CHAPTER 207 Moyamoya Disease
An increasingly recognized cause of stroke, including 6% of all strokes in children, moyamoya syndrome is a cerebrovascular disorder characterized by chronic progressive stenosis of the intracranial internal carotid arteries (ICAs), including the proximal anterior cerebral arteries (ACAs) and middle cerebral arteries (MCAs).1,2 This inexorable occlusion of the anterior circulation occurs simultaneously as characteristic arterial collateral vessels develop at the base of the brain in response to the resultant ischemia. Rarely, in advanced cases, this process can extend to involve the posterior circulation, including the basilar artery and posterior cerebral artery (PCA).
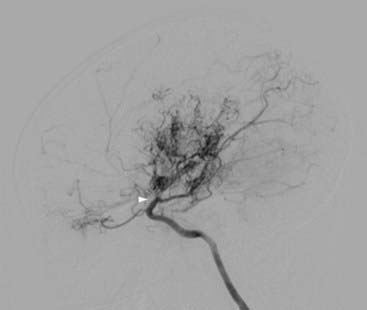
Takeuchi and Shimizu first reported a case of hypoplasia of the bilateral ICAs in 1957, although the term moyamoya (“something hazy, like a puff of smoke”), which describes the characteristic angiographic appearance of abnormally dilated collateral vessels in this condition, was not used until 1969 by Suzuki and Takaku (Fig. 207-1).3,4
Individuals with a well-recognized associated condition (see later) are categorized as having moyamoya syndrome, whereas idiopathic cases with no known risk factors have moyamoya disease. To have moyamoya disease, patients must have bilateral stenosis—patients with only unilateral findings have moyamoya syndrome.5 The term moyamoya, when used alone without the modifier of disease or syndrome, refers to the distinctive findings on arteriography, independent of etiology.
Epidemiology
First described in Japan, moyamoya has now been identified in patients worldwide.6 Although historically considered more prevalent in the Asian population, it affects individuals of many ethnic backgrounds, and there is increasing awareness of this disease in Europe and North America.7 In Japan, it is the most common pediatric cerebrovascular disease.1 In Europe, a recent study cited an incidence of 0.3 patients per center per year, which is approximately a 10th of the incidence in Japan.8 In the United States and Korea, reports have corroborated historical claims of a bimodal age distribution of moyamoya, one group in the pediatric age range (around the first decade of life) and a second group of adults in the 30- to 40-year-old range. Females are affected nearly twice as often as males.9–11 In the United States, moyamoya affects individuals differently, depending on ethnicity; when compared with white individuals, Asian Americans are more than four times as likely, African Americans are twice as likely, and Hispanic Americans are half as likely to have moyamoya.12
Associated Conditions
Particularly strong associations exist between moyamoya and radiotherapy of the head or neck (especially for optic gliomas, craniopharyngiomas, and pituitary tumors), Down syndrome, neurofibromatosis type 1 (with or without hypothalamic–optic pathway tumors), and sickle cell anemia.11,13–15 More tenuous links may exist between moyamoya and other disorders (Table 207-1).11,16
TABLE 207-1 Associated Conditions, Risk Factors, or Syndromes
| RISK FACTOR | NUMBER |
|---|---|
| No associated conditions (idiopathic) | 66 |
| Neurofibromatosis type 1 | 16 |
| Asian | 16 |
| Cranial therapeutic radiation | 15 |
| Hypothalamic–optic system glioma: 8 | |
| Craniopharyngioma: 4 | |
| Medulloblastoma with Gorlin’s syndrome: 1 | |
| Acute lymphocytic leukemia, intrathecal chemotherapy: 2 | |
| Down syndrome | 10 |
| Congenital cardiac anomaly, previously operated | 7 |
| Renal artery stenosis | 4 |
| Hemoglobinopathy (2 sickle cell, 1 “Bryn Mawr”) | 3 |
| Other hematologic: 1 spherocytosis, 1 ITP | 2 |
| Giant cervicofacial hemangiomas | 3 |
| Shunted hydrocephalus | 3 |
| Idiopathic hypertension requiring medication | 3 |
| Hyperthyroidism (one with Graves’ syndrome) | 2 |
Other syndromes, one patient each: Reyes’ (remote), Williams’, Alagille’s, cloacal exstrophy, renal artery fibromuscular dysplasia, and infection with congenital cytomegalic inclusion virus (remote). Two patients had unclassified syndromic manifestations. There were four African Americans, two of whom had sickle cell disease.
ITP, idiopathic thrombocytopenic purpura.
Pathophysiology
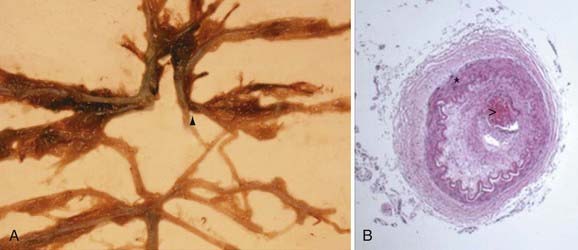
Pathologic analysis has demonstrated that affected vessels generally do not exhibit arteriosclerotic or inflammatory changes.17 Rather, vessel occlusion results from a combination of both hyperplasia of smooth muscle cells and luminal thrombosis (Fig. 207-2). The moyamoya collaterals are dilated perforating arteries believed to be a combination of preexisting and newly developed vessels.18,19 A number of growth factors, enzymes, and other peptides have been reported in association with moyamoya, including basic fibroblast growth factor, transforming growth factor-β1, hepatocyte growth factor, vascular endothelial growth factor, matrix metalloproteinases, intracellular adhesion molecules, and hypoxia-inducing factor-1α, among others.19–26 At present, it is difficult to discern which of these peptides are pathologic primary causal agents of disease and which are present merely as part of a normal response to ischemia.
Clues helping to distinguish between these possibilities have come from genetic studies. Associations between moyamoya and loci on chromosomes 3, 6, 8, and 17 (MYMY1, MYMY2, MYMY3), as well as specific HLA haplotypes, have been described.27–32 The familial incidence of affected first-degree relatives in Japan is 7% to 12%, similar to a rate of 6% reported in a recent U.S. series.11,33–38 However, despite evidence supporting a genetic basis of moyamoya, important caveats remain. Reports exist of identical twins with only one affected sibling.11,39 These data support the premise that some type of environmental factor must precipitate the syndrome’s clinical emergence in susceptible patients and suggest that the angiographic changes of moyamoya are the result of a complex interplay between genetic predisposition and external stimuli.
Clinical Findings
Accounting for approximately 6% of childhood strokes in Western countries, moyamoya affects young children in particular, with 50% of patients identified by 10 years of age.1,2 Some patients have rare, intermittent ischemic events or even extended periods of clinical stability, whereas other individuals exhibit rapid neurological decline.11,40 Children are usually initially seen with TIAs or strokes, as evidenced in the largest current report of pediatric moyamoya patients (Table 207-2).11 The much higher rate of stroke in children may be related to less developed verbal skills in this age group, thereby leading to delayed recognition of the underlying moyamoya.41
TABLE 207-2 Symptoms at Initial Evaluation in 143 Patients (Percentage of Patients with Symptoms)
| Stroke | 97 | (67.8%) |
| Transient ischemic attacks (including drop attacks) | 62 | (43.4%) |
| Seizures | 9 | (6.3%) |
| Headache | 9 | (6.3%) |
| Choreiform movements | 6 | (4.2%) |
| Incidental | 6 | (4.2%) |
| Intraventricular or intracerebral bleeding | 4 | (2.8%) |
Symptom totals are greater than patient numbers because some patients had multiple symptoms at initial evaluation.
Ischemic Symptoms
The symptoms of cerebral ischemia in moyamoya are generally related to the territory supplied by the ICAs, including the frontal and temporal lobes. Hemiparesis, dysarthria, aphasia, and cognitive impairment are common.11 Seizures occur frequently, and other, more subtle deficits may be present—an issue particularly problematic in younger patients because they may not be able to articulate their experiences as well as adults. These symptoms may be mistaken for psychiatric illness or developmental delay and include visual deficits, syncope, or personality changes.42–44
Once present, the ischemic symptoms may be transient or fixed. TIAs may be precipitated by events particularly common in children, such as hyperventilation with crying or exertion. The cerebral vessels, already maximally dilated in the setting of chronic ischemia, constrict in response to the decrease in PCO2 and can thus lead to a TIA or stroke.45 Dehydration, a problem in children after colds or fevers, may also give rise to ischemic symptoms.
Hemorrhage
Typically, hemorrhage is a hallmark of adult moyamoya, although children can also have this finding initially.11,46 Hemorrhage can be intraventricular, intraparenchymal (frequently in the region of the basal ganglia), or subarachnoid. Bleeding has historically been attributed to rupture of fragile collateral vessels unable to contain the increased flow shunted from progressive ICA stenosis.47,48 In addition to these friable vessels, studies have revealed that aneurysms in the circle of Willis may form in response to altered circulatory patterns, thus providing another potential cause of hemorrhage.49,50
Headache and Other Symptoms
Children with moyamoya will often complain of headache. Although the etiology is unclear, a recent review has speculated that dilation of meningeal and leptomeningeal collateral vessels may stimulate dural nociceptors.51 Typically, the headache is migraine-like in quality and refractory to medical therapies; although it may improve after surgical treatment of the moyamoya, often concordant with regression of the collateral vessels, it often persists as a troublesome symptom years after other symptoms remit.
Collateral vessels in the basal ganglia have also been implicated in the development of choreiform movements in individuals with advanced moyamoya.11,52 Similar to headache, these symptoms may regress after revascularization of the affected hemisphere.
Natural History and Prognosis
It is difficult to predict the natural history of moyamoya in a given patient. As discussed in the section “Clinical Findings,” patients can have isolated problems with lengthy periods of relative health or can exhibit fulminant deterioration in a very short time.11,40 However, moyamoya syndrome, in terms of both arteriopathy and clinical symptoms, inevitably progresses in untreated patients.4,53 A recent study revealed that the rate of disease progression is high even in asymptomatic patients and that medical therapy alone is of limited use.54 It has been estimated that two thirds of patients with moyamoya have symptomatic progression with poor outcomes if left untreated.55–57 This number contrasts strikingly with an estimated rate of symptomatic progression of just 2.6% after surgical treatment in a recent meta-analysis of more than 1000 patients.58 In general, neurological status at time of treatment, more so than age of the patient, predicts long-term outcome.11,59,60 The unpredictable but relentless course of this disease, coupled with the irreversible nature of deficits once present, dictates a need for early diagnosis whenever possible and prompt treatment once moyamoya is identified.
Diagnosis
Computed Tomography
In most patients with suspected stroke or intracranial hemorrhage, the evaluation typically begins with computed tomography (CT) of the head. In patients with moyamoya, hemorrhage or small areas of hypodensity suggestive of stroke are commonly observed in the cortical watershed zones, basal ganglia, deep white matter, or periventricular regions.61,62
Magnetic Resonance Imaging
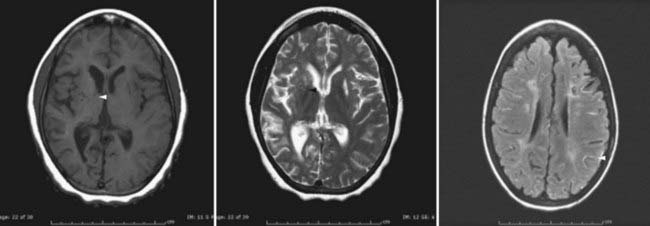
In recent years, magnetic resonance imaging (MRI) has become more widely available, which has led to substantial increases in its use as primary diagnostic modality for patients suspected of having a stroke—including patients undergoing evaluation for moyamoya.63–68 Acute infarction is best seen with diffusion-weighted imaging, whereas chronic infarction is better demonstrated on T1- and T2-weighted images (Fig. 207-3). Diminished cortical blood flow secondary to moyamoya can be inferred from fluid-attenuated inversion recovery (FLAIR) sequences, which demonstrate linear high signal following a sulcal pattern, the so-called ivy sign (Fig. 207-3).69–72 Reduced flow voids in the ICA, MCA, and ACA, coupled with prominent flow voids from the basal ganglia and thalamic collateral vessels, are considered by many to be essentially diagnostic of moyamoya.62,63,73–76 However, it is important to note that although MRI can often establish a diagnosis of moyamoya, it remains limited with regard to helping identify specific collateral networks and areas of marked stenosis—data frequently critical for successful surgical planning.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree