CHAPTER 371 Microsurgery of Vertebral Artery, Posterior Inferior Cerebellar Artery, and Vertebrobasilar Junction Aneurysms
Clinical Significance
Cruveilhier1 in 1829 was the first to report a vertebral aneurysm when he described a spherical aneurysm arising from the vertebral artery–PICA junction. Krayenbuhl2 was the first to show a vertebrobasilar aneurysm by positive contrast angiography in 1941. Dandy3 in 1944 described his surgical experience with treating three vertebrobasilar aneurysms. In 1947, Rizzoli and Hayes4 performed trapping of a vertebral aneurysm between two silver clips (reported in 1953), and in 1948, Schwartz5 described a suboccipital approach for direct surgical treatment of a posterior fossa aneurysm. Early surgical experiences with aneurysms in the vertebrobasilar system were met with varied success. High morbidity and mortality rates and complications plagued early neurovascular surgeons, making many reluctant to surgically treat vertebral and vertebrobasilar aneurysms aggressively. However, the introduction of the microscope ushered in new approaches to posterior circulation aneurysms.6 Drake7 was the first to report a successful large series of surgically treated vertebral and vertebrobasilar aneurysms. Subsequent advances in skull base approaches, as well as improvements in microneurosurgical technique and development of better pharmacologic brain protection, have contributed to better outcomes. A variety of surgical approaches have been developed to attack lesions at different locations in the vertebrobasilar system.8–11
Vertebral, PICA, and vertebrobasilar junction aneurysms are relatively uncommon lesions. In the study by Pia,12 posterior circulation aneurysms, including PICA and basilar aneurysms, accounted for about 8% to 9% of all intracranial aneurysms. Of these, aneurysms from the proximal vertebral artery, the vertebral artery–PICA junction, the distal PICA, and the distal vertebral artery make up 25% of all posterior circulation aneurysms and 2% of all intracranial aneurysms.12,13 More recently, the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage14 found 21 (0.8%) vertebral artery aneurysms, 4 (0.1%) vertebrobasilar junction aneurysms, and 13 (0.5%) PICA aneurysms from a total of 2672 single-site aneurysms; thus, vertebral artery, PICA, and vertebrobasilar junction aneurysms account for 1.4% of all intracranial aneurysms. In a Japanese 30-year autopsy study of 1230 consecutive cases,15 73 intracranial aneurysms were found in 57 cases (4.6%). Seven of the aneurysms were located at the vertebral artery or PICA, accounting for 9.6% of all intracranial aneurysms.
There is also a high incidence of fusiform nonsaccular aneurysms at the vertebrobasilar locations, much more frequent than in the anterior circulation. Many of fusiform aneurysms that occur at the vertebral artery are due to dissections and carry with them a high risk for bleeding and of rebleeding once hemorrhage has occurred. Yamaura and colleagues16 found vertebral dissections to account for 28% of posterior circulation aneurysms in their series. The rebleeding rate from ruptured dissecting aneurysms has been reported to be 21% to 71%,16–19 with the highest risk in the acute stage after initial bleeding. Many neurovascular surgeons believe that they cannot be left untreated. Because of their morphology, however, fusiform aneurysms, dissecting or nondissecting, often are not amenable to standard aneurysm clipping techniques. Neurovascular surgeons have been forced to devise alternative treatment strategies for these lesions. One surgical strategy has been deliberate therapeutic occlusion of the parent vertebral or basilar artery, otherwise known as proximal hunterian ligation.20–22 The advent of endovascular approaches has ushered in a new and more promising strategy for treating fusiform aneurysms.23–26 These techniques are described elsewhere in this text and involve proximal endovascular occlusion, lesion trapping, and stent reconstruction.
Indications
Although vertebral artery and vertebrobasilar aneurysms are relatively rare compared with intracranial aneurysms found at other locations, ruptured vertebral artery and vertebrobasilar aneurysms carry a high risk for rebleeding as well as high morbidity and mortality rates. Hernesniemi and colleagues27 reported fatal rebleeding in 10% of vertebrobasilar aneurysms, 3 times the number (3.4%) in their anterior circulation aneurysm group.
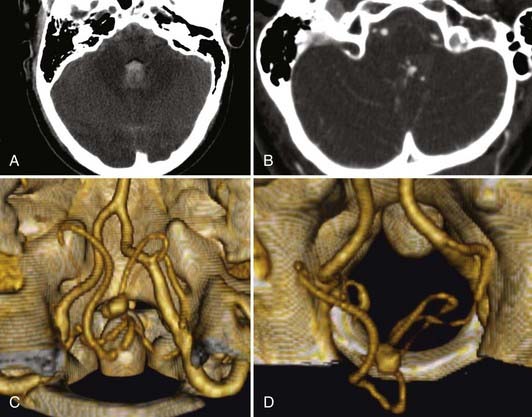
The 2-month mortality rate for untreated ruptured vertebrobasilar aneurysm in the Hernesniemi series was 63%, compared with 8% to 11% for the surgically treated group, and at 1 year, untreated vertebrobasilar aneurysms carried an 83.3% mortality rate.27 In Schievink and associates’ series of 136 aneurysmal subarachnoid hemorrhages (SAHs), the 48-hour mortality rate in ruptured vertebral artery aneurysms was noted to be 80%, compared with 23% in patients with ruptured anterior circulation aneurysms.28 For these reasons, it is generally believed that ruptured vertebral artery and vertebrobasilar aneurysms cannot be left untreated (Fig. 371-1).
Ruptured vertebral dissecting aneurysms carry a worse prognosis than saccular aneurysms of the same location. Kawagushi and associates17 reported in their series of vertebral dissecting aneurysms a 21% rebleeding rate in the first 72 hours with 100% mortality. Aoki and Sakai18 reported 30% rebleeding with 80% mortality occurring in the first few hours. Mizutani and colleagues19 reported 71% rebleeding, with the mortality of the rebleeding group being 46.7%, compared to a mortality of 8.3% in the non-rebleeding group. Some authors have advocated a conservative approach of anticoagulation and serial angiography in managing vertebral dissections. Kitanaka and associates29 managed six patients with unruptured vertebral dissections conservatively and followed them with serial angiography. There was no episode of SAH, and there was angiographic cure in four of the patients. All the patients had unruptured vertebral dissections, however, with no cases of ruptured vertebral dissections.
The timing of surgery for ruptured vertebral artery and vertebrobasilar aneurysms is still debated. The International Cooperative Study on the Timing of Aneurysm Surgery30,31 did not include enough vertebrobasilar aneurysm to make any significant conclusions about the timing of surgery for aneurysm at this location. Posterior circulation aneurysms differ from anterior circulation aneurysms in that rarely are emergent life-saving operations for hematoma evacuation necessary, whereas they more commonly are necessary with rupture of aneurysms in the anterior circulation. Ventricular drainage can be a necessary emergent procedure, however, because many patients with rupture of vertebral artery or vertebrobasilar aneurysms have SAH complicated by hydrocephalus. In the current era, early surgical or endovascular treatment of the ruptured aneurysm is generally the rule.
The high management morbidity and mortality associated with surgical treatment of ruptured aneurysms in this location have made neurovascular surgeons reluctant to treat these lesions aggressively in the acute setting. Early surgery often is confounded by a brain that is frequently more swollen with confining exposure, making the delicate perforating vessels difficult to visualize. Also, in the acute period, the aneurysm’s dome may be more friable and at greater risk for intraoperative rupture from manipulation. Although the general trend in the 1990s for anterior circulation aneurysms was early surgery, for posterior circulation aneurysms, most centers at that time adopted a protocol of delayed surgery. A few authors have advocated early surgery, however.2,32,33 The high morbidity and mortality rates associated with early rebleeding argue for early clipping of the ruptured aneurysm. In addition, with the aneurysm secured, the patient may undergo earlier and more aggressive vasospasm management. Hernesniemi and colleagues27 reported 63 patients operated on for ruptured vertebrobasilar aneurysms—36 patients in the first 6 days of SAH and 27 patients in 7 or more days after SAH. Outcomes at 1 year in the early-surgery group were 55.6% good recovery, 16.7% moderate disability, 19.4% severe disability, and 8.3% dead. Outcomes at 1 year in the late-surgery group were 59.3% good recovery, 11.1% moderate disability, 7.4% severe disability, and 22.2% dead. In an untreated group of 30 patients, 1-year outcomes were 6.7% good recovery, 6.7% moderate disability, 3.3% severe disability, and 83.3% dead. Lanzino and associates34 reported their results with a protocol for delayed surgery for vertebrobasilar aneurysms and concluded that early surgery would be preferable. Although they achieved 87% excellent or good surgical outcome and 8% surgical mortality, an additional 41% died under the delayed treatment protocol before reaching surgery. Peerless and associates32 reported an extensive series from 1959 to 1992 of 1767 patients with vertebrobasilar aneurysms. Because of their referral pattern, most of these aneurysms were operated on 14 or more days after SAH. Since 1970, these investigators operated on 206 ruptured vertebrobasilar aneurysm in 7 days or less after SAH. In the 206 early-operated patients, outcomes were 62.6% excellent, 18.9% good, 8.7% poor, and 9.7% dead. Bertalanffy and coworkers33 reported that their group operated on ruptured vertebrobasilar aneurysms in the acute stage. Of 17 vertebrobasilar aneurysms, they reported a 94% good result rate and a 5.9% mortality rate. Arguments for the timing of endovascular treatment of ruptured posterior circulation aneurysms are still in their infancy.
Small case series of mainly anterior circulation aneurysms have supported earlier intervention. Van Loon and others35 reported 11 patients presenting with SAH and poor neurological grades, of which 10 were treated with endovascular techniques within 24 hours. Mean follow-up at 12 months showed 4 patients with good Glasgow Outcome Scale (GOS) scores, 2 who were moderately disabled, 3 who were severely disabled, and 2 who died as a result of uncontrollable intracranial pressure. Of the 11 ruptured aneurysms, 2 involved the basilar artery. While the timing for posterior circulation aneurysms remains under investigation, the data from the surgical literature would suggest favoring earlier intervention. Endovascular techniques allow securing of the aneurysm to prevent the high risk for rebleeding while avoiding the technical challenges of working with a swollen and injured brain. Whether early or late, there is not much question that ruptured vertebral artery, PICA, and vertebrobasilar junction aneurysms should not be left untreated except in the extenuating circumstance in which the patient is medically unstable or the treatment comes at unacceptably high risk.
The natural history of unruptured vertebral artery, PICA, or vertebrobasilar junction aneurysms without treatment is less clear, however. Nevertheless, a 1998 international cooperative study36 of 1937 unruptured intracranial aneurysms found a 13.6 relative risk for rupture in the group of patients with vertebrobasilar artery and posterior cerebral artery aneurysms that had never had a history of SAH compared with patients with intracranial aneurysms of other locations (P = .007). The follow-up study in 2003 by the International Study of Unruptured Intracranial Aneurysms37 committee evaluated 1077 patients without history of SAH, of which 1591 were treated with open surgical repair and 409 were treated by endovascular techniques. Their review found that aneurysms involving the vertebrobasilar artery, posterior cerebral artery system, and posterior communicating artery had increased 5-year cumulative rupture rates in patients without history of SAH compared with anterior circulation aneurysms of comparable sizes. Specifically, aneurysms smaller than 7 mm, 7 to 12 mm, 13 to 24 mm, and larger than 25 mm had relative rupture risks of 2.5%, 14.5%, 18.4%, and 50%, respectively, in the posterior circulation, compared with 0%, 2.6%, 14.5%, and 40%, respectively, in the corresponding anterior circulation group. These rates were equaled or exceeded by risks associated with surgical or endovascular repair of comparable lesions. In the multivariate analysis of the unruptured aneurysms treated by open surgery, worse 1-year outcomes were predicted by age greater than 50 years (relative risk of 2.4 [1.7 to 3.3], P < .0001), size greater than 12 mm (relative risk of 2.6 [1.8 to 3.8], P < .0001), and location in the posterior circulation (relative risk of 1.6 [1.1 to 2.4], P < .0001). For the endovascular cohort, poor outcome was again predicted by size greater than 12 mm (relative risk of 2.4 [1.0 to 5.9], P = .03) and location in the posterior circulation (relative risk of 2.25 [1.1 to 4.4,], P = .02). In a 2007 meta-analysis of unruptured aneurysms,38 the relative risk for rupture from an aneurysm in the posterior circulation compared with aneurysms involving the internal carotid artery, including the posterior communicating artery, was 2.5 (1.6 to 4.1). Ruptured risks based on size showed increased risk in aneurysms 5 to 10 mm, with a relative risk of 2.3 (1.0 to 5.2), larger than 10 mm with a relative risk of 2.9 (1.5 to 5.7), larger than 12 mm with a relative risk of 7.5 (3.8 to 14.9), and larger than 15 mm with a relative risk of 11.9 (5.5 to 25.8). This study also found that age older than 60 years carried an increased relative risk of 2.0 (1.1 to 3.7).
Presentation
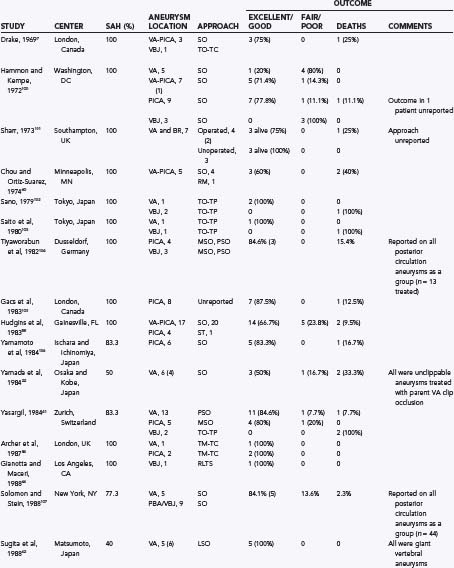
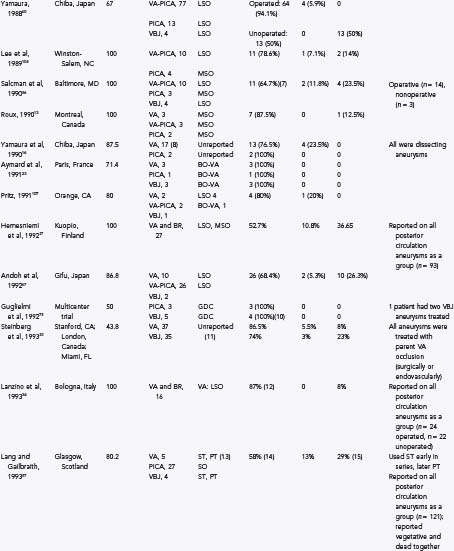
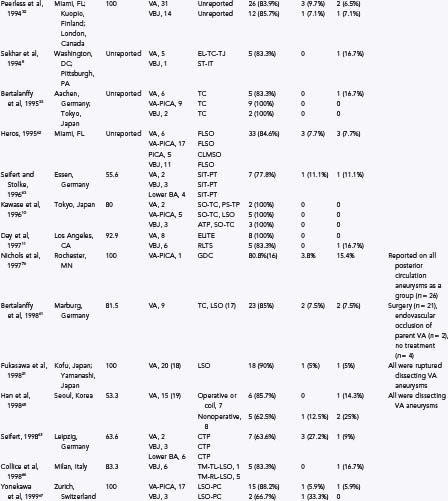
The most frequent clinical presentation of vertebral artery, PICA, or vertebrobasilar junction aneurysms is SAH. SAH was the primary presentation for all or most aneurysms at these locations in the published series in Table 371-1. These clinical conditions of patients with SAH from ruptured vertebral artery, PICA, or vertebrobasilar aneurysms can range from relatively good condition (Hunt-Hess39 grade I or II) to poor or comatose (Hunt-Hess grade IV or V). In the report by Peerless and associates32 of 206 ruptured vertebrobasilar aneurysms (this included basilar bifurcation, basilar trunk, and posterior cerebral artery aneurysms), 51.9% presented as Hunt-Hess grade I, 30.1% presented as Hunt and Hess grade II, 10.7% presented as Hunt-Hess grade III, 4.9% presented as Hunt-Hess grade IV, and 2.4% presented as Hunt-Hess grade V. In the series by Bertalanffy and coworkers of 27 patients with vertebral artery–PICA complex aneurysms, 22 (81.5%) presented with SAH. Of these patients, 2 presented with Hunt-Hess grade I (9.1%) disease, 2 presented with Hunt-Hess grade II (9.1%), 11 presented with Hunt-Hess grade III (50%), 4 presented with Hunt-Hess grade IV (18.1%), and 3 presented with Hunt-Hess grade V (13.6%). In Yamaura and coworkers’16 series of 24 vertebral dissections, 87.5% presented with SAH, and 12.5% presented with ischemic symptoms.
TABLE 371-1 Some Previously Reported Series of Vertebral, Posterior Inferior Cerebellar Artery, and Vertebrobasilar Aneurysms

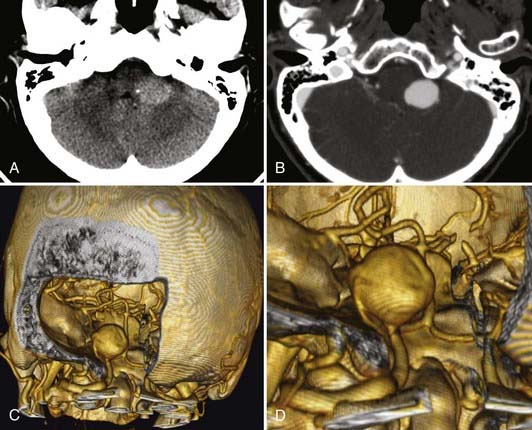
Unruptured vertebral artery, PICA, or vertebrobasilar aneurysms often present with signs and symptoms of ischemia or mass effect, such as lower cranial nerve deficits, brainstem compression, or posterior fossa symptoms. In Yamaura’s40 series of 94 vertebral artery aneurysms, 31 patients presented with unruptured aneurysms, of which 16 presented among multiple aneurysms (17%), 6 (6%) presented as mass effect, 3 (3%) presented with ischemic symptoms, and 6 (6%) presented incidentally during evaluation of unrelated disease. In Yasargil’s41 series, the patients with unruptured vertebral artery aneurysms presented with lower cranial nerve deficits (V through XII), hemiparesis, and bulbar deficits. In the series by Sugita and associates42 of giant vertebral artery aneurysms, the patients who did not present with SAH presented with lower cranial nerve deficits (dysarthria, dysphagia), cerebellar symptoms (ataxia), hemiparesis, or a combination of these (Fig. 371-2).
Diagnostic Evaluation
SAH in these patients is diagnosed first clinically, by history and examination, followed by confirmation with computed tomography (CT). As with the diagnostic evaluation for other forms of SAH, a CT scan that does not reveal hemorrhage must be followed by a lumbar puncture (except when contraindicated) with analysis of cerebrospinal fluid for the presence of blood. At our institution, all patients with SAH are evaluated with a computed tomographic angiogram (CTA) using 0.625-mm cuts on a 64-section multidetector. These thin-slice CT images are then used to reconstruct a three-dimensional computer model that allows for preoperative planning. The ease and low risks associated with CTA have made this technology our first line in evaluating patients with SAH. In a study of 29 patients with SAH by Pozzi-Mucelli and others,43 64-channel multidetector CTA had a sensitivity of 92.8% (26 of 28 aneurysms) and a specificity of 100% in detecting aneurysms, compared with conventional digital subtraction angiography. With the two false-negative results, the aneurysms were 1 to 2 mm in size. McKinney and colleagues44 evaluated 41 patients with 64-channel multidetector CTA and found 92.3% sensitivity in detecting aneurysms smaller than 4 mm and 100% sensitivity in locating aneurysms 4 mm and larger. The diagnosis of vertebral dissection relies on the angiographic findings of a string sign, rosette sign, pearl and string sign, tapered narrowing, occlusion, double lumen, or pseudoaneurysm.45 With angiography continuing to be our “gold standard,” suspicious cases of SAH in which no aneurysm or dissection is detected by 64-channel CTA are followed up with a conventional four-vessel angiogram with rotational angiography and three-dimensional reconstructions as needed.
A neuroradiologic review of CT scans46 of 44 ruptured PICA aneurysms showed that 95% were associated with radiologic hydrocephalus and 95% were associated with intraventricular hemorrhage (IVH). Supratentorial SAH was present in 70% of patients (isolated posterior fossa SAH occurred in only 30% of patients). In the series of 36 vertebral artery aneurysms reported by Andoh and colleagues,47 18 were ruptured saccular vertebral artery aneurysms, 10 were ruptured fusiform vertebral artery aneurysms, 5 were ruptured dissection aneurysms, and 3 were unruptured dissection aneurysms. In the subgroup of ruptured saccular vertebral artery aneurysms (n = 18), 78% (n = 14) showed CT evidence of IVH in addition to diffuse SAH in the basal cisterns, and 44% (n = 8) showed radiographic evidence of hydrocephalus. In the subgroup of ruptured fusiform vertebral artery aneurysms (n = 10), 90% (n = 9) had CT evidence of IVH in addition to diffuse SAH, and 30% (n = 3) showed hydrocephalus. In the subgroup of ruptured dissecting aneurysms (n = 5), all demonstrated IVH with diffuse SAH. In the unruptured dissecting aneurysm group (n = 3), 2 patients had pontine infarcts, and 1 had occipital lobe infarcts.
Preoperative Evaluation
The orientation and projection of the neck and dome are important considerations when planning for surgical clipping. The surgical approach to the aneurysm should be planned such that the neck is encountered before the dome. The size of a vertebrobasilar aneurysm can influence treatment strategy. For example, giant vertebrobasilar aneurysms can be difficult to treat, and many of them are not amenable to standard surgical clipping. Drake and others20,48,49 reported that clipping was impossible in 66% of their 354 giant vertebrobasilar aneurysms. Additionally, giant aneurysms of the posterior circulation have been associated with significantly worse outcomes.48,50,51 The finding of a giant aneurysm on angiography may necessitate further evaluation by CTA or magnetic resonance angiography. In more than 50% of cases of giant vertebral artery aneurysms, there are varying degrees of intraluminal thrombosis, and the angiographic opacity may not show the full size of the aneurysm appreciated on CT scan.48,52–55
Preoperative planning should also include careful attention to angiographic details, such as whether the PICA is reduplicated; whether the contralateral artery is present; whether the PICA territory is supplied by an alternative vessel (such as the anterior inferior cerebellar artery); and to what degree the posterior cerebral arteries are being supplied through the posterior circulation (i.e., whether there are fetal posterior cerebral arteries, and if not, what are the sizes of the posterior communicating arteries).56 The angiographic size of the posterior communicating artery has been found to be associated significantly with outcome in patients who have unclippable vertebrobasilar artery aneurysms treated by vertebral artery or basilar occlusion. The presence of a small posterior communicating artery was associated with worse outcome in these cases; the presence of two small posterior communicating arteries was associated with an even worse outcome.20,57
Technique
For vertebral artery, PICA, and vertebrobasilar aneurysms, various surgical approaches have been described. Most authors have used the lateral suboccipital or the far-lateral suboccipital approaches for vertebral artery, vertebral artery–PICA, and vertebrobasilar junction aneurysms; they have used midline suboccipital or paramedian suboccipital approaches for more peripheral PICA aneurysms (see Table 371-1). Subtemporal,34,58,59 retromastoid,60 pterional,59 extreme lateral-transcondylar-transjugular,8 subtemporal-infratemporal,8 transcondylar,33,61 combined lateral-medial suboccipital,62 supra/infratentorial-posterior transpetrosal,63 suboccipital transcondylar,9 presigmoid transpetrosal,9 anterotranspetrosal,9 extreme-lateral inferior transtubercular exposure,11 retrolabrinthine transsigmoid,11,64 combined transpetrosal,65 transmastoid-translabyrinthine (transsigmoid) and lateral suboccipital,66 transmastoid-retrolabyrinthine (retrosigmoid) and lateral suboccipital,66 and lateral suboccipital and partial condylectomy without laminectomy67 approaches also have been used.
Each approach has advantages and disadvantages. Some standard surgical approaches are hampered by a long working distance and by having to work around the brainstem and through the lower cranial nerve rootlets. For lesions located in the midline, medullary retraction or manipulation can occur, with the risk for stretching lower cranial nerve rootlets. Some approaches can be hazardous at times, especially in situations in which the aneurysm dome is encountered before the neck is fully visualized. The anterior midline approaches (transfacial or transoral transclival) are hampered by high rates of complications, particularly cerebrospinal fluid leak and meningitis. A review of the literature68 found a 50% incidence of cerebrospinal fluid leak, meningitis, or both in all published series using transclival approaches for aneurysms.
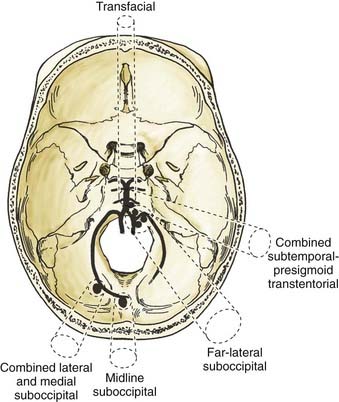
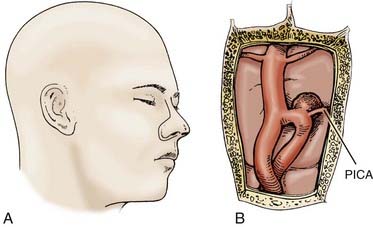
Based on the clinical experience at our institution, we use a handful of surgical approaches to gain access to various lesions of the vertebral artery, PICA, and vertebrobasilar junction. Figure 371-3 depicts the approaches we use. For distal peripheral PICA aneurysms located in the tonsillomedullary segment, we have used the combined lateral and medial suboccipital approach.62,69 For PICA aneurysms in the segment distal to the cerebellotonsillar and cortical segments, we have used a standard midline suboccipital craniectomy extending through the foramen magnum.62 We have used the far-lateral suboccipital approach described by Heros62,70 for most vertebral artery, proximal segment PICA, and vertebrobasilar junction aneurysms.69 In certain circumstances with midline vertebrobasilar junction aneurysms, we have used the transfacial transclival approach (Fig. 371-4).71 In unusually high vertebrobasilar junction aneurysms, we have used the combined subtemporal-presigmoid transtentorial approach.72
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree