CHAPTER 378 Microsurgical Management of Giant Intracranial Aneurysms
Historical Considerations
Cerebral aneurysms were not recognized as a pathologic entity until Biumi of Milan identified a ruptured aneurysm during an autopsy in 1765. Hutchinson described the first giant intracranial aneurysm in 1875, which was diagnosed by an audible bruit.1 The patient refused Hutchinson’s recommendation of carotid artery ligation. The diagnosis was confirmed 11 years later when the patient died of an aortic aneurysm and, at autopsy, a partially calcified aneurysm the size of a “bantam hen’s egg” was found in the middle fossa.
In the next century, as Standard and colleagues noted,2 the diagnosis of cerebral aneurysms improved significantly when Moniz developed cerebral angiography. Nonetheless, these lesions were still sometimes mistaken for brain tumors. In the early part of the 20th century, treatment included hunterian ligation of the internal carotid artery (ICA).3 According to Laws and Udvarhelyi,4 Dandy was the first to actually expose a cerebral aneurysm and ligate its neck by clipping.
Based on 6368 cases in a cooperative study, Locksley classified aneurysms that were 25 mm or greater as giant and observed a high rate of morbidity and mortality associated with these lesions.5 The first two significant studies of giant aneurysms were reported by Morley and Barr6 and by Bull.7 The size of the aneurysms in these series may well have been underestimated because the diagnosis was based solely on plain radiography and angiography. At that time, the natural history of these lesions was beginning to be understood, and surgical treatment was fraught with hazards. Morley and Barr concluded that “direct surgical attack on extracavernous giant aneurysms is seldom possible or successful except in the case of middle cerebral artery aneurysms.”6
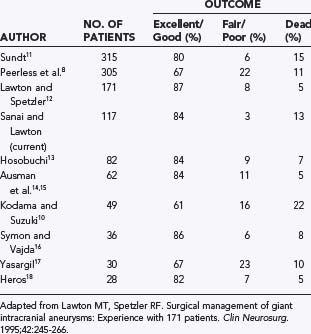
During the next several decades, several large series confirmed that significant morbidity and mortality rates were associated with these lesions. Peerless and associates reported mortality rates of 68% and 85% at 2 and 5 years, respectively, for untreated giant aneurysms, and even survivors suffered marked neurological dysfunction.8 Michel noted a 100% mortality rate at 2 years in this patient population,9 and Kodama and Suzuki reported that 75% of their untreated hospitalized patients died of subarachnoid hemorrhage (SAH).10 In contrast to this dismal natural history, several large surgical series demonstrated excellent to good outcomes in 61% to 87% of all patients treated surgically, with surgical mortality ranging from 5% to 22% (Table 378-1).8,10–18
Epidemiology and incidence
Giant aneurysms represent 2% to 5% of all intracranial aneurysms.6,19–22 As with other aneurysms, giant aneurysms have a female preponderance, although Kodama and Suzuki found an equal sex distribution.10 Most patients become symptomatic in the fourth through sixth decades of life.3,14,21,23
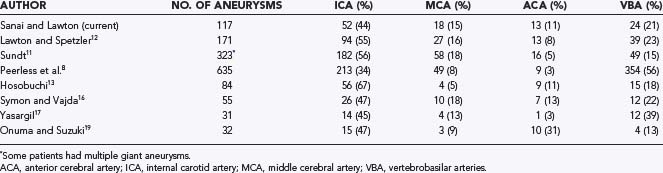
Giant aneurysms are found in all locations throughout the intracranial vasculature (Table 378-2).8,11–13,16,17,19 In general, 34% to 67% of giant intracranial aneurysms are associated with the ICA, 11% to 40% with the anterior cerebral artery and middle cerebral artery (MCA), and 13% to 56% with the vertebral and basilar arteries.3,8,11–13,16,17,19,21,24 In the senior author’s (M.T.L.) experience, posterior circulation aneurysms were treated more frequently in the group of patients with giant aneurysms (23%) than in the overall group of aneurysm patients (15%), which reflects a selection bias involving these complex aneurysms that favors microsurgery over endovascular therapy. Peerless and coauthors reported that 56% of their surgical series consisted of giant aneurysms of the vertebrobasilar system,8 a finding reflecting a referral bias to their institution.21
Pathophysiology
As early as 1930, Forbus proposed that aneurysms developed from defects in the medial layers of the vessel.25 Pathologic evaluation of giant aneurysms often demonstrates lack of a muscular layer and degeneration of the elastic laminar layers.26 Most giant aneurysms begin as saccular aneurysms located at arterial bifurcations, thus invoking hemodynamic factors in their formation. Stehbens was a proponent of acquired defects rather than congenital vessel defects contributing to the formation of cerebral aneurysms.27 Vessel weaknesses could be aggravated by flow-related stress or degenerative disease such as atherosclerosis. Some authors have postulated that giant aneurysms grow from repeated intramural hemorrhage within the aneurysm wall,20,21,28 followed by thrombus formation and neovascularization.29,30 Giant aneurysms developing de novo or from smaller aneurysms have been described.21,26,31,32 They have also been associated with underlying systemic arteriopathies.20,33 Giant aneurysms can result directly from trauma.20,33 More than one aneurysm may be present in 10% to 36% of patients.17,24,34
The morphology of giant aneurysms can be either fusiform or saccular, but their size and complexity may make it difficult to differentiate these two types. Saccular lesions most often occur at arterial bifurcations, probably the result of continuous hemodynamic stress. Fusiform or dolichoectatic lesions may result from atherosclerosis, congenital arteriopathies, or traumatic dissection.20,23,35,36 Numerous factors have been linked to the formation and rupture of aneurysms, including female sex, age, hypertension, connective tissue disease, and smoking.27,37,38
As aneurysms grow, wall tension must increase to maintain vessel integrity, as calculated from Laplace’s law. This equation relates the stress over the aneurysmal wall to the radius of the lesion.3 The same hemodynamic forces that prompt an aneurysm to grow may ultimately be responsible for its rupture.
A significant proportion of giant aneurysms have associated intraluminal thrombosis—as many as 60% in some series.3,20,21 Intra-aneurysmal thrombosis may develop in areas where blood outside the turbulent flow stream stagnates, thereby promoting thrombosis. The presence of a partially thrombosed aneurysm does not appear to lower the risk for rupture of the aneurysm.29,39
Clinical Findings
Although patients with giant intracranial aneurysms may initially be evaluated for SAH, signs and symptoms related to a mass effect develop in approximately two thirds.34,40 Only about a third of patients have SAH as the initial symptom,14,40 although Onuma and Suzuki reported that 80% of the patients in their series were initially seen because of SAH.19 As many as 50% of patients with giant fusiform aneurysms have signs of a mass effect, as opposed to 20% of patients with initial findings of SAH.36
The symptoms related to aneurysmal mass effect depend on the aneurysm’s location. In the anterior circulation, mass effect can be manifested as pain, visual field and acuity defects, and extraocular dysfunction. Dementia and mental disturbances, as well as hemiparesis and epilepsy, have also been described.20 Lownie and coworkers reported that an aneurysm had to be 3.5 cm to produce dementia and that those between 2.7 and 3.2 cm compressed the optic apparatus.41 In the posterior circulation, multiple cranial nerve dysfunctions may be present. If compression on the brainstem is significant, bulbar palsies and hemiparesis can also occur.20 The remainder of patients may have headache, syncope, sinusitis, epistaxis, confusion, cerebrovascular accidents, or symptoms related to head trauma.20,40
The risk for thromboembolism to distal vascular territories from a partially thrombosed giant aneurysm must be emphasized. In the senior author’s experience, 7% of patients with giant aneurysms initially had symptoms of distal thromboembolism, including transient ischemic attacks and stroke. Sano and associates reported that five conservatively treated patients all died of infarction.42 Naturally, only complete isolation of an aneurysm from the cerebral circulation eliminates the risk for rupture, continued enlargement, and thromboembolic phenomena.
Although SAH is less commonly associated with giant intracranial aneurysms than with small ones, it remains devastating. The annual rate of rupture of giant intracranial aneurysms is higher than that of smaller aneurysms. In several studies the annual risk for rupture correlated with increasing aneurysm size.43–45 Recent natural history studies have demonstrated an annual rupture rate for giant intracranial aneurysms of around 6%,46,47 higher than the 1% to 3% rate quoted for smaller aneurysms.12
SAH from giant aneurysms may be associated with more severe neurological deficits. According to Laplace’s law, the higher stress over the giant aneurysmal wall could result in a greater amount of SAH during rupture.2,21 As with smaller intracranial aneurysms, once SAH has occurred, more than 50% of patients die or experience significant complications.24,48 The goal is to diagnose aneurysms before they rupture or produce irreversible deficits.
Diagnosis
High-quality four-vessel cerebral angiography has long been considered the “gold standard” for the diagnosis of cerebral aneurysms; it provides information about an aneurysm’s location, anatomy, adjacent branch vessels, collateral circulation, and distal cerebral perfusion.40 It also reveals other intracranial aneurysms that might be treated simultaneously. Anteroposterior, lateral, and oblique views define the relationships between inflow and outflow vessels and the aneurysm neck. Superselective injections and manual compression techniques may clarify the anatomy or collateral blood flow. One caveat with traditional angiography is that it shows luminal filling but may fail to reveal the aneurysm’s true size if the aneurysm is thrombotic.
Computed tomography (CT) is useful in defining the outer dimensions of an aneurysm. Frequently, the aneurysm wall is thickened and calcified, which can influence the treatment strategy.20 When contrast material is administered, this eggshell border may appear as a “target sign,” with contrast material filling the center and the calcified rim appearing hyperdense.3 CT is essential in patients with SAH because it visualizes acute blood. CT also helps define the relationships between the aneurysm and skull base. CT angiography (CTA) avoids some of the risks of stroke associated with cannulation of the head and neck vessels during traditional angiography. Three-dimensional reconstructions demonstrate the aneurysm’s anatomy in exquisite detail but are not as helpful as angiography in visualizing the hemodynamics of the aneurysm. Still, angiography and CTA complement each other well and together give the neurosurgeon a clear picture of the anatomy of the aneurysm before surgery.49
Magnetic resonance imaging (MRI) offers another noninvasive technique for identifying and defining giant aneurysms. The breakdown products of blood, including deoxyhemoglobin, methemoglobin, and hemosiderin, have distinct signal characteristics on T1- and T2-weighted imaging that can be used to identify intraluminal clot. Signal flow voids, which indicate active blood flow within aneurysms, help define the filling component of an aneurysm. Magnetic resonance angiography (MRA) uses the flow void phenomenon to reconstruct an anatomic model of the vasculature.50 Even though MRI does provide information about the actual and luminal anatomy of the aneurysm, it is particularly helpful in evaluating the aneurysm’s compressive effects on adjacent brain and neural structures.51 Postoperative follow-up may be difficult with MRA, however, because of clip or coil metal artifacts.52
Management
Given the dismal natural history of giant cerebral aneurysms, a conservative or expectant approach should be considered only in patients with mitigating factors (e.g., poor medical grade, refusal of intervention). Patients with SAH should be managed according to accepted principles and protocols. In particular, we recommend aggressive control of hypertension, prompt institution of calcium channel blockers (e.g., nimodipine), and ventriculostomy if indicated.40 We recommend definitive treatment within 48 hours for ruptured aneurysms.53
Once the decision for intervention has been made, several treatment options are available in the neurosurgeon’s arsenal, including direct and indirect surgical attack, as well as endovascular procedures. Other considerations include the use of intraoperative electrophysiologic monitoring, intraoperative angiography, and specialized anesthesia techniques. Because of the potential for blood loss, all patients must have their blood typed and crossmatched, and central venous access should be available. If hypothermic circulatory arrest is being considered, a Swan-Ganz catheter should be placed for cardiac monitoring. Cerebral protective anesthetics such as isoflurane should be used. Cerebral protective agents such as barbiturates can be used during temporary vessel occlusion, and their efficacy can be monitored with electroencephalographic techniques.54,55 Intraoperative angiography may help determine whether residual luminal filling is present after the aneurysm has been clipped and can be useful in assessing the patency of bypass procedures. Furthermore, any stenosis or kinking associated with a clipped or reconstructed vessel can be confirmed immediately. Microvascular Doppler ultrasonography may be used to determine hemodynamically significant vessel stenosis. Videography with indocyanine green dye has been useful in assessing complete closure of aneurysms and the patency of parent and branch arteries, and it is faster and easier than catheter-based angiography.
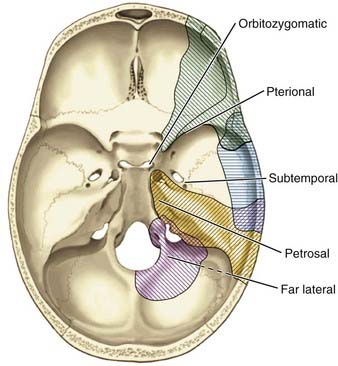
The optimal skull base approach to a giant aneurysm should offer a wide degree of exposure with full visualization of the aneurysm’s origin, outflow, associated perforators, adjacent vessels, and nearby neural structures (Fig. 378-1). The location of the aneurysm dictates the most appropriate skull base approach (Table 378-3). The basic principles of minimal brain retraction with maximal bone exposure apply. Increasingly, endovascular techniques are being combined with operative techniques to improve outcomes.56–61
TABLE 378-3 Selection of Surgical Approach Based on Location of the Giant Aneurysm
| SITE OF ANEURYSM | SKULL BASE APPROACH |
|---|---|
| Proximal internal carotid artery | Pterional, orbitozygomatic |
| Bifurcation of the internal carotid artery | Pterional, orbitozygomatic |
| Proximal anterior cerebral artery | Pterional, orbitozygomatic |
| Distal anterior cerebral artery | Pterional, orbitozygomatic, interhemispheric |
| Middle cerebral artery | Pterional, orbitozygomatic |
| Vertebral artery | Far lateral |
| Vertebrobasilar junction | Far lateral |
| Midbasilar artery | Petrosal, far lateral, orbitozygomatic |
| High basilar artery | Orbitozygomatic |
| Posterior inferior cerebellar artery | Far lateral, suboccipital |
| Anterior inferior cerebellar artery | Petrosal, far-lateral, orbitozygomatic |
| Superior cerebellar artery | Orbitozygomatic |
From Lemole GM Jr, Henn J, Spetzler RF, et al. Surgical management of giant aneurysms. Oper Tech Neurosurg. 2000;3:239-254.
Anterior Circulation Approaches
Orbitozygomatic-Pterional Approach
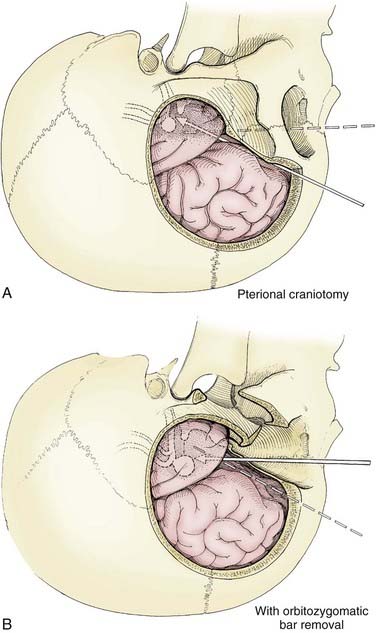
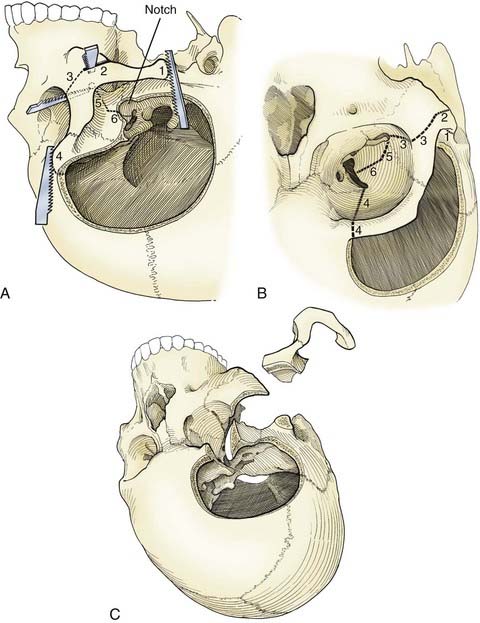
The pterional transsylvian approach is one of the most commonly used approaches to aneurysms of the anterior circulation. It provides access to the entire circle of Willis and its constituent branches. Exposure can be further enhanced by drilling the pterion and bony ridges over the floor of the frontal fossa.40 When combined with a transsylvian pterional approach, orbitozygomatic osteotomy can greatly increase the extent of exposure.62–65 Removal of the orbital rim, orbital roof, lateral wall, and zygomatic arch significantly enhances access to anterior skull base and upper clival lesions. Removing the orbitozygomatic bar provides the surgeon’s hands a wider corridor of access to the lesion and a shallower depth of field. Deep bypass procedures at the skull base are often technically difficult, and the added exposure can be essential for suturing vessels. This is particularly the case with giant MCA aneurysms that must be excised and the parent vessel reanastomosed. In addition, the orbitozygomatic approach provides a lower trajectory along the skull base, which reduces the need for cerebral retraction (Fig. 378-2).
Orbitozygomatic osteotomy is usually performed after a traditional pterional craniotomy is completed.66 A series of cuts through the lateral orbit, orbital roof, and maxillary and zygomatic roots frees the orbital bar from the adjacent skull base (Fig. 378-3). This piece of bone is preserved for later reinsertion. In addition, bone surrounding the superior orbital fissure can be removed with a high-speed drill or microrongeurs. Opening the dura with its flap based inferiorly allows the dural flap to gently depress the globe inferiorly and laterally, again enhancing exposure.40 In preparation for orbitozygomatic osteotomy, we also recommend subfascial or interfascial dissection of the temporalis fascia to protect the frontalis branch of the facial nerve.66,67
The risks associated with orbitozygomatic osteotomy include periorbital bruising and swelling, pulsatile enophthalmos, injury to the frontalis nerve, orbital entrapment, diplopia from injury to an extraocular muscle or nerve, and blindness. However, the overall incidence of these complications is low. Typically, the advantages provided by the orbitozygomatic approach more than offset its theoretical risks.66,68
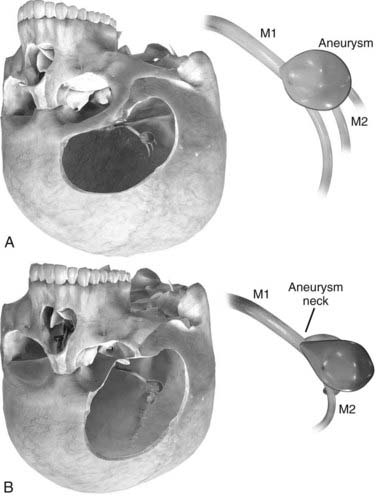
Orbitozygomatic osteotomy may be ideal for almost all giant and complex aneurysms originating from the circle of Willis, including ophthalmic, paraclinoid, superior hypophysial, posterior communicating, anterior choroidal, and ICA bifurcation aneurysms. Sometimes, additional exposure by drilling the anterior clinoid process and exposing the carotid artery in Glasscock’s triangle further enhances the exposure of certain lesions. At our institution, we also use orbitozygomatic osteotomy to approach giant and complex lesions of the MCA because the trajectory of the approach to the proximal M1 segment and the aneurysm neck is optimized (Fig. 378-4).
Posterior Circulation Approaches
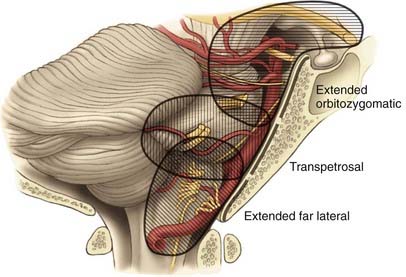
The skull base approaches used for giant aneurysms of the posterior fossa include the orbitozygomatic approach, transpetrosal approaches, and the far lateral approach. Combinations of these approaches can be used for more extensive lesions. Selection of the appropriate skull base approach depends on the aneurysm’s location (Fig. 378-5). Conceptually, the basilar artery can be divided into thirds. The orbitozygomatic approach is optimal for lesions of the upper third, and the vertebrobasilar area is best accessed with the far lateral approach. Lesions involving only the midbasilar zone may require transpetrosal or extended retrosigmoid approaches.
Orbitozygomatic Approach
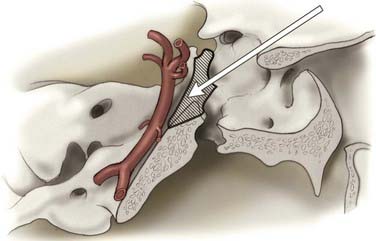
The particulars of the orbitozygomatic osteotomy were described previously, but modifications can improve access to the upper basilar artery. Drilling the anterior and posterior clinoid processes and the clivus itself allows visualization down toward the midbasilar zone (Fig. 378-6).69 The orbitozygomatic approach is also useful with high-riding giant aneurysms of the basilar artery because it provides exposure of the upper interpeduncular space without requiring excessive frontal lobe traction. Important with any approach to aneurysms in this area is adequate visualization of all inflow and outflow vessels, including the bilateral posterior cerebral arteries and superior cerebellar arteries, as well as the proximal basilar artery.
Transpetrosal Approaches
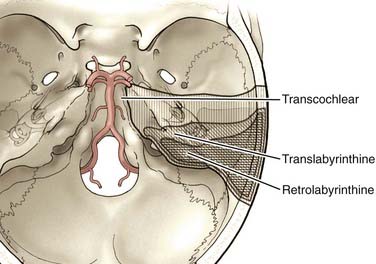
The anterior clivus and brainstem can be accessed by progressive removal of the petrous ridge. Depending on the degree of removal, the approaches can be defined as retrolabyrinthine, translabyrinthine, and transcochlear (Fig. 378-7).70–75 The amount of petrosal drilling dictates the degree of exposure of the anterior brainstem and clivus. Drilling the semicircular canals sacrifices hearing, whereas drilling the facial canal and transposition of the facial nerve may produce temporary facial nerve palsy. The expected morbidity involving cranial nerves VII and VIII with the more aggressive petrosal approaches must be weighed against the advantages of increasing exposure in the posterior fossa. At our institution, transpetrosal approaches are reserved for complex lesions of the midbasilar zone. If possible, we prefer to use the orbitozygomatic or far lateral approach or a combination to access lesions that extend into the midbasilar zone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree