CHAPTER 130 Metastatic Brain Tumors
Brain metastases represent a significant source of morbidity and mortality in patients with systemic cancer, as first reported by Bucholz1 in 1898. They are neoplasms that originate in tissues outside the central nervous system (CNS) and spread secondarily to the brain. In adults, cerebral metastases are by far the most common intracranial tumors, and their incidence seems to be rising as systemic cancer therapies have improved, thereby extending patients’ lives. This chapter describes current thought on the epidemiology of brain (parenchymal) metastases and strategies for their treatment by surgery, radiation therapy, radiosurgery, and chemotherapy.
Epidemiology
The incidence of brain metastasis is difficult to determine with precision. The bulk of older estimates originated from historical neurosurgical series, and because neurosurgeons were reluctant to operate on patients with known systemic cancer, these series grossly underestimated the actual incidence of brain metastasis. Similarly, major ascertainment and underreporting problems are limiting factors in obtaining accurate epidemiologic data from large patient populations. In the national survey for intracranial neoplasms reported by Walker and associates,2 only 20% of the metastatic cases diagnosed during 1973 and 1974 were verified by tissue examination. Estimates of incidence from earlier studies of large populations in the United States, Iceland, and central Finland ranged from 2.8 to 11.1 per 100,000 individuals.2–5 More recent series and autopsy studies indicate a much higher incidence of brain metastasis. These studies place brain metastases first in frequency among all intracranial tumors.2,6,7 It is currently estimated that between 100,000 and 200,000 patients will develop brain metastases each year in the United States (the wide range reflects the uncertainty of the estimates). It is also estimated that between one fourth and one fifth of patients with cancer will have brain metastases at autopsy.8–10 This prevalence at autopsy translates into 112,620 to 140,775 cancer patients per year who will die with brain metastases, based on the American Cancer Society’s 1999 estimate of 563,100 cancer deaths11—an increase over previous figures. How much of this increase is real is unclear. An increased incidence of lung cancer and melanoma, longer survival times of patients with cancer, and an aging patient population may have resulted in a true increase. However, a more adequate representation of brain metastasis in more recent neurosurgical series, advances in neuroimaging techniques, and routine staging that assesses the CNS may have artificially inflated the figures.
The incidence of brain metastasis and the spectrum of metastasizing primary cancers vary with patient age.9,12–14 Brain metastases occur more frequently in adults than in children.6,8,15–19 Among adults, the highest incidence is observed in the fifth to seventh decades.9,15 The most common sources of brain metastases in this patient group are cancers of the lung, breast, and skin, in descending order. In children, the most common cause of brain metastasis is leukemia, followed by lymphoma.9 Osteogenic sarcoma and rhabdomyosarcoma are the most frequent causes of solid brain metastases among children younger than 15 years, whereas germ cell tumors are the most frequent producers of brain metastases in patients 15 to 21 years old.15
The overall incidence of brain metastasis is not affected by the patient’s gender, nor is the incidence of brain metastasis from a given primary tumor. The only apparent exception is melanoma, which is more likely to spread to the brain in male patients.9,20,21 The fact that melanoma primaries in males develop in locations that are more likely to spread to the brain, namely the head, neck, or trunk, could explain this observation.22,23 Overall, differences in the incidence of primary cancers between the two sexes result in differences in the sources of brain metastasis in male and female patients. For example, lung cancer is the most common source of brain metastasis in men, whereas breast cancer is the most common source in women.2,9
The primary tumor’s histologic type appears to be the most important dictator of the frequency and pattern of intracranial extension. Lung cancer, breast cancer, melanoma, renal cancer, and colon cancer account for most brain metastases and are listed in order of decreasing relative frequency. Primary lung tumors account for 30% to 60% of all brain metastasis cases.9,24–32 Breast cancer ranks second, contributing 10% to 30% of all brain metastases among women.9,24,25,27,30,32–34 Melanoma ranks third; of patients with brain metastases, approximately 5% to 21% have melanoma as the primary tumor.16,27,28,30–32,35 Renal and colon cancers infrequently metastasize to the brain. Metastases to the brain are even rarer from other types of cancers, such as sarcoma and genitourinary primaries.36–44 Virtually any malignancy can metastasize to the brain, however, and patients with no known history of cancer frequently present with symptoms caused by a brain metastasis from an undiagnosed primary malignancy. The frequency of such presentation varies.45
The picture is different when one considers the ability of a primary tumor to spread to the brain. Interestingly, malignant melanoma, which represents only 4% of all cancers,46 has the highest propensity of all systemic malignant tumors to metastasize to the brain.23,35,47 The incidence of brain metastases among patients with malignant melanoma varies from 6% to 43% in clinical series48–50 and from 12% to 90% in autopsy series23,35,47,51; it was 6.9% in a recent large, population-based study of the incidence of brain metastasis from single primary cancers.52 Lung cancer ranks second in overall number of brain metastases produced. Of patients with lung cancer, 18% to 65% will develop brain metastasis,9,53–55 and the primary tumor histology is very important in determining metastatic frequency. Indeed, more than 40% of patients with small cell lung cancer (SCLC) and lung adenocarcinoma have brain metastases at autopsy, a prevalence of more than twice that found in the other types of lung carcinoma such as squamous cell carcinoma.9,56,57 Breast cancer ranks third in overall contribution to brain metastases. Historically, it has been suggested that approximately 20% to 30% of patients with breast cancer will develop brain metastasis.6,12,33,35,53,58 However, a large population-based study by Barnholtz-Sloan and colleagues52 showed that only 5.1% of breast cancer patients with a single primary tumor developed brain metastasis and that renal cancer had a slightly higher cumulative incidence of 6.5%.
Treatment Modalities
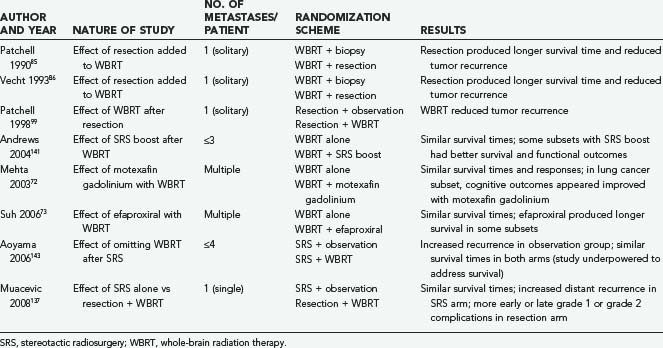
High-dose corticosteroids constitute the initial treatment of patients with symptomatic brain metastases, with the objective of decreasing the edema that typically surrounds these tumors and helping to restore neurological function. Systemic chemotherapy is not very effective against the most common types of primary tumors metastasizing to the brain, which tend to be chemoresistant; however, it appears to be a useful adjunct to other therapies against metastases from SCLC and germ cell tumors. The major weapons in the clinician’s arsenal against brain metastases include whole-brain radiation therapy (WBRT), surgical resection by open craniotomy, and stereotactic radiosurgery (SRS). Table 130-1 lists some key randomized clinical trials relevant to the management of brain metastases.
Radiation Therapy
For the past 50 years, radiation therapy has played a major role in the palliation of metastatic brain disease. In 1954 Chao and coworkers59 were the first to report the use of WBRT for the treatment of brain metastases. Subsequently, numerous publications (reviewed by Sawaya and associates60) have considered the role of WBRT in treating brain metastases. WBRT (20 to 40 Gy delivered over 1 to 4 weeks) results in a median survival time of 4 to 6 months, as established by trials conducted by the Radiation Therapy Oncology Group (RTOG)61 (Table 130-2). In terms of improved symptoms, the published response rate ranges from 70% to 90%.61,62 Headaches, seizures, or symptoms of increased intracranial pressure show a complete response to WBRT in more than 50% of cases, but the durability of that response at 1 year is 65%. Cranial nerve deficits also improve in more than 40% of patients.62
Patient Parameters and Prognostic Factors
Although WBRT can provide effective palliation of brain metastases and can reduce the likelihood of death due to neurological causes, which translates into improved quality of life, patient-related factors such as age, performance status, presence of extracranial metastases, and status of the primary tumor remain the primary determinants of patient outcome.63,64 Patient parameters are an important database for the evaluation of WBRT treatment response and for the prediction of patient outcome when similar patient groups are compared. To identify favorable subgroups of patients for future protocols, Diener-West and colleagues63 used multivariate analysis in a large RTOG study (RTOG 7916) and identified four factors associated with improved survival: Karnofsky Performance Scale (KPS) score of 70 or greater, an unknown or controlled primary tumor, age younger than 60 years, and metastatic spread limited to the brain. Patients with all four favorable characteristics had a predicted 200-day survival of 52%. Patients with none of the favorable factors had a predicted survival time of 1.8 months.63 These prognostic factors were identified again when a database from three consecutive RTOG trials examining dose escalation and radiosensitizers was subjected to recursive partitioning analysis.64 Patients were sorted into three classes. Class 1 included patients with a KPS score of 70 or greater who were younger than 65 years and had a controlled primary tumor and no extracranial metastases; these patients experienced a median survival time of 7.1 months. Class 3 included patients with a KPS score less than 70; they survived for a median of only 2.3 months. Class 2 included all remaining patients.64
Dose-Fractionation Schemes for Whole-Brain Radiation Therapy
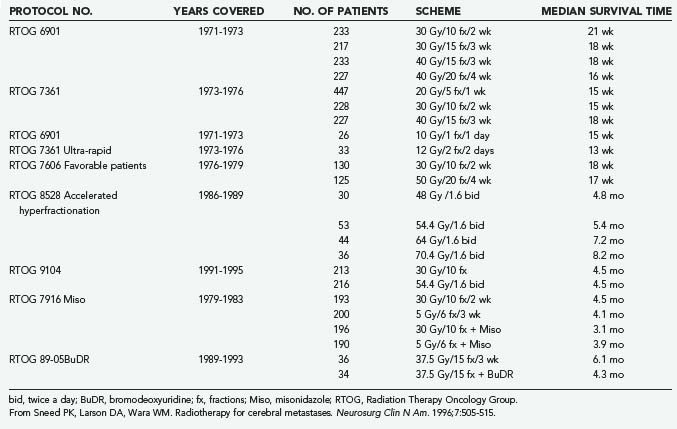
The optimal dose-fractionation schedule was studied by the RTOG using cobalt 60 and linear accelerator equipment (4 to 6 MV). Two studies using five different fractionation schemes were reported together: RTOG 6901 and RTOG 7361 (see Table 130-2).61 All treatment schedules were comparable with regard to frequency and duration of improvement, time to progression, survival, and palliative index.61 The median survival times in these two studies were 18 weeks and 15 weeks, respectively. WBRT improved neurological function in approximately 50% of patients.61 An optional “ultra-rapid high-dose irradiation schedule” was used to randomize patients to treatment with either 10 Gy in 1 fraction or 12 Gy in 2 fractions.65 These patients were compared with control patients receiving 20 to 40 Gy during a period of 1 to 4 weeks. The improvement in neurological function in patients receiving the ultra-rapid treatment was comparable to that of patients receiving more protracted schedules. Promptness of response, morbidity, and median survival time were also comparable. However, duration of improvement, time of progression to improved neurological status, and rate of complete disappearance of neurological symptoms were generally less favorable for patients receiving 10 to 12 Gy, leading the authors to conclude that ultra-rapid schedules may not be as effective as higher-dose schedules in palliating brain metastases.65
A follow-up study of patients from the first two studies who had a favorable prognosis was performed by the RTOG.66 Its purpose was to test the hypothesis that for selected patients with a favorable prognosis, the duration of palliative effect is greater with higher total doses of radiation. The study found no advantage in treating patients with more than 20 Gy in 1 week; thus, it was concluded that this schedule could be used for effective palliation with less inconvenience and cost to the patient, although late effects of radiation were not addressed. Gelber and coworkers66 classified ambulatory breast cancer patients with no soft tissue metastases, ambulatory lung cancer patients with the primary not found or with no extracerebral metastases, and ambulatory patients with other primaries and no extracerebral metastases as favorable subgroups who had a median survival of 28 weeks, in contrast to 11 weeks for the remaining patients. Many radiation oncologists commonly prescribe 30 Gy of WBRT in 10 fractions and adjust fractionation based on expected prognosis. A hypofractionated course of WBRT should be reserved for patients with severely limited life expectancies, and more protracted courses can be given to patients with more favorable prognoses.
Altered Fractionation Schemes
An RTOG phase I-II trial of accelerated fractionation for brain metastases suggested that dose escalation may improve survival.67 An incremental nonstatistically significant improvement in survival was noted with escalating doses. A follow-up randomized phase III study was performed that assigned 445 patients who had not undergone resection and whose KPS scores were 60 or greater to undergo either accelerated hyperfractionation (at a dose of 1.6 Gy twice a day to an end point of 54.4 Gy) or accelerated fractionation (30 Gy in 10 fractions). The phase III trial failed to demonstrate any improvement in survival in the group receiving 54.4 Gy.68
Radiosensitizers
The RTOG evaluated the radiation sensitizer misonidazole by randomly assigning patients to four treatment arms: 3 Gy in 10 fractions with or without a 1 g/m2 dose of misonidazole versus 5 Gy in 6 fractions with or without a 2 g/m2 dose of misonidazole. Patient survival times did not vary significantly among the four treatment arms.69
Bromodeoxyuridine, a halogenated pyrimidine that has been studied in the treatment of malignant gliomas, was evaluated for use against brain metastases in an RTOG trial in which 72 patients were randomly assigned to treatment with WBRT (37.5 Gy in 15 fractions) either with or without bromodeoxyuridine at a dose of 0.8 g/m2 per day for 4 days on each of 3 consecutive weekends.70 Although the drug caused significant grade 4 and grade 5 hematologic and skin toxicity in five patients, there was no significant difference in patient survival between the two treatment arms.
Further investigation of radiosensitizers has continued with the evaluation of gadolinium texaphyrins.71 Motexafin gadolinium (MGd) was used in a randomized controlled trial that evaluated survival as well as neurological and neurocognitive function in 401 patients with multiple brain metastases (including 251 with non–small cell lung cancer [NSCLC]) who underwent WBRT (see Table 130-1).72 Patients were randomly assigned to receive 30 Gy with or without 5 mg/kg per day of MGd. Although overall survival times and responses were similar in the two trial arms, in the subset of patients with lung cancer, MGd administration appeared to produce better cognitive outcomes.
Subsequently, Suh and associates73 conducted a phase III study of the use of efaproxiral, a noncytotoxic radiosensitizer, as an adjuvant to WBRT in 515 patients with multiple brain metastases (see Table 130-1). Again, overall survival times were not significantly different between the groups receiving and not receiving the radiosensitizer, but the subset of patients with breast cancer who received efaproxiral had better survival (although not significantly so).
Prophylactic Cranial Irradiation for Small Cell Lung Cancer
Patients with SCLC may be considered for prophylactic cranial irradiation (PCI) because of their high likelihood of developing brain metastases and consequent neurological deficits. About 10% of SCLC patients have metastasis to the CNS at diagnosis, and another 20% to 25% will develop CNS metastasis later on.74 There is debate regarding the use of PCI because of concerns that it may contribute to neurological deficits. The pertinent issues are quality of life and survival. The Prophylactic Cranial Irradiation Overview Collaboration Group reported a meta-analysis in which data on 987 patients with SCLC in complete remission were collected in seven trials that randomized patients to receive or not receive PCI.75 The main end point of the study was survival. The risk of death in the treatment group relative to the control group was 0.84 (P = .01), corresponding to a 5.4 percentage point increase in the rate of survival at 3 years (15.3% in the control group versus 20.7% in the treatment group). PCI also decreased the relative risk (RR) of recurrence or death to 0.75 (P < .001) and decreased the cumulative RR of brain metastasis to 0.46 (P < .001). Larger doses of radiation led to greater decreases in the risk of brain metastasis, according to an analysis of four total doses (8 Gy, 24 to 25 Gy, 30 Gy, and 36 to 40 Gy) (P for trend = .02), but the effect on survival did not differ significantly by dose. Critics point to the neurocognitive impairment seen in patients who have undergone PCI. However, the two largest trials in this meta-analysis used neuropsychological tests to evaluate most patients before, during, and after treatment; neurocognitive impairment was often detected at initial diagnosis, but no deterioration was found after PCI.76,77 This meta-analysis makes a strong case for using PCI as standard treatment in all patients with SCLC in complete remission. To minimize neurological toxicity, PCI should not be given concurrently with chemotherapy.78 Determining the optimal sequencing and dose of PCI to maximally reduce the incidence of brain metastasis while minimizing toxicity will be the goal of future trials.
Complications of Whole-Brain Radiation Therapy
Acute effects of WBRT include mild fatigue, reversible hair loss, mild scalp erythema, and hyperpigmentation. Somnolence syndrome, described as persistent fatigue, anorexia, and irritability (especially in children), may occur 3 to 10 weeks after WBRT and resolve within 6 weeks.79,80 In long-term survivors with metastatic brain disease, long-term toxicities associated with WBRT can become apparent. DeAngelis and colleagues81 reported a series of 12 patients who developed progressive dementia, ataxia, and urinary incontinence within 5 to 36 months of treatment with WBRT, causing severe disability and leading to death in 7 patients; computed tomography (CT) scans showed cortical atrophy and hypodense white matter in all 12 patients. Nevertheless, Patchell and Regine82 have suggested that the frequency of long-term neuropsychological side effects of WBRT in adult patients with brain metastases may be overestimated. Although 5 of 47 patients (11%) in DeAngelis’s study experienced dementia 1 year after WBRT for nonrecurrent brain metastases, all 5 received either abnormally high daily radiation fractions (3 to 6 Gy—a dose not currently given) or radiation-sensitizing agents, potentially increasing damage to normal tissue.81 Yet none of the 15 patients who were treated with more modern fractionation schemes (<3 Gy/fraction) had dementia at 1 year. Moreover, Langer and Mehta83 recently showed that the risk of neurological decline induced by recurrent disease outweighs the potential loss of neurocognitive function after WBRT. Although the untoward long-term effects of WBRT are probably less significant than previously thought, neurocognitive decline remains a possible complication, and it may be reasonable to consider administering WBRT in daily fractions of 1.8 to 2 Gy to a total of 40 to 45 Gy to reduce long-term sequelae in patients with more favorable prognoses.
Surgical Resection
Surgical resection is an important component in the therapeutic arsenal for cerebral metastases. Although initial reports from the early 20th century concluded that surgery was not warranted because of high morbidity and poor postoperative survival,84 advances in surgical technique since the 1970s have dramatically decreased the operative complication rates and increased survival times. Most important, two prospective randomized trials from the early 1990s (see Table 130-1) demonstrated that surgery followed by WBRT is superior to WBRT alone for patients with single brain metastases and good neurological performance scores.85,86 Moreover, recent reports have suggested that surgery may benefit patients with multiple or recurrent metastases, who have traditionally been excluded from surgical intervention.87,88 Thus, in the modern era, surgery is often considered the primary and optimal treatment of brain metastases.
Surgery has certain advantages over other treatments. First, complete excision of a metastatic lesion provides palliation by immediately eliminating the effects of increased intracranial pressure and the direct irritation of surrounding brain tissue. This effect may be greater for metastases than for primary intraparenchymal tumors because metastases grow by expansion and compression rather than by infiltration and often produce a large amount of edema. Although corticosteroids may provide immediate palliation of symptoms, their effects are not long lasting. Second, surgery provides tissue to confirm the diagnosis of metastasis. This is important because as many as 10% to 15% of patients with a clinical diagnosis of metastasis may actually have nonmetastatic lesions such as abscesses or primary tumors.85 Last, surgery may provide local cure if all the tumor cells are removed. These advantages must be weighed against the requisite invasiveness of surgery, which subjects patients to potential intraoperative and postoperative problems, including bleeding, wound infection, pulmonary emboli, myocardial infarction, and sepsis.
Patient Selection and Prognostic Factors
Radiographic Features
Tumor Number
Patients with single brain metastases are the most appropriate surgical candidates. Oldberg89 was the first to recognize that surgery for single brain metastases could result in longer survival times than other treatments. Multiple retrospective surgical series consistently verified this finding, but it was not until Patchell and colleagues85 and Vecht and associates86 reported the results of their randomized prospective trials that surgical resection became the standard treatment for single brain metastases. These studies demonstrated that patients with single metastases who were treated with surgery and radiation lived statistically longer, had fewer recurrences, and had a better quality of life than patients treated with WBRT alone. One caveat about these studies is that they included patients with limited systemic disease and those with a KPS score greater than 70.90 This is important because a randomized study by Mintz and coworkers91 that failed to demonstrate any advantage of surgery over WBRT included many patients with extensive systemic disease and poor performance status. Thus, the value of surgery for single brain metastases may apply only to patients with the potential for long-term survival (see later).
For patients with multiple metastases, the role of surgery is more controversial. The historical bias against resecting multiple brain metastases was questioned by Bindal and coworkers,87 who retrospectively reviewed 56 patients who underwent resection of two or three brain metastases at The University of Texas M. D. Anderson Cancer Center. Among these patients, 30 had one or more lesions left unresected (group A), and 26 underwent resection of all lesions (group B). These patients were compared with 26 matched controls with single surgically resected metastases (group C). There was no difference in surgical mortality (3%, 4%, and 0% for groups A, B, and C, respectively) or morbidity (8%, 9%, and 8%), regardless of treatment group. Patients with multiple metastases that were all resected (group B) had a significantly longer survival time (median, 14 months) than patients with multiple metastases in whom some lesions were not resected (group A; median survival, 6 months). The survival of patients in group B was similar to that of patients with resected single metastases (group C; median survival, 14 months). Thus, the authors concluded that resecting multiple brain metastases (typically two to four) is as effective as resecting a single brain metastasis as long as all the lesions are resected. Although intriguing, these results have not been confirmed by a prospective randomized trial. Moreover, it is important to note that no more than three metastases (per patient) were treated in Bindal’s study, and most patients received WBRT after surgery.
Tumor Size
The size of a metastasis also influences surgical decision making. Although tumor size has never been shown to influence survival after surgery, it has become an increasingly important factor in decision making because of the possibility of treating metastases with SRS (discussed later). Tumors can be divided into three groups, according to size. First are tumors greater than 3 cm in maximal diameter. For such large tumors, surgical resection is the primary and best option because these lesions are too large for SRS. Second are very small tumors measuring less than 5 mm in maximal diameter. For these lesions, SRS is probably most appropriate (see later), particularly if they are located deep within the brain. In our experience, the trend toward screening cancer patients for brain metastases using contrast-enhanced MRI has resulted in the early detection of small, asymptomatic lesions that would not have been detected in the past, even with CT. It can be argued that these tumors are most suitable for SRS, considering the potential difficulties associated with locating such small lesions during surgery.92 Last are intermediately sized metastases that typically range from 1 to 3 cm in greatest diameter. Decision making is particularly challenging for these lesions because in many cases surgery and SRS may be considered equally appropriate. This is the group for which a randomized prospective study is required to determine which approach is more efficacious (see later). However, until such a trial is completed, the decision for surgical intervention must rely on an assessment of other variables such as the potential for surgical morbidity, the need to reverse neurological deficits, the extent of systemic disease, and the presence of medical comorbidities.
Clinical Assessment
The most significant determinant of a patient’s ultimate outcome is the status of the systemic disease, which is defined as the activity and extent of the primary tumor and systemic (noncerebral) metastases. The importance of systemic disease status in determining outcome has been emphasized in nearly all the studies examining factors that predict survival. Moreover, in the prospective randomized trial of Patchell and colleagues,85 as many as 70% of patients undergoing surgery for single brain metastases died from progression of systemic disease rather than from neurological causes. The importance of the extent of systemic disease was further illustrated in the prospective study of Mintz and coworkers,91 who failed to find any benefit of surgery compared with standard fractionated WBRT for patients with single brain metastases. In this study, more than 45% of patients had external metastases, and 41% had KPS scores of 50 or less. In contrast, only 38% of patients in Patchell’s study had external metastases, and all patients had KPS scores of 70 or greater.85 Thus, these studies suggest that extensive external disease may make it more difficult to detect a survival advantage for surgery. It has been suggested that to reap the benefits of surgical resection, patients should have a life expectancy of more than 3 to 4 months, based on the extent and activity of their systemic disease. To state it another way, surgery is most beneficial to patients with absent, controlled, or limited systemic disease.
Postoperative Whole-Brain Radiation Therapy
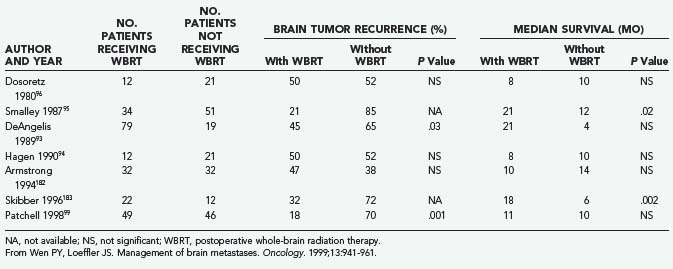
The role of postoperative WBRT has not been clearly defined. WBRT theoretically destroys residual cancer cells at the site of resection, as well as microscopic deposits at other sites. However, it is unclear whether postoperative adjuvant WBRT should be given to all patients after surgical resection. Although several retrospective studies examining WBRT after resection of single brain metastases have shown a beneficial effect,93–95 others have not shown any benefit (Table 130-3).31,96,97 Moreover, WBRT is associated with a significant risk of dementia and other long-term neurotoxicities. Looper and associates98 noted a high incidence of severe neurological problems after combined modality therapy (chemotherapy and WBRT) in long-term survivors with SCLC. Sundaresan and Galicich31 noted that after surgical resection, 50% of 2-year survivors developed hydrocephalus ex vacuo or evidence of leukoencephalopathy on CT scans; almost 17% developed evidence of radiation necrosis.
Patchell and colleagues99 reported the results of a randomized prospective trial examining the benefits of adjunctive WBRT in the surgical treatment of single brain metastases (see Table 130-1). After surgery, patients were randomly assigned to either treatment with 50.4 Gy over 5.5 weeks or observation (median follow-up, 43 weeks), and the patients were classified according to extent of disease and primary tumor type. The patients who received WBRT showed a striking reduction in tumor recurrence (distant and local) relative to the observation group (18% versus 70%; P < .001). The local recurrence rate was 20% in the surgery only group and 3% in the surgery plus WBRT group, and patients in the radiotherapy group were less likely than those in the observation group to die of neurological causes (14% versus 44%; P = .003). Nevertheless, overall patient survival was not improved by adjunctive WBRT. Moreover, the KPS scores for patients undergoing WBRT declined at the same rate as the scores of those in the surgery only (observation) group, raising the possibility that the toxicity of WBRT offsets its beneficial effect. An unexplained result was that among patients who died from systemic disease, those not receiving WBRT survived longer than those in the observation group. Although the authors concluded that WBRT is a valuable adjunct to surgical resection (partly on the basis of preventing death from neurological causes), the lack of overall survival improvement, the use of a higher than standard radiation dose (50 Gy rather than 30 Gy), and the potential for radiation toxicity leave some unresolved questions regarding the best treatment for patients with single brain metastases. Confirmation of these findings with more careful assessments of cognitive function would be helpful.
For patients with tumors having so-called radioresistant histologies, including metastatic melanoma and RCC, postoperative WBRT is controversial. Based on the study by Patchell and colleagues,99 it is difficult to draw conclusions for patients with RCC or melanoma because each arm of the study contained only one melanoma patient and an unspecified number of RCC patients. A randomized trial of postoperative WBRT exclusively for RCC or melanoma patients is needed to resolve the controversy.
Surgical Techniques
Metastasis Anatomy
As viewed microscopically, brain metastases are composed of a solid tumor mass without intervening brain tissue. There may be some degree of infiltration, but this typically does not extend beyond a radius of 5 mm from the solid tumor.31,60,100 There may be a central area of necrosis in larger lesions. At the macroscopic level, typical cerebral metastases are round and well demarcated from the surrounding edematous brain. Tumor cysts may also be present, particularly in metastases from bronchogenic carcinoma. At surgery, a gliotic pseudocapsule is often identifiable surrounding the metastasis. Dissection in this gliotic plane generally ensures gross total resection because there are typically no tumor cells in this zone. The tumor mass corresponds to the region of contrast enhancement seen on CT or MRI.101
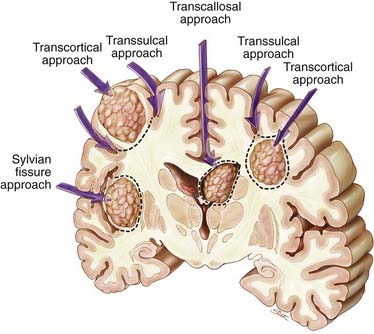
Supratentorial metastases can be surgically defined by their relationship to adjacent sulci and gyri (Fig. 130-1).101–103 Metastases may occur superficially just below the cortex, filling a gyrus (subcortical); deep within a sulcus, either at the side of the sulcus (subgyral) or at its base (subsulcal); or deep within the white matter, independent of a single sulcus or gyrus (lobar). These same patterns may arise near the cerebral fissures. For example, tumors in the subinsular cortex are deeply located relative to the sylvian fissure. Midline metastases, such as those in the cingulate gyrus, should be viewed in relation to the interhemispheric fissure. Metastatic tumors occasionally arise within the ventricles (see Fig. 130-1).
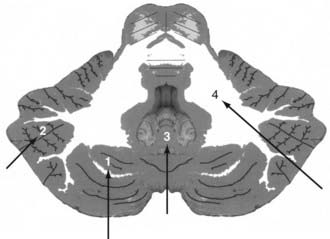
The less common cerebellar metastases can be divided into deep and hemispheric locations. Hemispheric lesions can be categorized as lateral or medial; a subset arises directly within the vermis. Cerebellar tumors can be subdivided into those occurring in superior and inferior locations (Fig. 130-2).
Surgical Approaches
Surgical approaches are based on the anatomic location of the brain metastasis.101,102 Supratentorial subcortical lesions are best resected by an incision in the apex of the sulcus and circumferential dissection of the tumor (transcortical approach; see Fig. 130-1). Removal of a cortical plug above the lesion improves exposure. This may be problematic when the lesion arises within eloquent cortex. In these situations, a longitudinal incision dictated by local mapping with direct brain stimulation and, for large metastases, an “inside-out” piecemeal (rather than en bloc) resection may minimize injury to the surrounding brain.
Lesions in the subgyral or subsulcal location are best approached by splitting the sulcus leading to the lesion. Subgyral tumors are removed by making an incision in the side of the split sulcus, whereas subsulcal lesions are entered at the sulcal base (transsulcal approach; see Fig. 130-1).
Metastases located deep within the white matter, independent of a single sulcus or gyrus (lobar), can be approached either transcortically or transsulcally (see Fig. 130-1). Tumors in the subinsular cortex can be approached by splitting the sylvian fissure. Midline metastases are best approached by splitting the interhemispheric fissure. Tumors can then be resected by further splitting or entering a deep gyrus (see Fig. 130-1). Intraventricular lesions can be approached transcallosally or transcortically (Fig. 130-3; see also Fig. 130-1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree