Figure 9.1. Under physiological conditions, the brain maintains CBF within normal limits through autoregulation when the CPP is between 40 and 140 mmHg. Other factors that independently affect CBF are the PaO2 and PaCO2. This diagram, drawn by the author, is based on a figure taken from [5].
Factors | Changes in CBF |
CPP | CPP increase (usually >25 mmHg) or significant decrease in mean arterial pressure (<60 mmHg) leads to decreased CBF |
PaCO2 | PaCO2-arteriolar response mediated by changes in extracellular pH. CBF changes 3-4% for every 1% change in PaCO2. The relationship is almost linear up to 100-120 mmHg PaCO2 |
PaO2 | CBF increases dramatically when PaO2 falls to <50 mmHg |
Brain temperature | Tissue temperature increases CMRO2 6-7% per degree C rise in temperature. Hypothermia decreases CMRO2 |
Innervation-sympathetic nervous system | Sympathetic nerve activation of the sympathetic alpha adrenergic nerves shifts the autoregulation curve to the right (more vasoconstriction), while denervation shifts it to the left |
Chronic hypertension | Chronic hemodynamic changes lead to changes in the structure of arterioles. The self-regulatory curve is shifted to the right. There is greater tolerance to increases in blood pressure but lower tolerance to decreases |
Blood viscosity | In normotensive conditions, a fall in hematocrit from 35 to 25% leads to an increase of 30% in CBF. Falling pressure leads to a loss of the relationship. Compensatory vasodilation mechanisms are lost when hematocrit is decreased by 19% |
Table 9.1. Physiological and pathological factors affecting CBF autoregulation.
9.3 Techniques to Measure Cerebral Blood Flow
Practical and efficient measurement of CBF is difficult. Such impediment limits the information needed to determine the evolution of deleterious factors which may affect neuronal viability. One potential adverse events of this would be a delay in therapeutic interventions that could potentially reverse or prevent ischemic insults. AAn ideal technique of CBF measurement must meet the following requisites: portable, repeatable, and inexpensive. Since no currently available technology meets all these requirements, we are forced to use methods that are not always optimal (Table 9.2).
Method | Advantages | Disadvantages |
Neurologic examination | May be repeated as needed | Cannot detect early changes in CBF |
CPP | Can be measured continuously | Needs ICP catheter insertion and depends on the CVR |
EEG | Not invasive and can be continuously measured | Interference may be an issue and costly equipment is needed for data analysis |
Fick’s Principle | Allows direct measurement of CBF and is considered the gold standard | Invasive, determines global CBF and requires manual sampling |
Xenon133 | May be repeated as needed | Poor resolution and does not allow proper evaluation of CBF in subcortical structures |
Xenon-enhanced CT | Measures regional CBF and may be repeated as needed | Expensive, technically difficult, and has the anesthetic effects of xenon |
PET | Allows measurement of cerebral blood volume, tissue oxygen extraction, and CMRO2 | Expensive, not readily accessible; requires skilled personnel and patient transport out of the neurocritical unit |
MRI and perfusion CT | Measures CBF and cerebral blood volume | Allows qualitative measurement; depends on comparison of data from the two cerebral hemispheres |
TCD | Portable, inexpensive, and allows continuous measurement | Measures the speed of blood flow but not CBF; is operator-dependent and acoustic windows; allows only study of the basal arteries of the circle of Willis |
Thermal diffusion flowmetry | Allows continuous and quantitative real-time CBF measurement in the area of interest | Requires insertion of a catheter and allows only measurement of local CBF |
Table 9.2. Comparison of different methods of measuring CBF in a neurocritical care unit.
CMRO2 = cerebral metabolic rate for oxygen; CPP = cerebral perfusion pressure; CVR = cerebral vascular resistance; CT = computed tomography; EEG = electroencephalography; ICP = intracranial pressure; PET = positron emission tomography; MRI = magnetic resonance imaging; TCD = transcranial doppler sonography.
The neurologic examination can reveal altered level of consciousness or changes indicating that CBF is below acceptable thresholds for normal neuronal function. Early assessment is vital because when clinical changes are evident, irreversible changes have probably already occurred [2].
Measurement of CPP has been used as a substitute for evaluating CBF as this is directly related to CPP and inversely related to cerebral vascular resistance (CVR) according to the following equation:
CBF = CPP / CVR
From a practical standpoint, CVR is not easy to determine. Therefore, the CPP is derived from the difference between mean arterial pressure (MAP) and ICP:
CPP = MAP – ICP
Normal CPP values are between 55 and 70 mmHg [6]. It should be noted that a normal CPP can be used as a surrogate marker of normal CBF as long as the CVR is also normal. For example, if the CVR is high, as occurs in cerebral vasospasm, then a normal CPP may be associated with cerebral ischemia. Similarly, if the CVR is low, a normal CPP could lead to cerebral hyperemia.
Continuous electroencephalography (EEG) monitoring can furnish data complementary to neurological findings to detect early changes in CBF. Because CBF changes in a predictable manner in situations of cerebral ischemia, such alterations may prompt the neurointensivist to intervene early [7,8]. At CBF levels between 16 and 22 ml/100 g/min there is a predominance of slow waves, followed by a decrease in the amplitude when the CBF is 11-19 ml/100 g/min which can reach an almost total reduction on the EEG tracing when levels are <10 ml/100 g/min.
The first technique for the quantitative measurement of CBF, as devised by Kety and Schmidt, was based on the Fick method described above and used nitrous oxide as a marker as this gas is metabolically inert and diffuses quickly in the brain [9]. The gas is inhaled and blood samples are obtained from an artery or a jugular vein. The technique relies on two conditions: a continuous CBF and freedom from contamination of jugular samples with extracerebral blood. Inhaled or intravenous administration of xenon133 has also been used for similar measurements. The advantage of this technique is that you have a small portable unit, but the disadvantage is that the partition coefficient of the blood-brain barrier for this material is abnormal in pathological conditions.
Xenon-enhanced computed tomography (CT) offers the advantages that once the gas is inhaled it acts as a contrast medium for cerebral CT and it has a higher spatial resolution, so that regional CBF can be measured [10] (Figure 9.2).
Other advances in diagnostic imaging have allowed us to use positron emission tomography (PET) and magnetic resonance imaging (MRI). Although advances in these techniques continue, there remain limitations to their use (Table 9.2).

Figure 9.2. Xenon-enhanced CT scan in a patient with severe traumatic brain injury. Note the significant decrease in CBF in the left frontal region which, if not corrected, can lead to cerebral ischemia with subsequent infarction. Courtesy of Dr. Claudia Robertson, Ben Taub Hospital, Houston, Texas.
The last two CBF measurement tools we will mention are transcranial Doppler ultrasound (TCD) and thermal diffusion flowmetry [11-16]. TD measures CBF velocity, from which CBF can be estimated if the amount of blood flow through the cerebral vessels is measured simultaneously. However, the cerebral blood flow velocity rate is used in clinical practice. A detailed analysis of blood flow velocity, the pulsatility index are obtained to detect cerebral vasopasm in patients with subarachnoid hemorrhage (Figure 9.3), the pattern of collateral circulation in the circle of Willis in patients with vascular stenosis, arterial reperfusion status after thrombolysis in patients with acute ischemic stroke, and progression to cerebral circulatory arrest.
Thermal diffusion flowmetry allows us to calculate an index of cortical blood flow by measuring the dispersion of heat delivered through a catheter per unit of time. The major advantage of this technique is that it can continuously determine CBF based on a very simple principle (Table 9.2).
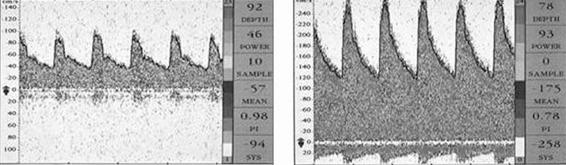
Figure 9.3. Transcranial Doppler of a patient with a subarachnoid hemorrhage. The left panel shows the tracing on day 3 and the right panel the tracing on day 7 when the patient was in a confusional state, with double vision and tetraparesis. The right panel shows a basilar artery vasospasm.
9.4 CBF in Pathological Conditions
Secondary injury resulting from a decrease in CBF to a critical level can lead to poorer clinical outcomes. The following paragraphs present the data from studies in which CBF was measured in such common brain diseases as severe traumatic brain injury, seizures, subarachnoid hemorrhage, acute ischemic stroke, and stroke or intracerebral hemorrhage. Importantly, there is considerable controversy regarding the use of data from the CBF measurement methods discussed above. For instance, there have been reports of important variability in the methodology and the definition of CBF thresholds for the diagnosis of cerebral ischemia in several clinical conditions such as severe traumatic brain injury and acute ischemic stroke [17,18].
In the initial phase of severe traumatic brain injury, there is a decrease in CBF in 50-60% of patients [19]. However, this does not always indicate that the patient is suffering from cerebral ischemia. To determine this, AVDO2 data are also needed, which show that in approximately 27% of cases the delivery does not meet the demand. It has also been shown that in the first days after trauma, some patients have hyperemia, i.e., increased CBF beyond the metabolic requirement [20]. Both ischemia and hyperemia may increase ICP, leading to disastrous results for the patient. It follows that proper management of CBF is essential, and most researchers agree that a CPP of 55-70 mmHg is sufficient to maintain brain function.
In patients with subarachnoid hemorrhage and altered level of consciousness there is a greater decrease in CBF than in those who arrive alert. This reduction can occur in the first two days after the initial event and may gradually continue for the first weeks. The critical point in the management and monitoring of CBF in these patients is during cerebral vasospasm. The most commonly used method, as discussed above, is TCD which demonstrated increases in flow rate (usually >200 cm/sec). The absolute value is not necessarily the best indication of vasospasm but instead the changes with increases >50 cm/sec over a 24-hour period. The usual management of vasospasm includes hypertensive therapy according to the hypothesis that it will increase CBF. However, we must emphasize that the autoregulation mechanism is altered, and, whether global or focal, it is difficult from a clinical perspective to determine if the therapy causes an increase or a decrease in CBF steal from areas with normal vasoreactivity [11,12].
PET studies in patients with acute stroke [21] have shown that CBF can follow various patterns:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







