time (aPTT)) should immediately be checked, as underlying coagulopathy or thrombocytopenia are potentially treatable culprits for expansion of an ICH. Rapid action is the key: rapid reversal of an elevated INR in patients with ICH has been shown to reduce hemorrhage progression and mortality [5]. Normalizing the INR as soon as possible is also a very important determinant for the success of the INR reversal; every 30 minutes of delay in the first dose of FFP in one study was associated with a 20% decreased odds of INR reversal within 24 hours [6]. A target INR of 1.2–1.5 is usually considered adequate reversal [7,8]. If a patient presents with a subtherapeutic INR, it is less clear whether and how much their risk of hemorrhage expansion is increased. The bulk of experience is derived from patients with therapeutic or supratherapeutic INR values. Data regarding the risk of bleeding with subtherapeutic INR are very limited.
Pharmacologic options for INR reversal include the administration of vitamin K, FFP, prothrombin complex concentrates (PCC), and recombinant activated factor VII (rFVIIa). While FFP used to be the standard therapeutic, there are several unfavorable factors that lead to exploration of different reversal strategies: FFP is a blood product with risk of transfusion reactions; the process of obtaining and thawing FFP prior to infusion often results in a longer time to reversal than desired – this was the case for this patient. Even if FFP is ordered immediately, there will be a delay before receiving and administering it. Furthermore, FFP transfusions lead to considerable amounts of volume being given to a patient. For patients with heart failure, this can lead to increased congestion and respiratory problems. PCC have recently been introduced to reversal protocols, and available data so far are favorable. PCC have several advantages: they can be readily administered, are of low fluid volume, and their effect on INR reversal is faster than that of FFP. The most feared side effects of PCC are thromboembolic complications. With all reversal agents, vitamin K should always be given as an additional therapeutic to boost production of coagulation factors. Given its slower time of onset, it is not sufficient to serve as the only therapy. For newer anticoagulants, such as direct thrombin inhibitors (e.g., dabigatran) or direct Xa inhibitors (e.g., rivaroxaban, apixaban, edoxaban), there are no specific reversal agents and experience in patients with ICH taking these medications is limited. There is some evidence that PCCs may have limited effectiveness in reversing the effect of rivaroxaban but not of dabigatran [9]. The use of rFVIIa in ICH patients on dabigatran has shown theoretical potential [10].
For antiplatelet therapy, data vary regarding the impact on hematoma expansion and outcome for patients presenting with ICH. Overall, increased risk of hematoma growth while on these agents is suggested [11]. However, there are no clear data on whether and how to treat patients who are known to take antiplatelet medication, but have a normal platelet count. Practice for utilization of platelet transfusions or other therapeutics varies widely. Possible therapeutics for reversal of antiplatelet therapy include platelet transfusions, desmopressin, and rFVIIa [12]. The current AHA guidelines state that “the usefulness of platelet transfusions in ICH patients with a history of antiplatelet use is unclear and is considered investigational” [2]. A complicating factor may be the risk that is imposed on a patient when an attempt is made to reverse the antiplatelet effect. The patient in our case example had had recent drug-eluting coronary stents placed. Treatment with antiplatelet therapy is usually necessary for several months due to a risk of stent thrombosis. With reversal of anticoagulation and antiplatelet therapy, this possible complication has to be considered, and the patient needs to be monitored appropriately.
Case 4. Infective endocarditis
Case description
This 61-year-old man had undergone bioprosthetic aortic valve replacement surgery for heart failure 2 months prior to presentation. He had since then resided at a rehabilitation facility where he continued to make progress with physical therapy; however, he was still too weak to walk without assistance. Other relevant medical problems included arterial hypertension, coronary artery disease, and chronic kidney disease. For the heart disease, he had been maintained on dual antiplatelet therapy with aspirin and clopidogrel. During his stay at the rehabilitation facility, he developed shortness of breath along with fever of 39.5°C and was transferred to a community hospital for evaluation. Findings were consistent with pneumonia, and treatment was initiated with antibiotics. However, his respiratory condition worsened and he became lethargic and confused, eventually requiring intubation and mechanical ventilation. Neurological examination at that time was limited by sedation in the setting of mechanical ventilation, but had revealed a markedly depressed level of alertness and attention, suggestive of delirium, and a mild right hemiparesis. For further evaluation, a CT of the head was performed (Figure 17.5), which showed an acute hematoma in the left precentral gyrus as well as several smaller hemorrhagic foci. The patient’s blood pressure and coagulation parameters were within normal limits.
CT of the head, non-contrasted. Axial images. Acute hematoma in the left precentral gyrus, several smaller intracranial hemorrhages, and scattered small areas of subarachnoid hemorrhage in both hemispheres.
This unexpected imaging result prompted referral and transfer from the community hospital to a tertiary center with a neurological intensive care unit. Physical examination on arrival after transfer showed an ill-appearing, afebrile male who was intubated and obtunded. General physical examination revealed a sternotomy scar that was erythematous and mildly swollen, but without dehiscence or drainage. There was a systolic murmur over the left sternal border. On his right foot, there were raised, erythematous lesions on his second and third toe. On neurological examination, he would open his eyes spontaneously, but would not look at the examiner and had a right gaze preference. He was not able to follow commands. Corneal, oculocephalic, and gag reflexes were intact. He displayed some spontaneous movement of his left arm, but no movements were observed in the other limbs. There was minimal withdrawal of his limbs to external stimulation.
Discussion
This patient appears very ill, and has a markedly abnormal neurological examination. The findings on brain imaging show several small intracranial hematomas. These per se would not be expected to cause such fundamental neurological impairment. In a clinical scenario like this, such global neurologic dysfunction could more likely be caused by a severe medical disease such as sepsis or metabolic derangement, or non-convulsive status epilepticus. In a patient with prior heart surgery, especially valve replacement, and intracranial hemorrhage, a diagnosis of septic bacterial endocarditis needs to be investigated and ruled out. Diagnostic work-up will include an echocardiogram and microbiologic blood culture data. If a transthoracic echocardiogram is not revealing, it should be followed by a transesophageal echocardiogram. In this patient, the transthoracic echocardiogram showed large vegetations on both aortic and mitral valves as well as a new dehiscence of the prosthetic valve. Blood cultures grew methicillin-sensitive Staphylococcus aureus. Diagnosis of infective endocarditis is based on the Duke criteria, which define the likelihood of presence of infective endocarditis based on pathologic and major and minor clinical criteria [13]. According to the new modified Duke criteria, our patient displayed two major clinical criteria fulfilling a diagnosis of definite infective endocarditis: blood cultures were positive for infective endocarditis with a typical microorganism on two separate occasions drawn more than 12 hours apart, and he had a positive echocardiogram. He also fulfilled four of the minor criteria: he had a known predisposing heart condition, a fever with temperature greater than 38°C, and Osler’s nodes (tender, erythematous subcutaneous raised lesions that are located on the pulp of the digits of hands or feet, and caused by immune complex depositions), and he was found to have intracranial hemorrhages.
Neurologic complications in patients with infective endocarditis include strokes, intracerebral hemorrhage, brain abscess, meningitis, encephalitis, seizures, and encephalopathy. Their occurrence is common: up to 35% have symptomatic cerebrovascular complications [14], but up to 80% may be found to have cerebrovascular complications on imaging studies. Hemorrhagic strokes can be the consequence of embolic strokes, septic necrotic arteritis, or ruptured mycotic aneurysms. Mycotic aneurysms arise from penetration of septic emboli through the vessel wall which then may rupture and cause ICH or SAH [15]. Identification of neurologic involvement, especially of cerebrovascular disease, often starts with a CT scan of the head, as in our patient. MRI is more sensitive than CT scan, particularly for the detection of small infarcts and microhemorrhages [15]. The appearance of microhemorrhages found in infective endocarditis is different from those in cerebral amyloid angiopathy or hypertensive vasculopathy: Infective endocarditis leads to mostly homogeneous microhemorrhages predominantly located in cortical areas and of small (<5 mm) size. While the mainstay of treatment is systemic antibiosis, urgent cardiac surgery may become necessary if antibiotic therapy fails to stop embolic events, in the setting of heart failure or large abscesses or vegetations. Due to the requirement of cardiopulmonary bypass with heparinization for cardiac surgery, surgery usually has to be postponed for at least a month after occurrence of an ICH. If a patient requires cardiac surgery, screening for microhemorrhages and mycotic aneurysms is usually recommended even in the absence of overt ICH. In a case with presence of ICH, identification of possible mycotic aneurysms is mandatory for treatment planning. Mycotic aneurysms can be detected by both CT angiography and MR angiography (MRA) with high sensitivity (90–95%). While unruptured aneurysms commonly shrink and eventually disappear with antibiotic therapy, ruptured aneurysms require neurosurgical or endovascular treatment in most cases.
Infective endocarditis is a severe illness that often is fatal. The presence of neurologic complications heightens morbidity and mortality in patients with infective endocarditis. This applies especially to staphylococcus endocarditis with neurologic complications, as was the case in our patient, with an estimated mortality of higher than 70%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree