CHAPTER 38 Medical and Surgical Management of Chronic Subdural Hematomas
Chronic subdural hematoma (CSDH) is seen frequently in daily neurosurgical practice. Initially, it was called “chronic subdural haemorrhage of traumatic origin” by Trotter.1 This term anticipates two main aspects of the disease:
Previously, CSDH was seen simply as the chronic form of acute subdural hematoma. It was thought that its development was continuous from acute to subacute and then to chronic subdural hematoma.2 These entities, however, do not share much more than the anatomic location where the hematoma is found. Even though 1% to 6% of patients with untreated acute subdural hematoma experience transformation to CSDH,3,4 the latter develops its own dynamics. Until now, there was only limited understanding of its pathophysiology, which is discussed elsewhere in this volume (see Chapter 37). Major differences between these entities are risk factors, typical age groups, and outcome.
Dramatic improvement in the outcome of CSDH has been achieved as a result of the wide availability of modern imaging methods and refinement of operative techniques.5 Currently, CSDH is considered to be a rather benign disease of the elderly. Nevertheless, the relatively high mortality rates of up to 13% reported in the contemporary literature reflect the fact that as many as four deaths per year are still related to this ailment in a typical neurosurgical department.
History
As early as the Neolithic Period, humans started to open the skull for uncertain reasons. It is unclear whether CSDH was a common condition at a time when people died in their fourth decade. If so, CSDH might have been one of the ailments with a spectacular course of salvation after trephination. There is no doubt that head injury afflicted humanity from its earliest beginnings. Most likely, Stone Age humans had already realized that injury to the head could render an adversary unconscious.6 It has been a matter of debate, however, whether our ancestors from the initial stages of neurosurgery were able to establish a logical relationship between trauma and CSDH on the one hand and trephination on the other.7,8 Clearly, science and magic were interwoven at that time.9 Various diseases were ascribed to evil spirits, and cure was achieved by allowing these spirits to escape from the skull.10–12 Indian folktales, for example, tell us about extraction of worm-like structures from the skull.13 It is conceivable that CSDH, in contrast to other disorders, might have been a basis for such magic embodiment in folktales and myths.
The first pathologic description of CSDH was attributed to Wepfer, who described two cases of “serum accumulation” between the dura mater and pia mater in 1675.14,15 About a century later, Morgagni, the “father of modern pathology,”16 added more cases of “bleeding between the meninges” and speculated about the cause of the “delayed apoplexy.”17 Initially, it was assumed that CSDH was caused by spontaneous bleeding17,18 or trauma.19 Subsequently, the concept of an inflammatory process with lethal hemorrhage into a pseudomembranous sac was favored. The term pachymeningitis hemorrhagica interna was introduced by Virchow.20 Decompression of CSDH was sought via the auditory meatus. The failure of surgical treatment trials, in general, led to the assumption that “pachymeningitis” was an incurable disorder. In the following years, a series of publications reported on lifetime sufferers of pachymeningitis,21–23 and a large number of unfortunate patients spent their life in almshouses and asylums.24
Successful neurosurgical treatment was marked by the publication of Hulke in The Lancet in 1883.25 However, only in the early 20th century did neurosurgery become important in the treatment of CSDH.1 It was the work of Cushing that fueled the development of neurosurgical treatment strategies and advocated for early diagnosis.26 At that time the diagnosis of CSDH depended on the skills of the individual performing the neurological examination. This was limited by the overall low familiarity with the entity of CSDH and the diagnostic dilemma of CSDH not providing pathognomonic signs or symptoms. Osler reported on an autopsy study in which 197 of 1185 patients were found to suffer from CSDH.24 The cardinal symptom was dementia in more than a third of the patients. Underestimation of the incidence was obvious, and scientists as well as physicians tried to find certain characteristics providing diagnostic clues.21,23,27,28
Enabled by the introduction of pneumencephalography by Dandy29 and angiography by Moniz,30 radiologic diagnosis had an enormous impact on diagnostic decision making. As a consequence, CSDH was diagnosed much earlier and surgical outcome improved continuously.31
In 1937, Horrax and Poppen reported a cure rate of 83% after surgery. During the following decades, similar results were achieved by less invasive methods in larger series. Cure rates after simple bur-hole craniotomy (BHC) with saline rinsing2,32 approximated those of large craniotomies with marsupialization of the membranes,33 but the former technique reduced the risk for perioperative morbidity and mortality.34
The problem of reexpansion of the brain was identified as a major cause of recurrence. Treatment concepts were aimed at direct expansion of the brain by provoking cerebral edema,35 reducing venous outflow,31 or expanding cerebrospinal fluid (CSF) volume.36,37 Fitting the skull to its content was another rarely performed procedure in pediatric patients.38 The introduction of closed system drainage eased the problem,39–41 and the establishment of cross-sectional imaging techniques was the last milestone in the treatment of CSDH because it allowed further minimization of neurosurgical approaches.
Definition
CSDH is defined as a fluid collection within the layers of the dura mater.42–44 It must be distinguished from other entities that might have a similar appearance on cerebral computed tomography (CT), including subdural hygroma, formerly called subdural hydroma,45 and external hydrocephalus.46 Although the former has a watery or xanthochromic consistency and often lacks a surrounding membrane, the latter is a variant of hydrocephalus and may develop after tearing of the arachnoid layer in cases of internal hydrocephalus. It is often seen after vascular surgery.47 Subdural hygroma can transform into CSDH. A comprehensive literature review on the relationship of CSDH and subdural hygroma showed that up to 58% of cases of subdural hygroma evolved into CSDH, depending mainly on the length of observation.48
Epidemiology
The incidence of CSDH has been estimated to be 1 to 2 cases per 100,000 inhabitants per year in publications in the early CT era.49,50 According to newer studies, however, the incidence appears to be as high as 13.1 cases per 100,000 inhabitants,51 which might reflect primarily an increase in diagnosis. An increasing frequency is observed in all advanced societies throughout the world, which might also have primarily been driven by an increasing percentage of the population older than 65 years.52–54 Patients older than 40 years account for 80% of cases.32,55 The peak incidence currently occurs in the eighth decade. There is a clear gender difference. In older publications, at least 70% to 80% of patients with CSDH were male.56,57 Newer studies describe a male-to-female ratio of 3 : 2.55,58–61 The higher incidence of CSDH in men most likely reflects the overrepresentation of men with head injury.53
Trauma is probably the most important risk factor for the development of CSDH, with two thirds of CSDH patients remembering some type of minor trauma.53,55,62 Patients with seizures are also thought to be at higher risk.63 Traumatic or postsurgical communication of the subarachnoid space with the subdural space is thought to increase the risk for CSDH considerably.64 As a consequence, neurosurgical treatment of vascular disorders involving opening the subarachnoid space bears the highest risk for surgery-related CSDH.65 CSDH is rarely a complication in sports with a propensity for cranial trauma, such as boxing,66 capoeira,67 soccer,68 and snowboard racing.69
Craniocerebral disorders and therapeutic procedures that alter the volume ratios of intracranial compartments and thereby result in intracranial hypotension also increase the risk for CSDH. Thus, CSDH is seen in conjunction with dehydration70 and with brain atrophy71 or degenerative cerebral diseases such as Huntington’s disease.72,73 CSF shunting is another well-recognized risk factor.74 Up to 8% of patients undergoing shunting for normal-pressure hydrocephalus may suffer from this complication.75 Other procedures that reduce the volume of CSF acutely, such as lumbar puncture,76 spinal anesthesia,77 or transventricular approaches for tumor surgery,65 are seldom followed by the formation of CSDH.
Primary coagulopathies in children,78 as well as secondary coagulopathies or anticoagulant treatment in adults, are further risk factors for the development of CSDH.79 In acute subdural hematoma, the risk increases 7-fold in men and 26-fold in women.80 Similar mechanisms might be involved when CSDH complicates leukemia,81 thrombopathies,82 or hepatogenic coagulopathies.83
Chronic alcoholism is often cited as a major risk factor for CSDH. Kremiansky had already noted the importance of alcoholism in 1868 and provided an animal model in which it was demonstrated that feeding 45% brandy to dogs could cause CSDH.84 In an epidemiologic study from Helsinki, alcoholism was present in as many as 50% of CSDH patients.49 The pathoetiologic mechanisms are not known precisely, but the propensity of alcohol-dependent individuals to experience trauma is considered the single most important cause.53 Other possible factors are alcohol-induced hepatopathy, generalized vascular fragility as a result of avitaminosis, dysfunction of thrombocytes, brain atrophy with intracranial hypotension, and increased serum levels of estrogens.85
Older age predisposes to the development of CSDH in a multifaceted fashion. Older patients suffer falls more often,86 treatment with anticoagulative medications is frequent,87 the risk for concomitant bleeding rises with age88 and, finally, brain atrophy is a physiologic phenomenon in advanced age.89
Diagnosis
Patient History
The clinical picture of CSDH varies widely. There are no pathognomonic signs or symptoms that allow one to make the diagnosis solely on the basis of clinical data. It is not even clear which pathophysiologic aspect causes the clinical symptoms. The variety of mechanisms may include cortical dysfunction, which is responsible, for example, for Gerstmann’s syndrome associated with CSDH,90 adverse effects on the basal ganglia resulting in parkinsonian symptoms,91 conduction failure of fiber tracts leading to hemiparesis,92 or simply decreased regional cerebral blood flow.93–95
According to more recent data, CSDH is asymptomatic in a large number of patients, but it may also cause high intracranial pressure resulting in coma. Between these extreme states, nearly every constellation of speech, sensorimotor, neuropsychiatric, or mood disturbances may occur. It might not be surprising that CSDH was called the “great imitator” in the past.96
Common symptoms in the largest series with medical reports on 2300 patients were simple refractory headache and sensorimotor and neuropsychiatric changes such as amnestic deficits or lack of concentration.62 The development of symptoms is slow in the majority of patients, but stroke-like onset can occur.97,98 A relevant number of patients have seizures of different semiology.63,99–101 Diagnostic evaluation for CSDH has improved in some areas such that no individual in a group of 560 demented patients was referred to a neurological ward with dementia caused by CSDH.102
The use of clinical grading systems for CSDH is reserved for scientific purposes and has no impact on therapeutic decision making (Tables 38-1 and 38-2).103,104
TABLE 38-1 Original Clinical Score Introduced by Bender and Christoff103
| 1 Fully alert and conscious, normal mental function, few or no focal signs |
| 2 Drowsy or lethargic, organic mental syndrome, focal neurological signs |
| 3 Very drowsy or stuporous, conspicuous organic mental syndrome, pronounced focal signs |
| 4 Coma or signs of herniation |
TABLE 38-2 Original Clinical Score Introduced by Markwalder and Reulen104
| 0Patient neurologically normal |
| 1Patient alert and oriented, mild symptoms such as headache, absent or mild neurological deficits such as reflex asymmetry |
| 2Patient drowsy or disoriented with variable neurological deficits such as hemiparesis |
| 3Patient stuporous but responding appropriately to noxious stimuli, severe focal signs such as hemiplegia |
| 4Patient comatose with absent motor responses to painful stimuli, decerebrate or decorticate posturing |
Imaging
Preoperative CT
The introduction of cross-sectional imaging revolutionized the diagnosis of CSDH.105 Because of the widespread use of modern imaging techniques in developed countries, the clinical picture of CSDH has changed within the past 30 years,106 a process that also occurs in developing countries after the introduction of CT.107,108 Cranial CT and magnetic resonance imaging (MRI) made former techniques obsolete. Cerebral angiography and ventriculography, which look for deviation of a calcified pineal body on radiographs of the skull, no longer play a role in the diagnostic evaluation for suspected CSDH. Today, experience with the superior image quality of modern multislice computed tomographic scanners allows one to estimate that the rate of false-negative scans might approximate zero. As a consequence, CT of the cranium is the diagnostic tool of choice in many hospitals.109 It enables establishment of the diagnosis within a few minutes.
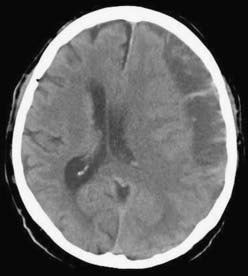
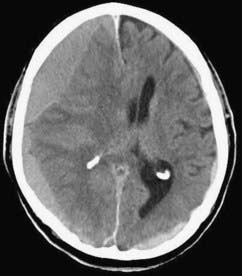
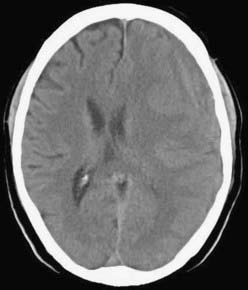
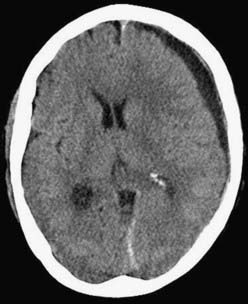
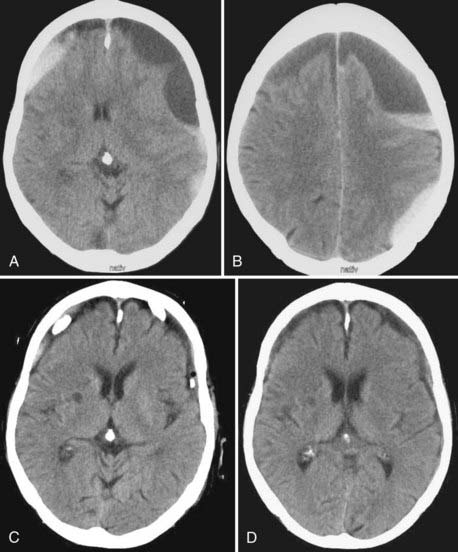
CSDH appears as a sickle-shaped lesion between the cortical surface and the calvaria. The gyri beneath the hematoma may still be visualized, a finding indicating that the arachnoid space is preserved. Compression of the ipsilateral gyri or the ventricle and midline shift are frequent observations. Although in acute subdural hematoma, a midline shift is regularly correlated with signs of elevated intracranial pressure, it is not uncommon in CSDH that shifts of greater than 1 cm are compensated for without neurological deficit. In one study, an average of 90 mL of hematoma fluid was compensated for without a detrimental rise in intracranial pressure.110 The computed tomographic appearance of CSDH varies widely (Figs. 38-1 to 38-5) and has been categorized by different classification systems (Tables 38-3 and 38-4).111,112 The scales were positively correlated with the age of the hematoma, rate of recurrence,113 amount of exudation,114 and the state of consciousness before surgery.115 It was emphasized that the highest risk for recurrence is seen with a mixed-density or layering type of hematoma on cranial CT because both types may be due to recent rebleeding into the cavity.111 An isodense appearance on CT can hamper establishment of the diagnosis, especially in patients with bilateral hematomas, which are characterized by no asymmetry of ventricles or midline shift, although intracranial pressure may be elevated because of the bilateral extracerebral space-occupying lesions. Bilateral hematomas are noted in up to 25% of cases.62
TABLE 38-3 CT Classification According to Nomura and Colleagues111
| 1Hyperdensity |
| 2Isodensity |
| 3Hypodensity |
| 4Mixed density |
| 5Layering type |
TABLE 38-4 CT Classification According to Naganuma and Colleagues112
| 1Homogeneous density |
| 2Laminar type |
| 3Layering or separated type |
| 4Trabecular density type |
Postoperative CT
It is useful to repeat CT after surgery before the drains are removed because there is a recurrence rate of up to 29% after BHC and up to 76% after twist drill craniostomy (TDC). Notably, recurrence after TDC is tacitly accepted as a complication because it is the least invasive technique. Caution must be exercised not to misdiagnose any remaining subdural fluid collection as recurrence after surgery. Residual fluid was present in 78% of cases on day 10 and in 15% in the sixth week after surgery.116 It is therefore recommended that one reoperate only in cases in which the hematoma increases and the patient deteriorates as a result of the recurrent hematoma. In the first days after surgery, intracranial air can be detected as black hypodense areas within the hematoma cavity. The amount of intracranial air after surgery was positively correlated with recurrence.117–119 Severe complications may arise from tension pneumocephalus after decompression of CSDH.120 In bilateral CSDH, a collapsed brain, especially in its frontal aspect, leads to the characteristic “Mount Fuji sign” on CT.121,122
MRI
MRI has the advantage of higher resolution, which allows better differentiation of tissue and fluids.123,124 In children with CSDH, MRI is the preferred diagnostic tool. In the elderly, MRI is considered a second-line option for diagnostic evaluation,109 possibly because of historical, technical, and practical reasons. CT was the first widely available imaging modality that allowed visualization of CSDH, and neurosurgeons may be more familiar with interpretation of blood and its degradation products on CT than on MRI. Furthermore, MRI is a time-consuming and more expensive method, and it depends on a cooperative patient. In that regard, it should be considered that 18% of patients with CSDH have neuropsychiatric deficits when CSDH is diagnosed. Up to 15% of patients are comatose and need intensive care equipment,62 which often is not compatible with the high magnetic fields of MRI scanners.
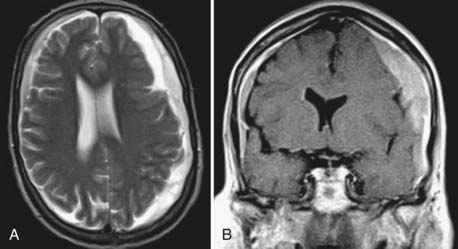
In the majority of cases, CSDH appears hyperintense on T2-weighted (Fig. 38-6A) or proton-weighted images because blood degradation products, especially methemoglobin, give a hyperintense signal on such images. The variability in signal intensity is greater on T1-weighted images. Although nearly 50% of CSDH images appear hyperintense (Fig. 38-6B), hypointense, isointense, and mixed intensities are also seen in CSDH. Hypointense or isointense signals were interpreted as fresh rebleeding into the cavity.123 Therefore, it is not surprising that the recurrence rate is higher with the latter than with high-intensity hematomas on T1-weighted images. The inner architecture of CSDH noted on MRI suggests similarities with the findings on CT, but no correlation was found between the appearance in the two different techniques.109 Hematomas with low density, isodensity, and mixed density on CT may all appear as homogeneous hyperintense lesions on MRI.
CSF is characterized by a hypointense signal on proton-weighted images. Subdural fluid collections with low intensity on proton-weighted images may therefore be lesions other than CSDH.109 Because subdural hygroma is an important differential diagnosis, MRI can provide elementary information to differentiate both entities.
Contemporary Treatment
The approach to management of patients with CSDH ranges from a simple “watch and wait” strategy to large craniotomies with marsupialization of hematoma membranes.5 Pharmaceutical treatment was suggested in the past.103,127,128 Spontaneous resolution of CSDH occurs only rarely.112 Scientific reports on successful nonsurgical treatment of CSDH are also rare and date back to the 1960s and 1970s. The results are controversial and in reality cannot be construed as providing an alternative to surgical treatment. Conservative treatment has thus far included corticosteroids and bed rest. Glover and Labadie demonstrated a reduced rate of membrane formation in an animal model with corticosteroid treatment.129 The effect of dexamethasone was thought to be anti-inflammatory129 or antiangiogenic.130 Another approach included long-term application of mannitol and bed rest.131 The promising results of a pilot study, however, could not be reproduced in a randomized investigation because the side effects of long-term immobilization became more prominent in elderly patients.132 No parameters were defined in the past that might help decide in favor of a nonsurgical approach. It is common sense to carefully monitor asymptomatic patients in whom the presence of CSDH was proved by CT or MRI. Once clinical symptoms develop, however, surgical management is mandatory in the majority of cases.
New pathophysiologic aspects might have an impact on conservative treatment in the future. In particular, detection of the angiogenic cytokines responsible for development of the well-known leaky vessels within the outer membrane of a hematoma might offer new and promising targets to be blocked by pharmacologic agents. Recently, it was shown that the antiangiogenic properties of angiotensin-converting enzyme inhibitors could reduce the rate of recurrences in CSDH, as well as levels of vascular endothelial growth factor within the hematoma.133 Prospective trials with other antiangiogenic substances are under way.
An important issue in the perioperative and postoperative management of patients with CSDH is the question of whether anticonvulsive prophylaxis should be administered. The literature on this topic does not provide any guidelines. As a consequence, a Cochrane review came to the conclusion that because of the controversial findings in mainly retrospective studies, no formal recommendation could be given.100 Detailed data on the frequency of early or late seizures caused by CSDH are not available to date. However, posttraumatic or postoperative epilepsy is thought to have a low incidence in patients with CSDH.134 In a retrospective analysis of their own patient data and data obtained from a literature survey, Rubin and Rappaport found a 5.6% incidence of preoperative seizures versus 3% in the literature and a 4.3% incidence of postoperative seizures versus 1.8% in the literature.101 This is in accordance with a generally low standardized incidence ratio of between 1.5 and 2.8 for epilepsy in patients with mild head injury.135 From a pathophysiologic point of view, epileptic foci develop mainly from cortical injuries.136 Subdural hematoma in the chronic state, however, is an exclusively extracerebral lesion in the majority of cases. Pathoanatomically, a fibrous visceral membrane separates potentially epileptogenic blood degradation products within the hematoma from the cerebral cortex.101 Presumably, a higher risk for seizures might exist in cases in which the inner membrane was opened during surgery. Other risk factors are alcohol abuse and age older than 65 years.101,135
Patient posture in the early days after surgery was also thought to influence the rate of recurrence. In the first prospective study no difference was found with regard to recurrence and outcome.137 A recent randomized controlled trial, however, found an increased incidence of CSDH recurrence when patients did not maintain a flat position in the first 3 days after surgery. The number of adverse events caused by the flat position was equal in both groups. No effect on final outcome was demonstrated.138
Postoperative reexpansion of the brain was found early on to be a factor associated with recurrence and hence considered to influence outcome. Generous hydration of patients with intravenous fluids postoperatively was thought to increase the brain’s volume and decrease the risk for recurrence. However, attention must be paid to elderly patients because postoperative hyperemia in areas of cortex that were covered by hematoma was demonstrated in up to 41% of patients older than 75 years after rapid decompression of CSDH.139 Hyperemia might be one reason for postoperative subcortical hemorrhage140–145 or postoperative hyperperperfusion syndrome in agitated or delirious patients.126
Surgical Treatment
Surgical treatment of CSDH in symptomatic patients is still the “gold standard” of therapy because it allows immediate decompression of the space-occupying lesion and significantly improves outcome.106 However, a standardized approach to the treatment of CSDH does not exist,5 in part because of the fact that until recently, the advances in determining the pathophysiology of CSDH discussed earlier were of little practical relevance for treatment.
CSDH is still one of the most frequent problems encountered in neurosurgery. The progress in its surgical treatment is not comparable, however, to the sophisticated development of treatment concepts and surgical techniques in other subspecialities of neurosurgery, such as functional, spinal, or vascular neurosurgery. Unfortunately, even in light of the relatively frequent occurrence of unilateral and bilateral CSDH, there has been no definitive study or studies to define the best primary treatment. The number of randomized controlled trials on topics concerning CSDH has increased since the beginning of the 21th century, but there is still a surprisingly low level of scientific evidence for the different surgical approaches used for the treatment of CSDH.5 All these studies report successful resolution of hematomas, albeit in a varying number of cases, and there are two possible explanations. First, resolution of hematomas occurs independently of the treatment chosen. Second, CSDH is not a uniform entity, and different manifestations of the disease might demand different approaches. If the latter is true, it offers the possibility of tailoring treatment to an individual patient. One challenge, therefore, is to characterize individual cases according to relevant parameters.
Parameters identified thus far aim at predicting risk for recurrence and complications. Both risks were appreciated by the appearance of CSDH on cross-sectional images. However, to date, the specificity of parameters is not high enough to allow more precise prediction of outcome or to tailor treatment on the basis of these parameters. Furthermore, to the best of our knowledge, no study has compared different approaches for different appearances of CSDH on cross-sectional imaging. Moreover, the very definition of recurrence may vary substantially from site to site. For example, Torihashi and colleagues defined a recurrent CSDH as one in which the increased hematoma volume resulted in a neurological deficit.146 Many neurosurgeons would intervene earlier for recurrence, before the development of a neurological deficit, if the patient suffered severe and progressive headache, which correlates with reaccumulation of fluid and a mass effect in the subdural space. Finally, the issue of recurrence cannot be considered in an isolated fashion. One must also consider the complication rate and morbidity associated with the various treatments, and given the lack of large controlled studies, this is quite difficult. We have therefore tried to treat the problem according to the principles of evidence-based medicine while always keeping in mind that such medicine is not “cookbook medicine”147 and what appears to be the best treatment for a patient population is not necessarily the best option for an individual patient.
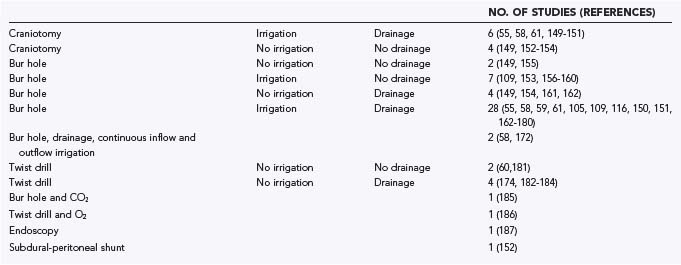
A systematic review of 48 publications from the MEDLINE database and from reference lists was conducted. The articles were written in English or German. Pediatric series and series with more than 10% of patients lost to follow-up were not included. The articles were classified as providing class I, class II, or class III evidence according to the criteria of the American Academy of Neurology.148 The various surgical treatment options are summarized in Table 38-5 and range from single-needle trephination without intraoperative irrigation or postoperative drainage to large craniotomies with marsupialization of the membranes.55,58–61,105,148–187 No study met the criteria for class I evidence, and six studies provided class II evidence. For analysis and comparison of results, the following uniform criteria were defined: morbidity—any complication during or after surgery other than recurrence; mortality—any death reported between surgery and discharge from the hospital; recurrence—clinical or radiologic deterioration requiring further surgery; and cure—complete patient autonomy after surgery (grade 0 or 1 in the classification of Markwalder and associates116 or Bender and Christoff103 and grade 5 in the Glasgow Outcome Scale).188 Morbidity and mortality can be considered as a measure of the safety of a procedure, whereas the cure rate reflects the efficiency of a particular surgical method.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree