Video 44.1). While INO is presented here, any isolated brain stem syndrome, including diplopia, facial weakness, and trigeminal neuralgia, particularly in a young person with no other risk factors, MS should be highly considered (see Chapter 10).
3. Partial myelitis. MS commonly affects the spinal cord but rarely affects the entire cross-section of the cord, creating a partial myelitis clinical picture rather than a complete myelitis (which is more common in neuromyelitis optica [NMO]). Patients often will report numbness involving both feet (due to the layering of dorsal columns fibers with the feet being closest to the midline and the upper extremities more laterally located). Any patient who reports numbness that extends above the level of the pelvis (a sensory level) should be assumed to have a spinal cord process until proven otherwise. Patients should be queried regarding “Lhermitte’s sign” (which is really a symptom: an electric shock-like sensation traveling down the back induced by neck flexion). Patients should also be asked about bladder and sexual dysfunction (particularly erectile dysfunction in young men) which can indicate spinal cord pathology.
4. Cognitive dysfunction and fatigue. While the differential diagnosis of cognitive dysfunction and fatigue is broad, in a young person MS should be considered. Many patients with MS report fatigue that is out-of-proportion to the level of activity, and may note mild difficulty with cognition, particularly multitasking. While fatigue may be multifactorial, it may also be related to the reduced efficiency of demyelinated pathways resulting in a higher energy demand.
1. Fatigue. Patients with MS often report fatigue that is out-of-proportion to level of activity. It may be related to increased energy demands due to demyelination. Patients often report increased fatigue in the latter part of the day, concordant with a natural rise in body temperature. There is no single highly effective treatment. Patients should be counseled to plan their day accordingly. Temperature modification can be helpful. Patients may notice worsening of fatigue with higher temperatures (heat intolerance). Use of cooling devices (cooling scarves, cooling vests) that lower body temperature by as little as 1 degree can improve neural function. Patient should be screened for sleep disturbances. Medications that may have a modest effect on fatigue include amantadine, modafinil, and methylphenidate.
2. Spasticity. Muscle stiffness is particularly common due to involvement of the corticospinal tract. Patients often report muscle tightness especially later in the day when they are increasingly fatigued. Symptoms usually respond best to medications, with stretching providing only temporary relief. Muscle relaxants such as baclofen are used as first-line therapies. Patients need to be counseled as to the potential for withdrawal with sudden cessation of baclofen. Alternative oral therapy options include tizanidine, dantrolene sodium, and benzodiazepines. Patients with focal spasticity may benefit from botulinum toxin (Botox). More severe spasticity may be alleviated with an intrathecal baclofen pump, which seem to work better for lower extremity spasticity. Medical marijuana, while illegal at the federal level in the United States but legalized by many individual states, may theoretically be helpful for spasticity.
3. Gait impairment. Dalfampradine (4-aminopyradine or Ampyra) specifically approved for walking speed in MS, was shown to improve the average 25-foot timed walk. The medication is contraindicated in those with a history of seizures or significant renal impairment.
DIAGNOSIS
Despite many technological advances to aid in the diagnosis of neurologic disease, the core clinical diagnostic features for the diagnosis of MS, a CNS demyelinating disorder with attacks separate in space and time, laid out in the 1965 “Schumaker Criteria,” still hold true today. MS is a clinical diagnosis with paraclinical support, which should be considered in a patient with objective abnormalities on the neurologic examination, or highly suggestive symptoms by history, attributable to dysfunction of two or more parts of the CNS involving white matter tracts that occur at separate points in time with no better explanation. It is notable that the Schumaker Criteria did not require any specific testing other than a history and neurologic examination. While this may seem “antiquated” to a current reader, it is worth highlighting that a patient presenting with objective evidence of >2 attacks, or objective evidence of 1 attack with a reasonable historical evidence of a prior attack, requires no additional testing for the diagnosis of MS in the 2010 McDonald Criteria. MS is, after all, a clinical diagnosis, and paraclinical measures are used to support the diagnosis.
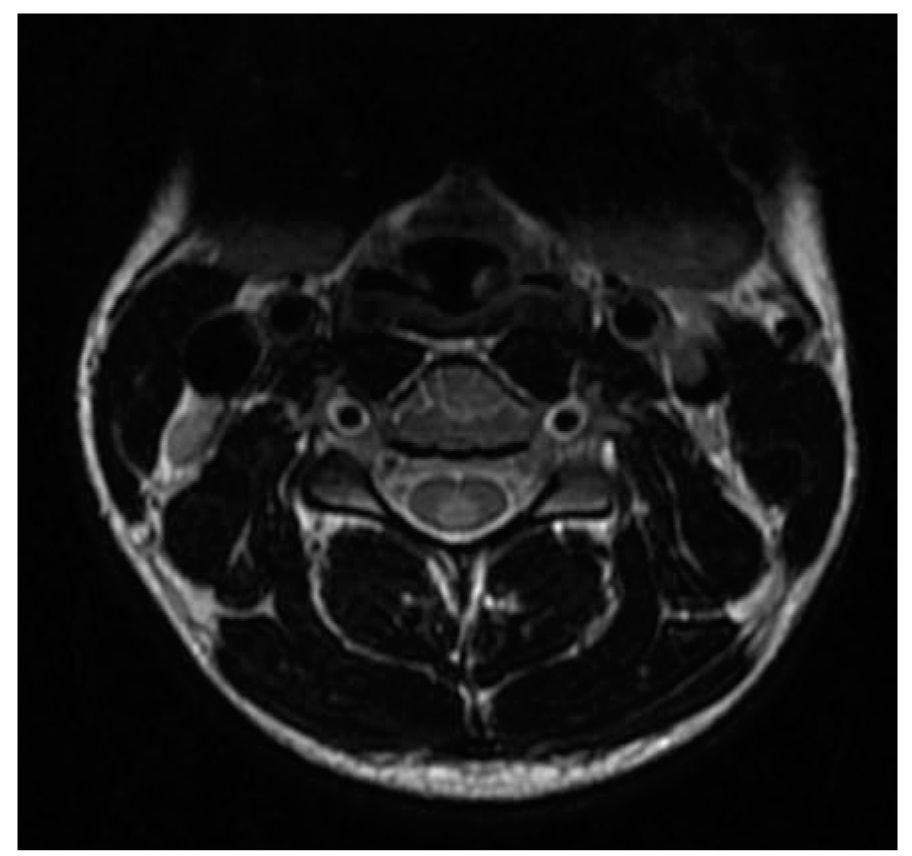
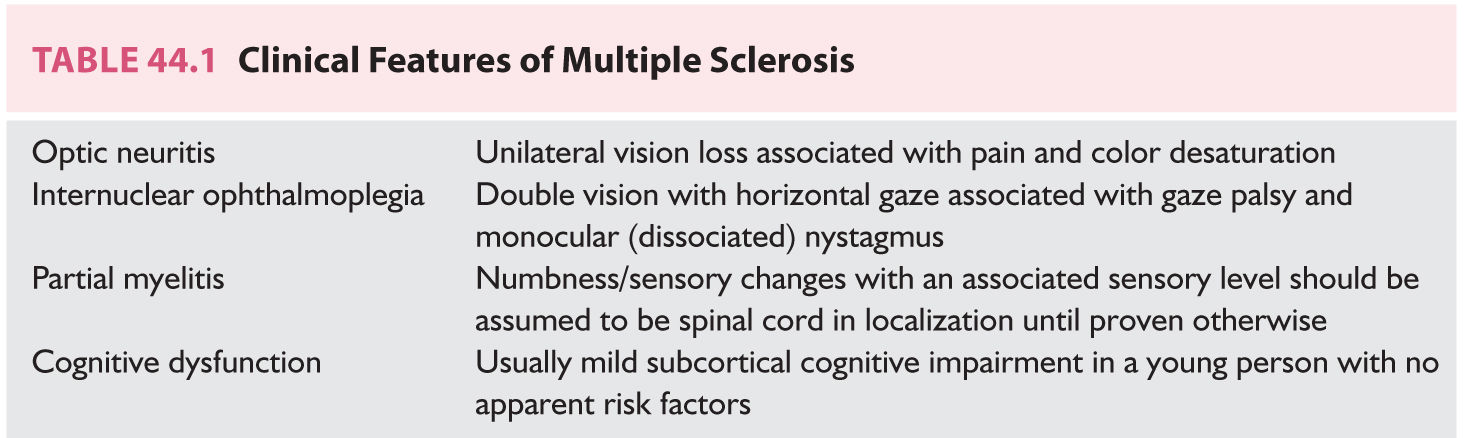
FIGURE 44.1 Spinal cord lesion involving the dorsal columns abutting the pial surface.
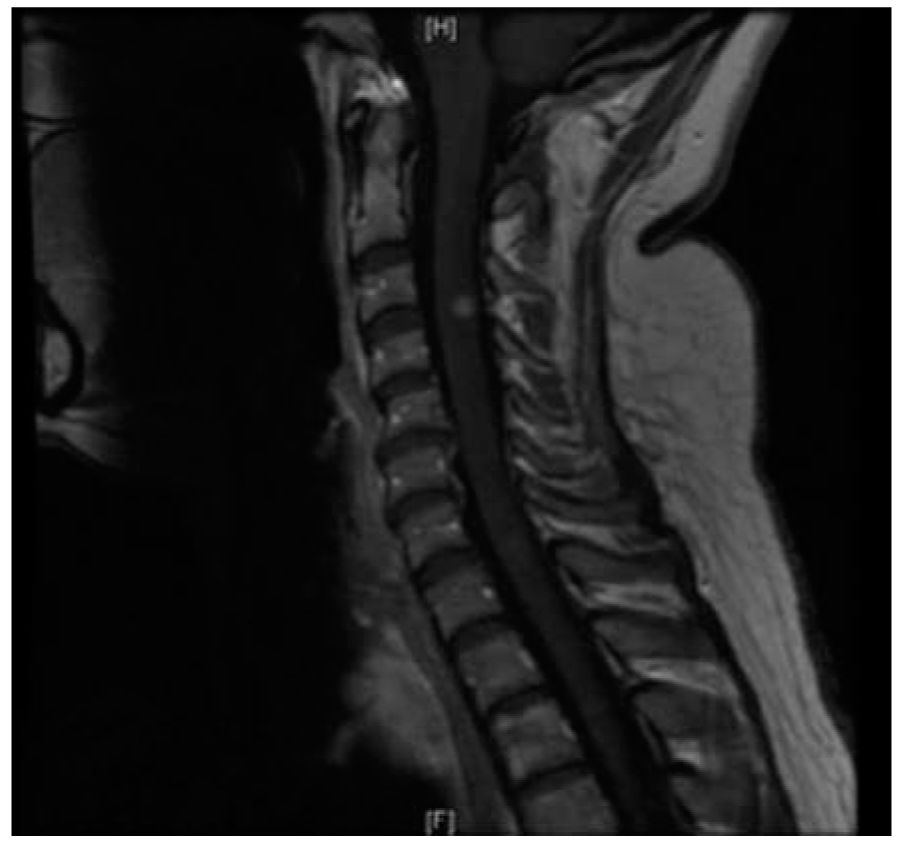
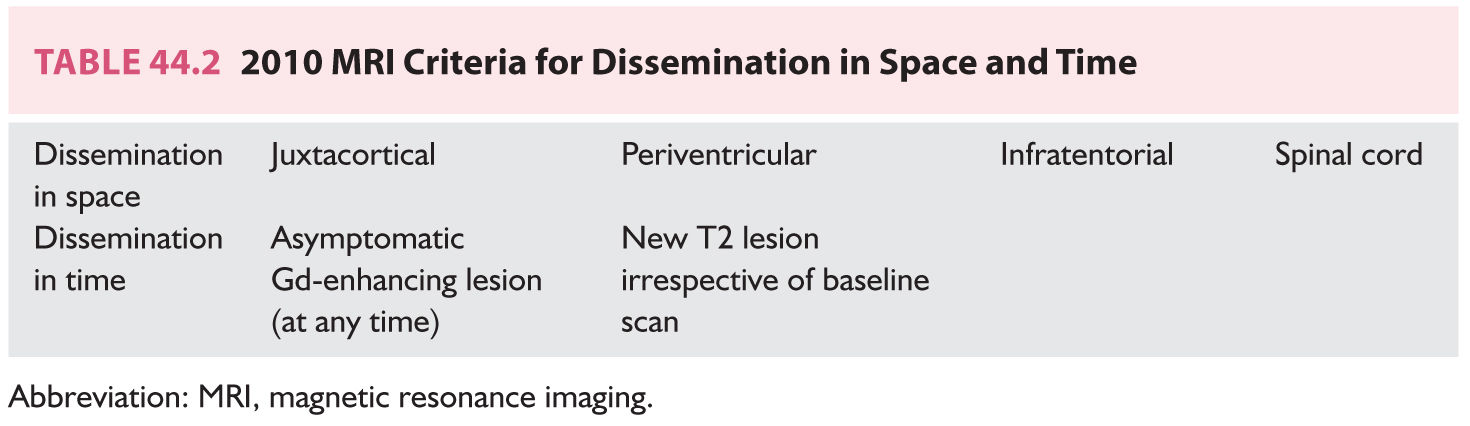
FIGURE 44.2 Gadolinium-enhancing spinal cord lesion <1 vertebral level, typical of MS. MS, multiple sclerosis.
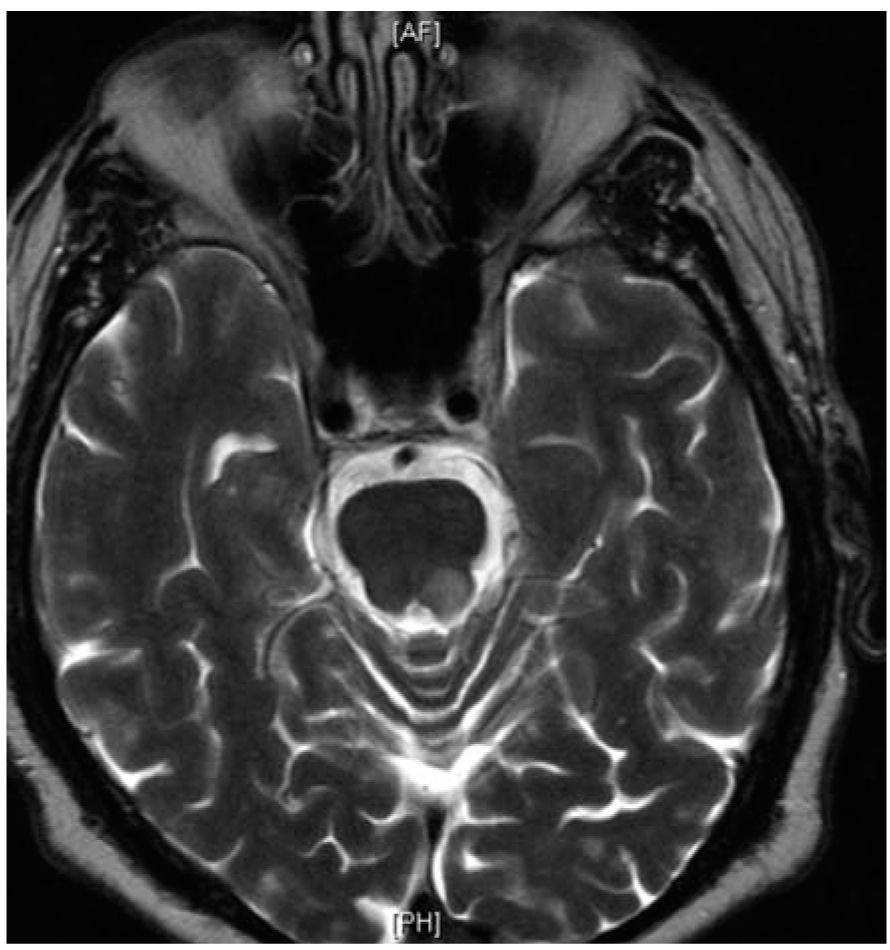
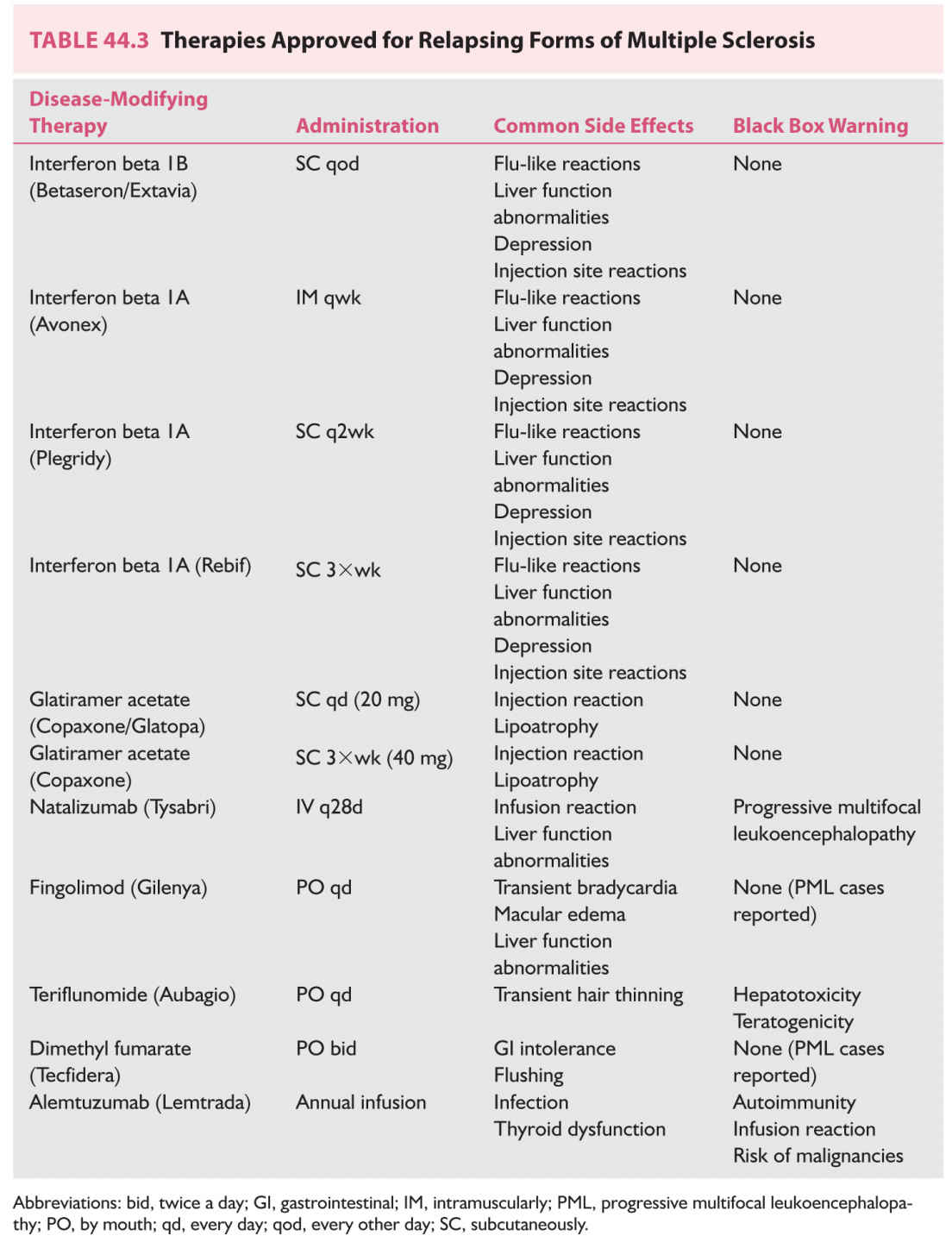
FIGURE 44.3 Infratentorial brainstem lesion close to the pial surface of the brainstem.
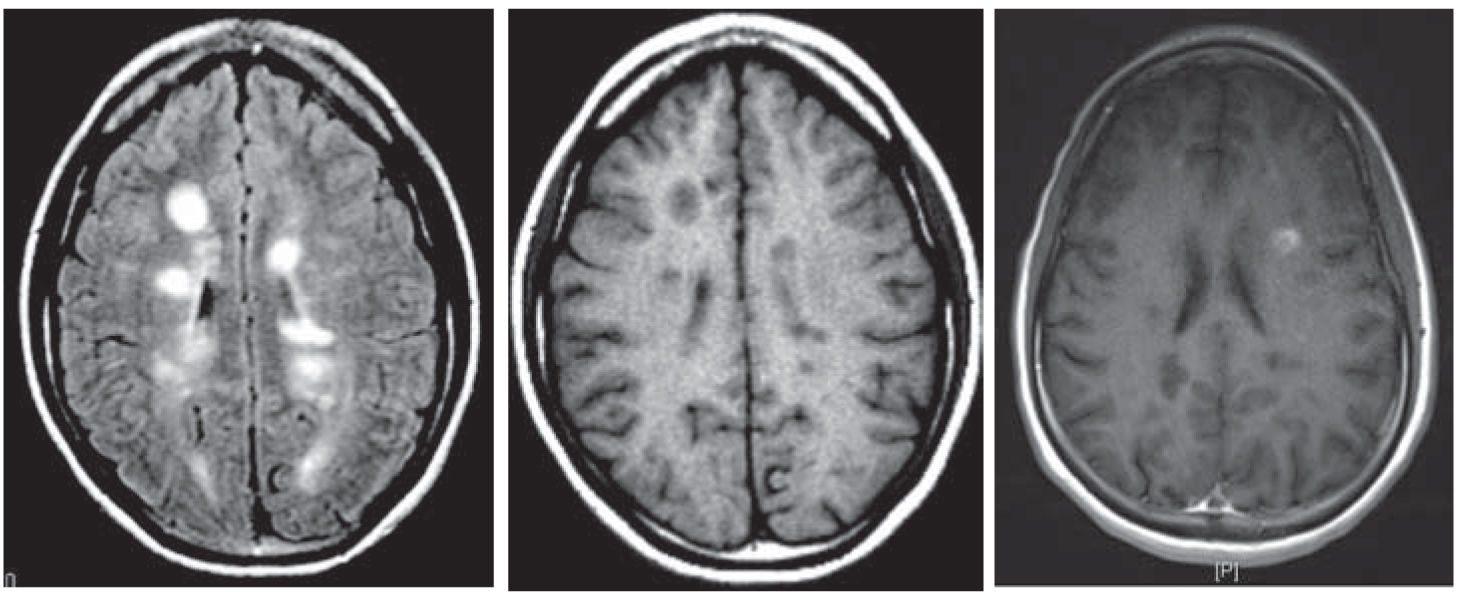
FIGURE 44.4 Periventricular lesions (FLAIR, T1 hypointense “black hole,” and characteristic gadolinium-enhancing lesion). FLAIR, fluid attenuation inversion recovery.
However, it is also evident that paraclinical studies can be helpful in reasonably confirming the diagnosis in a patient presenting with suspicious clinical features. Brain and spinal cord magnetic resonance imaging (MRI) has been the most helpful ancillary study in the diagnosis of MS in appropriately selected patients, and can be used in the setting of a single clinical event to demonstrate prior demyelinating events involving different parts of the CNS (radiographic dissemination in space and time) that suggest a risk for future clinical relapses. Radiographic lesions due to MS usually have a typical size, appearance, and location (Figs. 44.1 to 44.5). Most lesions are >3 mm (though occasionally being as large as 2 cm or more in tumefactive MS), and have an ovoid appearance. To fulfill the 2010 McDonald Criteria for dissemination in space, lesions should be present in at least two out of four areas: periventricular, juxtacortical, infratentorial, or spinal cord (only asymptomatic spinal cord lesions are counted; Table 44.2). MS lesions tend to accumulate close to the pial surface near the ventricular system. Periventricular lesions are often best seen on sagittal fluid attenuation inversion recovery (FLAIR) imaging that demonstrates a perpendicular orientation of the lesions to the ventricle in close proximity or involving the corpus callosum (“Dawson’s fingers”, first described by J. W. Dawson in 1916). Infratentorial lesions, unlike vascular lesions that appear deep in brainstem structures, tend to accumulate near the 4th ventricle, along the pontine surface or in the cerebellar peduncles, and are usually best seen on T2-weighted imaging. Spinal cord lesions are often seen in the periphery of the spinal cord, particularly in the dorsal columns, are typically <1 vertebral segment in length, and seldom involve the entire cross-section of the cord. It is unusual for any disease other than MS to affect the brain and spinal cord. Dissemination in time is demonstrated by the appearance of a new T2 lesion at any point in time compared to a reference scan, irrespective of the timing of the first scan (older criteria required a 30-day gap), or the presence of an asymptomatic gadolinium-enhancing lesion on even the baseline scan. Gadolinium-enhancing lesions are often incompletely enhancing, and indicate recent (several days to several weeks) or active demyelination. The presence of T1 hypointense lesions, though suggestive of chronic lesion formation that would support dissemination in time, is not included in the criteria.
The concept of “radiographically isolated MS” (RIS) has been introduced in the literature, referencing a situation in which a patient is imaged for presumably alternative reasons (such as headaches) that are not felt to be demyelinating in nature, and the imaging reveals lesions characteristic in appearance for MS. RIS is not currently included as an MS phenotype, though patients may require close clinical and radiographic follow-up to assess for signs and symptoms suggestive of MS.
MRI has become clearly the leading paraclinical study, and most other “classic” paraclinical studies now having diminishing clinical relevance. For example, though a historic biomarker for the disease, spinal fluid analysis is no longer considered mandatory for the diagnosis of MS and is not included in the 2010 McDonald Criteria for relapsing forms of MS. While this may induce chest pain and rage in older neurologists, its inclusion was not felt to be necessary. In the past, “positive” cerebrospinal fluid (CSF) could be used to reduce MRI requirements for the diagnosis of MS. As the 2010 McDonald criteria simplified imaging requirements, further “liberalizing” MRI requirements with CSF was felt to be unnecessary.
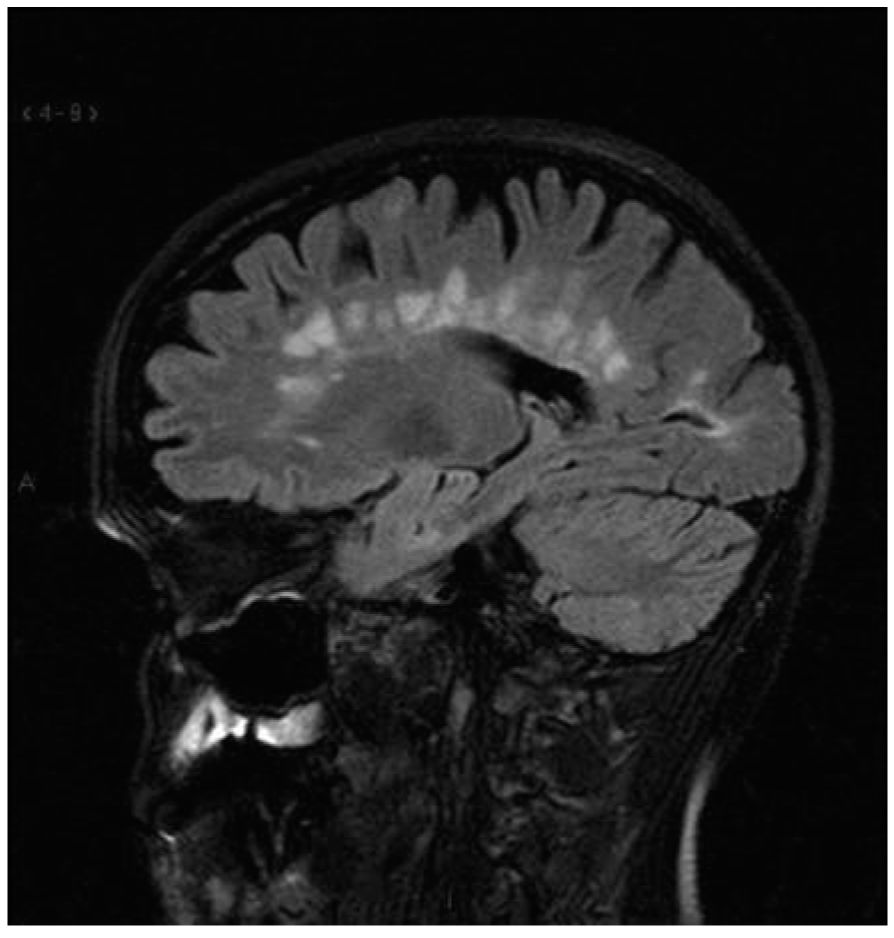
FIGURE 44.5 Perpendicularly oriented periventricular lesions (Dawson’s fingers).
Though CSF is not required, it can still provide important information and should be used in appropriate situations where the information may influence clinical decisions. The basic CSF profile (white blood cell count [WBC], protein, glucose) in MS is usually normal, though mild elevations in the WBC count and protein content can be seen. A higher than normal (>5 × 106/L) WBC count is seen in upwards of one-third of MS patients and a mildly elevated protein count (>54 mg/dL) in as many as one-fourth. However, extreme elevations such as a very elevated WBC count (>50 × 106/L) or protein count (>100 mg/dL) are unusual and should prompt consideration of an alternate diagnosis. Oligoclonal bands (OCBs) represent IgG unique to the CSF, and suggest immunologic activity of clonally expanding lymphocytes within the CNS. Qualitative assessment of CSF for IgG is likely the most informative analysis, and is considered superior to quantitative IgG analysis (IgG index). While commonly assumed to be “diagnostic” of MS, OCBs are not unique to MS and may alternatively be absent in patients with MS. OCBs may have some prognostic role, with the presence of OCBs possibly being associated with an increased risk of converting from clinically isolated syndrome (CIS) to MS. Other historic markers, such as myelin basic protein (MBP) which may be a marker of acute demyelination, are nonspecific. There has been great interest in other CSF biomarkers for diagnostic and prognostic purposes in MS but, to date, have shown minimal real-world clinical promise.
Evoked potentials have also been classically used as a paraclinical support for clinically silent CNS demyelination. The American Academy of Neurology (AAN) has published recommendations that visual evoked potentials (VEPs) are “probably” useful to identify patients at risk to develop MS, somatosensory evoked potentials (SSEPs) are “possibly” useful, and brainstem auditory evoked potentials (BAEPs) have insufficient evidence be recommended as useful. With advances in MRI technology, and the emergence of ocular coherence tomography (OCT), VEPs will likely take a placed next to “hot bath tests” as a footnote to the diagnostic history of MS.
Perhaps the key questions for the neurologist facing a patient with possible MS are: (1) Does the patient have concerning signs or symptoms for MS? (2) Does the MRI of the brain and spinal cord appear characteristic for MS? and (3) Is there any more reasonable explanation? If the answers are satisfactory for each, the patient has MS. It is also important to remember that diagnostic criteria exist to aid the diagnosis, and the clinician is not completely beholden to criteria in the face of a clear clinical presentation. As Admiral John Kurtzke noted, “MS is what a good clinician would call MS.”
DEFINING THE COURSE OF MULTIPLE SCLEROSIS
Patients with MS have MS independent of the relatively arbitrary labels placed on them by clinicians. It is clear, however, that some patients with MS have intermittent inflammatory relapses that recover in time, some patients have steady progression of symptoms without clear recovery, and some patients have both inflammatory relapses and concomitant progression.
In 1996, several subtypes of MS were defined by the United States National Multiple Sclerosis Society (NMSS). Four clinical courses were defined at that time: (1) relapsing–remitting (RR) (2) secondary progressive (SP) (3) primary progressive (PP), and (4) progressive relapsing (PR). At that time of publication, it was noted that the subtypes lacked objective biomarkers such as MRI (which was still in its relative infancy, and not even included in the existing diagnostic criteria for MS).
The subtypes of MS were updated in 2013. Key updates include the following:
1. Clinically isolated syndrome. The first clinical presentation of an inflammatory CNS demyelinating condition suggestive of MS, but not meeting full criteria. Perhaps ironically, though now “officially” recognized, the changes in the diagnostic criteria for MS have made the use of the term “clinically isolated syndrome” somewhat academic as patients with a single clinical event meeting current MRI criteria can simply be defined as having MS based on a single scan.
2. Assessment of disease activity by clinical or radiographic features as “active” or “not active.” Evidence of disease activity and clinical progression, either clinically or radiographically, reflects ongoing risk of inflammatory disease or neurodegenerative progression. Disease activity can be assessed by annual clinical assessment and annual cranial imaging for patients deemed to be at risk of inflammatory attacks. The role of annual imaging for patients with progressive disease is less clear.
3. A patient with RR-MS with a clinical relapse or evidence of a gadolinium-enhancing lesion would be considered “RR-active”; a patient with RR-MS without clinical or radiographic activity would be considered “RR-not active.”
4. The use of disease activity as a modifier allows for the elimination of the PR-MS subtype. A patient previously considered PR-MS would not be classified as PP-active or PP-not active.
5. Assessment of disease progression, independent of relapses. Progression can be determined by history or objective measure of change. Though labeled “progressive,” not all progressive forms of MS progress over a specified period of time, and no two cases necessarily progress at the same rate.
6. A patient with SP-MS without progression would be considered SP-MS-not progressing.
7. A patient with SP-MS with new lesions and gradual clinical worsening would be considered SP-MS-active and progressing.
What remains to be seen is how quickly, if at all, the 2013 revisions to the clinical course of MS are incorporated into regular clinical practice. The four subtypes of MS (RR-MS, SP-MS, PP-MS, PR-MS) seem to have become relatively ingrained in the MS vernacular, despite their existence for barely two decades. However, the 2013 revisions do emphasize a key point—is the patient at risk for future clinical or radiographic relapses, which is a pivotal decision point in the treatment algorithm. The revisions are also seemingly one of the first “authoritative” recommendations for annual imaging in patients with relapsing forms of MS as a means of surveillance.
TREATMENT OF ACUTE RELAPSES
An MS relapse has classically been defined as a new or worsening neurologic deficit lasting >24 hours in the absence of a fever or infection. The goal of treating acute inflammatory MS attacks is to restore neurologic function as quickly as possible. Treatment is not absolutely necessary—many patients will regain function within several months independent of treatment. Before treatment, concurrent infection should be excluded. Infections or other active medical issues can cause recurrence of prior MS symptoms, often termed a “pseudo-relapse.”
The mainstay of acute treatment has been high-dose corticosteroids, administered in one of several fashions. The first medication proven to be effective at treating an MS relapse in a well-designed, multicenter randomized clinical trial was corticotropin gel (ACTH). ACTH is still available for use, but is often used only in patients who have had an inadequate response to high-dose steroids or cannot tolerate steroids for some reason.
Intravenous (IV) methylprednisolone (MP) has become the “standard” treatment for MS relapses based on clinical trial experience in the 1980s. IV MP was found to be comparable to ACTH (and at a lower cost) in several head-to-head trials. The Optic Neuritis Treatment Trial in 1992 seemed to suggest that IV MP was superior to oral prednisone. The IV MP had faster recovery of visual function and the oral prednisone group had a higher relapse recurrence rate than IV steroids. However, it is entirely possible the response was dose-dependent and not route-dependent. In the study, patients received 1,000 mg of IV MP (divided 250 mg every 6 hours for 3 days) followed by an oral course of prednisone for 11 days, or 1 mg/kg/day of oral prednisone for 14 days—a substantially lower dose. IV MP has close to a 1:1 conversion rate to oral steroids. Interestingly, low-dose IV MP (40 mg/day for 7 days, 20 mg/day for 4 days, and 10 mg/day for 3 days) was not shown to be as effective in the treatment of relapses in 1989. Several studies have shown that equally dosed oral and IV steroids have a similar impact on recovery and rate of recurrence. For patients who can tolerate high-dose oral prednisone, oral treatment may be an option.
For patients who do not respond to high-dose oral steroids, plasma exchange (PLEX) may be an option. The American Academy of Neurology published guidelines in 2011 in support of PLEX for steroid-refractory MS relapses, recommending that PLEX be considered for the adjunctive treatment of exacerbations in relapsing forms of MS, and that it may be considered in the treatment of fulminant CNS demyelinating diseases that fail to respond to high-dose corticosteroid treatment. Intravenous immunoglobulin (IVIG) has also been used, though the efficacy data are less clear with conflicting data in the literature.
DISEASE-MODIFYING THERAPIES
A cure for MS has long been sought for, and has long been equally elusive. Charcot once remarked regarding treatment for MS that “I can only tell you of some experiments, the results of which have, unfortunately, not been very encouraging.” This was largely true in MS until the early 1990s when interferon beta-1b was shown to reduce the risk of MS relapses and radiographic lesion formation. As Dr. Barry Arnason editorialized, though not “the long-awaited cure. . . . half a loaf is better than no bread.” Since the early 1990s, there has been a significant change in the landscape for the treatment of MS. Whereas in the past physicians had little to offer patients in the way of treatment, the current challenge is in trying to navigate all of the treatment options.
There is no “perfect” treatment for MS, and the variability of the disease makes an algorithmic approach problematic. There are few head-to-head studies comparing treatment options to guide decision making. Cross-study analysis is fraught with statistical peril, as MS clinical trials have changed substantially in the last 30 years in regards to patient demographics, in part reflective of changes in the diagnostic requirements for MS (the placebo annualized relapse rate [ARR] for the original interferon trial was >1.0; in many modern studies, the ARR is closer to 0.5).
Many factors should be taken into account when selecting a therapy. Reasonable therapeutic questions include:
1. Is the medication effective at treating MS? Most clinical trials have measured the effectiveness of MS therapies on the relative reduction in the relapse rate compared to placebo, the reduction in the formation of new radiographic lesions (new or unequivocally enlarging T2 lesions or new gadolinium-enhancing lesions), and the reduction in risk of disability progression. While data on the long-term impact of treatment on disease impact are somewhat lacking (in part because therapies have only been available just over 20 years), extension data from the original Interferon (IFN) clinical trial clearly show a mortality and quality-of-life benefit from treatment.
2. Is the medication reasonably safe? Many patients who start therapy are young, often in child-bearing years, and can reasonably expect to be on treatment for many years, possibly many decades. A reasonable risk–benefit analysis must be favorable in the treatment of a disease that can cause significant disability but a less clear impact mortality.
3. Is the medication reasonably tolerated? A patient with a “high” relapse rate may have new symptoms due to MS as infrequently as once a year, and many patients having relapses “only” once every 2 to 3 years. Medications must be taken with regularity to be effective. If the day-to-day experience of a medication is worse than the disease, few patients will take it.
4. Will the patient be compliant with the medication? Few medications work less well than the one not taken. Physicians should discuss at length with patients lifestyle factors including family life, work, personal preferences, in selecting a therapy for a patient.
APPROVED THERAPIES FOR RELAPSING MS (TABLE 44.3)
A. Injectable therapies.
1. Interferons (IFN). Though the exact mechanism of action is unknown, IFNs are believed to prevent the migration of self-reactive immune cells across the blood brain barrier. All IFNs have similar side-effect profiles, most notably including the potential for liver function abnormalities, flu-like reactions following injections, and depression. Monitoring requirements include baseline liver function tests (LFTs) and routine blood counts, which should be periodically repeated (usually at 3 months, then 6 months, then 12 months, and every 6 to 12 months thereafter). IFNs are most notably differentiated by the dosing regimen. There are very limited head-to-head studies comparing the individual therapies.
a. IFN beta-1b (Betaseron, Extavia) is given subcutaneously (SC) every other day
b. IFN beta-1a (Avonex) is given intramuscularly (IM) once weekly
c. IFN beta-1a (Plegridy) is given IM once every 2 weeks
d. IFN beta-1a (Rebif) is given SC 3 days weekly
2. Glatiramer acetate (GA). The exact mechanism of action is unknown, but is believed to induce a shift from a proinflammatory state to an anti-inflammatory state within the CNS. Notable side effects include the potential for a chest-tightening reaction (usually within a few minutes of injection and lasting several minutes), and focal lipoatrophy at injection sites. No routine laboratory monitoring is required. There are currently three formulations of GA available.
a. GA (Copaxone) 20 mg given SC on a daily basis
b. GA (Copaxone) 40 mg given SC three times weekly
c. GA (Glatopa) 20 mg given SC on a daily basis
B. Oral therapies.
1. Dimethyl fumarate (Tecfidera). Fumarates have been used for many years for the treatment of psoriasis. In part due to reports of patients with MS and psoriasis responding well, dimethyl fumarate (DMF) was studied in relapsing forms of MS. The mechanism of action of DMF is unknown, but it has been theorized that it may interfere with the NrF2 pathway, a proinflammatory pathway. Notable side effects include gastrointestinal upset that typically occurs for the first few days to a week after initiation, and may possibly be ameliorated by taking the medication with foods high in fat content, and a flushing reaction variably described as a reddening of the skin and/or itching that often randomly occurs and usually lasts for several minutes following ingestion. There have been, to date, a very limited number of case reports of progressive multifocal leukoencephalopathy (PML) in patients taking DMF, none of whom had known prior exposure to or concomitant use of medications associated with PML. Monitoring includes a baseline complete blood count (CBC) and follow-up studies every 6 months. A small percentage of patients may show a significant and sustained drop in lymphocyte count that may be a risk factor for opportunistic infection.
a. Dimethyl fumarate (Tecfidera) 240 mg by mouth (PO) twice daily
2. Fingolimod (Gilenya). Fingolimod was the first oral therapy approved for the treatment of relapsing MS. It is believed to affect sphingosine-1-phosphate (S1P) receptors. S1P is involved in the egress of lymphocytes from lymph nodes. The presumed mechanism of action is the reversible sequestration of autoreactive lymphocytes from lymph nodes. S1P receptors are also found on cardiac tissue, and may be cause for the transient drop in heart rate following first dose. Notable side effects include the potential for macular edema (particularly in those with diabetes mellitus), which typically occurs within the first few months of starting the medication, transient cardiac changes, and the potential for some infections. Monitoring requirements include baseline LFTs and CBC, examination of the fundus (either formal funduscopic examination or OCT), reasonable confirmation of prior varicella-zoster virus (VZV) exposure (either by history or, more commonly, testing for serum VZV antibodies to confirm immunologic surveillance), and first dose observation (FDO). FDO includes a baseline electrocardiogram (ECG) to evaluate for a prolonged QT interval and second-degree, type 2, or third-degree heart block (both of which are contraindications to fingolimod and should prompt immediate cardiology consultation). After taking the medication, the patient is monitored for 6 hours with periodic evaluation of the heart rate and blood pressure. Following FDO, patients should undergo a repeat fundus examination after 3-to-4 months to evaluate for macular edema. Follow-up blood studies include periodic surveillance of LFTs. CBC surveillance often reveals a drop in the total WBC count and lymphocyte count, sometimes to dramatically low levels (<500). However, the clinical significance of the lymphopenia is unclear; based on the available clinical trial data, there was not an association between lymphopenia and infection, and is not a reason to reflexively discontinue treatment. There have been, to date, a small number of case reports of individuals taking fingolimod developing PML. The majority of cases have occurred in those who switched from natalizumab to fingolimod, often with a short interval between the last dose of natalizumab and first dose of fingolimod, and virtually all having a known John Cunningham virus (JCV) positive antibody status. There are also a smaller number of cases of cases in which individuals with no prior exposure to natalizumab who were on fingolimod who developed PML.
3. Teriflunomide (Aubagio). Teriflunomide is a derivative of leflunomide, which has been used for the treatment of rheumatoid arthritis (an autoimmune inflammatory condition directed against joints). Teriflunomide is given as a once-daily pill. Its presumed mechanism of action is inhibition of the enzyme dihydro-oratate dehydrogenase (DHOD), an enzyme critical in the de novo cell synthesis pathway. Notable side effects include the potential for LFT abnormalities, transient hair thinning (occurring around 3 months after treatment initiation and usually resolving around 6 months after treatment initiation), and a possible increased risk for peripheral neuropathy (especially mononeuropathies such as median neuropathy at the wrist). Effective forms of birth control are recommended as the medication has been classified as pregnancy Category X by the Food and Drug Administration (FDA). Though the medication has a considerably long half-life, and can theoretically linger as long as 2 years, it can be eliminated with cholestyramine, usually over the course of 11 days. Activated charcoal would also theoretically eliminate the medication and is included in the package insert, but its availability and tolerability make it a moot point. Monitoring requirements include baseline and monthly LFTs for the first 6 months of therapy, baseline and periodic CBC, screening for tuberculosis (either tuberculin skin testing or quantiferon gold testing), and exclusion of pregnancy.
C. Intravenous therapies.
1. Natalizumab (Tysabri). Natalizumab is a once-every-28-day monoclonal antibody that is believed to bind to integrin, which prevents the adherence of WBCs to the blood vessel wall, a necessary step for access to the CNS across the BBB. While considered a highly effective therapy for MS, it was briefly pulled from the market after its approval in 2004 due to the occurrence of treatment-related PML. Natalizumab has been definitively associated with the risk of PML. Patients considered at the highest risk include those with history of prior immunosuppressant exposure, on therapy for greater than 2 years, and who have a positive JCV Ab status.
2. Alemtuzumab (Lemtrada). Alemtuzumab is a CD52 monoclonal antibody that causes the rapid depletion of T- and B-cell lines, with a slow return to baseline over many months. It is uniquely dosed in annual courses, with five consecutive daily infusions given in year 1 and three consecutive daily infusions in year 2. The need for a third course or beyond is case dependent, but many patients do not require re-treatment in year 3 or 4. Notable side effects include the potential for an infusion reaction that may be reduced with concomitant IV MP for at least the first three infusions. Interestingly, patients can develop delayed secondary autoimmunity. Rare but significant side effects include immune thrombocytopenic purpura (ITP) and rapidly progressive glomerulonephritis. Autoimmune thyroid dysfunction, either hypo- or hyperthyroidism, occurs more commonly (30% to 40%), usually between 1 and 4 years from the last infusion. As a result, a comprehensive risk mitigation program is required that includes monthly blood and urine studies for 48 months after the last infusion.
D. Emerging therapies.
Rapid evolution of MS treatment options often relegate virtually all literature outdated almost as soon as it is published. At the present time, at least two therapies are close to approval for use, including ocrelizumab and daclizumab. Several others are under investigation including laquinimod and ofatunamab. A number of treatments are showing promise for remyelination including anti-LINGO, RhMIg22, and high-dose biotin. Some of these therapies will eventually be available for use, while others may never bear out the promise of initial studies. But for patients, it is clear that more and more options are readily emerging.
MULTIPLE SCLEROSIS AND PREGNANCY
As MS commonly affects women of child-bearing potential, it is fairly common for the neurologist to offer an opinion in regard to obstetric management. MS appears to have little impact on pregnancy and pregnancy little impact on MS. While there is an increase in relapses in the 3 months after delivery, the relapse rate declines during pregnancy and during the “pregnancy year” (pregnancy plus 3-month postpregnancy period) the relapse rate is unaffected. Factors that may influence the intra- and postpregnancy relapse rate include the prepregnancy relapse rate and higher expanded disability status scale (EDSS). Pregnancy does not appear to impact the long-term course of the disease. There is no evidence that MS increases the risk of miscarriages, ectopic pregnancies, premature delivery, or fetal malformations. There is no medical reason to discourage women with MS from considering pregnancy.
If a woman does experience a relapse during pregnancy, a decision can be made as to whether it is significant enough to require treatment. Prednisone is classified a Category C medication. It may cross the placenta and could possibly contribute to miscarriage, preterm labor and cleft palate if used in the first trimester. It is probably safe to use in the 2nd or 3rd trimester. IVIG does not appear to affect the fetus though it does cross the placenta and could be used if deemed clinically appropriate (see Chapter 62).
Most patients with MS do well during their pregnancy and do not require disease-modifying therapies (DMTs). The majority of DMTs are pregnancy Category C medications, with glatiramer acetate classified a B, leflunomide an X, and mitoxantrone a D. While there are no clear data, patients are generally advised to stop DMTs before becoming pregnant. Medications can be found in the breastmilk. As there are no adequate studies, resumption of a DMT is usually held until after the mother has stopped breastfeeding.
MS should have no impact on most obstetric decisions, including pain management. There are outdated, flawed reports that epidural anesthesia may increase the risk of a postpartum relapse. Consistent studies have shown this to not be true. Epidural analgesia does not increase the risk of relapse of the level of disability following pregnancy. There is no contraindication to vaginal or caesarean delivery. Most patients with MS will have routine deliveries. Patients with significant pelvic floor weakness (due to spinal cord lesions) may have prolonged labor, and may experience increased muscle fatigue due to a rise in body temperature.
• MS is an inflammatory demyelinating condition of the CNS
• The hallmark of MS is the occurrence of acute, focal neurologic symptoms (relapses) with gradual, though sometimes incomplete, recovery of function (remission)
• In time, after repeated inflammatory attacks, progressive neurologic deterioration may occur (progressive multiple sclerosis)
• The diagnosis is made based on the presence of characteristic clinic events corroborated by paraclinical evidence (specifically MRI) demonstrating a disease that is disseminated in space and time
• There is an ever-expanding arsenal of treatment options to slow, or halt, the disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree