Chapter 20 Myoclonus
Phenomenology, etiology, physiology, and treatment
Literally, myoclonus means “a quick movement of muscle.” Sudden, brief jerks may be caused not only by active muscle contractions, positive myoclonus, but also by sudden, brief lapses of muscle contraction in active postural muscles, negative myoclonus or asterixis (Shibasaki, 1995).
The history of myoclonus has been described by Marsden and colleagues (1982), Hallett (1986), and Fahn (2002). Friedreich first defined myoclonus as a discrete entity in a case report published in 1881 of a patient with essential myoclonus. He wanted to separate the involuntary movement that he saw from epileptic clonus, a single jerk in patients with epilepsy, and chorea, which was the only previously described type of involuntary movement. For the next 10–20 years, many other types of involuntary movements, such as tic and myokymia, were also called myoclonus, but in 1903 Lundborg proposed a classification of myoclonus that cleared up much of the confusion. Lundborg classified myoclonus into three groups: symptomatic myoclonus, essential myoclonus, and familial myoclonic epilepsy.
Myoclonus is a common movement disorder. Caviness and Maraganore (Caviness et al., 1999) reviewed the record linkage system for Olmsted County at the Mayo Clinic, Rochester, Minnesota for the years 1976–1990 and found an average annual incidence of myoclonus of 1.3 cases per 100 000, and a prevalence in 1990 of 8.6 cases per 100 000.
Classification of myoclonus
Myoclonus can be classified on the basis of its clinical characteristics, its pathophysiology, or its cause (Table 20.1) (Marsden et al., 1982; Hallett et al., 1987; Fahn, 2002).
Table 20.1 Classification schemes for myoclonus
| Clinical | Pathophysiology | Etiology |
|---|---|---|
| Spontaneous Action Reflex Focal Axial Multifocal Generalized Irregular Repetitive Rhythmic | Cortical Focal Multifocal Generalized Epilepsia partialis continua Thalamic Brainstem Reticular Startle Palatal Spinal Segmental Propriospinal Peripheral Ballistic | Physiological Essential Epileptic Symptomatic Storage diseases Cerebellar degenerations Basal ganglia degenerations Dementias Viral encephalopathies Metabolic encephalopathies Toxic encephalopathies Hypoxia Focal damage |
Pathophysiology
The clinical features of myoclonus and the results of electrophysiologic investigation allow a relatively precise prediction as to its site of origin in the nervous system (Shibasaki and Hallett, 2005; Hallett and Shibasaki, 2008). On this basis, myoclonus may be shown to arise in the cerebral cortex (cortical myoclonus); in the brainstem (brainstem myoclonus); or in the spinal cord (spinal myoclonus). Rarely, lesions of spinal roots, nerve plexi, or peripheral nerves can cause myoclonus (peripheral myoclonus). Hemifacial spasm might be considered a form of peripheral myoclonus, due most often to neurovascular compression.
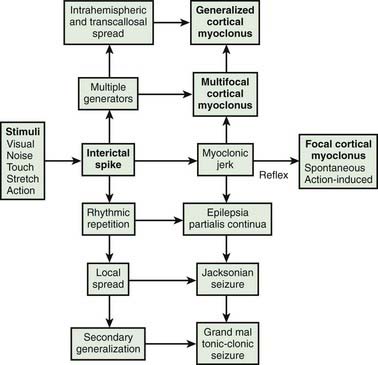
Cortical myoclonus, in which the abnormal activity originates in the sensorimotor cortex and is transmitted down the spinal cord in pyramidal pathways, may manifest as focal jerks, sometimes repetitive (epilepsia partialis continua), which can propagate into focal motor seizures; with or without secondary generalization (Hallett et al., 1979; Shibasaki and Hallett, 2005; Hallett and Shibasaki, 2008).
Myoclonus arising in the brainstem may take different forms (Hallett, 2002). One employs the pathways responsible for the startle reflex, causing exaggerated startle syndromes and the hyperekplexias. Another is independent of startle mechanisms, but causes generalized muscle jerks (brainstem reticular myoclonus). A third is the palatal myoclonus (tremor) syndrome.
Finally, one pathophysiologic type of essential myoclonus takes the form of spontaneous or action-induced ballistic electromyographic (EMG) bursts in muscles, with inappropriate overflow into other muscles (ballistic movement overflow myoclonus) (Hallett et al., 1977b).
Cause
With regard to etiology, so many neurologic conditions may produce myoclonus that such a classification runs to a textbook of neurology (Table 20.2) (Marsden et al., 1982; Hallett et al., 1987; Fahn, 2002; Hallett and Shibasaki, 2008). It is, however, useful to consider several broad categories.
Table 20.2 Etiologic classification of myoclonus
| I. Physiological myoclonus (normal subjects) |
| II. Essential myoclonus (no known cause other than genetic and no other gross neurologic deficit) |
| III. Epileptic myoclonus (seizures dominate and no encephalopathy, at least initially) |
| IV. Symptomatic myoclonus (progressive or static encephalopathy dominates) |
Physiologic myoclonus refers to muscle jerks occurring in certain circumstances in normal subjects. These include sleep jerks (hypnic jerks) and hiccup. Essential myoclonus consists of multifocal myoclonus in which there is no other neurologic deficit or abnormality on investigation. Epileptic myoclonus refers to conditions in which the major clinical problem is one of epilepsy, but one of the manifestations of the epileptic attacks is myoclonic jerks. Symptomatic generalized myoclonus refers to those many conditions in which generalized or multifocal muscle jerking is a manifestation of an underlying identifiable neurologic disease. Psychogenic myoclonus refers to myoclonus produced as a conversion symptom or as “voluntary” or “simulated” myoclonus (Thompson et al., 1992; Monday and Jankovic, 1993).
In the survey by Caviness et al. (1999), symptomatic myoclonus was most common, followed by epileptic myoclonus and essential myoclonus. Dementing illnesses were the commonest cause of symptomatic myoclonus.
Neurophysiologic assessment
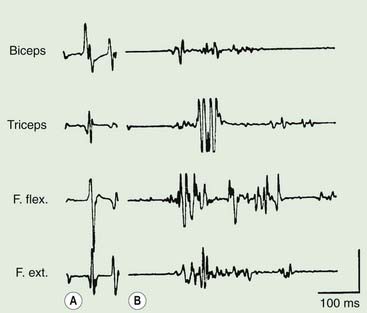
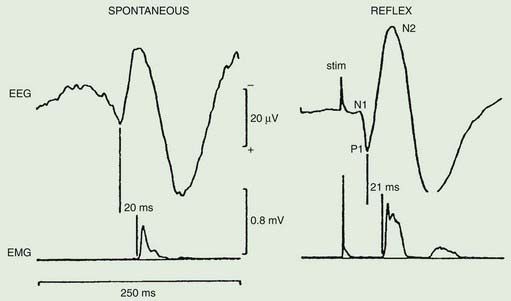
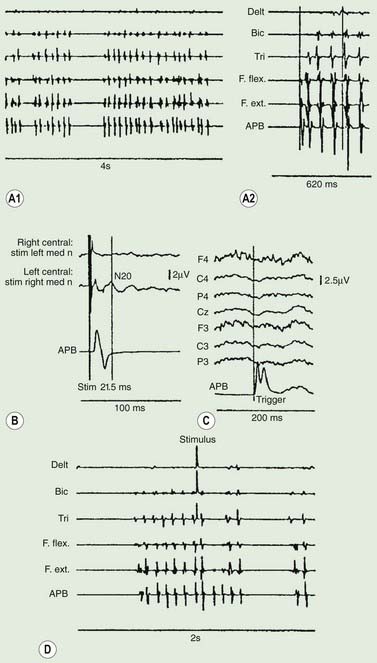
Polymyography (recording the duration, distribution, and stimulus sensitivity of EMG activity in affected muscles) is the first step in assessing a patient with myoclonus (Toro and Hallett, 2004; Shibasaki and Hallett, 2005; Hallett and Shibasaki, 2008). Most myoclonic jerks are due to brief EMG bursts of 10–50 ms. EMG bursts in the 100 ms range are seen in some situations such as essential myoclonus. Longer jerks of more than 100 ms are likely to be dystonic. Agonists and antagonists usually fire synchronously (Fig. 20.1).
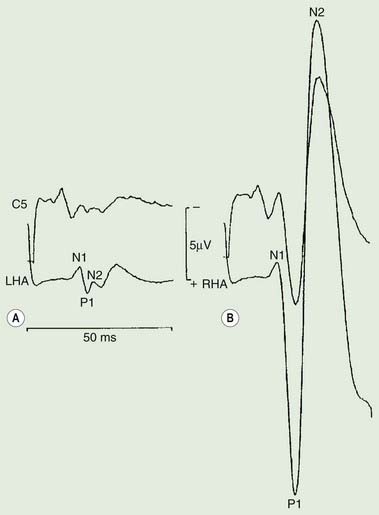
The distribution of muscles involved may suggest that it arises as a result of a lesion of a peripheral nerve, part of a plexus, a spinal root or a restricted number of segments of the spinal cord (segmental myoclonus). Myoclonic muscle jerks affecting axial muscles (neck, shoulders, trunk, and hips) may arise in the brainstem as an exaggerated startle response or brainstem reticular myoclonus, or in the spinal cord as propriospinal myoclonus. In brainstem myoclonus, there is no preceding cortical discharge. Cranial nerve muscles are usually activated from the XI nucleus up the brainstem; limb and axial muscles are activated in descending order. In propriospinal myoclonus, the first muscles activated are usually in the thoracic cord, with slow upward and downward spread. Cortical myoclonus is indicated when somatosensory evoked potentials produced by peripheral nerve stimulation are pathologically enlarged, and a cortical correlate can be back-averaged in the ongoing EEG by triggering from the EMG of the muscle jerk (Hallett et al., 1979; Shibasaki and Hallett, 2005). Stimuli generating giant somatosensory evoked potentials often provoke a subsequent EMG burst of myoclonic activity (the C reflex), at a latency compatible with conduction through fast corticomotoneuron pathways from the motor cortex to muscle. The giant somatosensory evoked potentials usually consist of an enlarged P25/N33 component; the first major cortical negative peak (N20), reflecting arrival of the sensory volley in the cortex, usually is of normal size. The motor volleys in cortical myoclonus activate the cranial and limb musculature in descending order via fast conducting corticospinal pathways. Abnormal corticomuscular and intermuscular coupling can also be a sensitive physiologic feature in cortical myoclonus (Grosse et al., 2003). The increased cortical excitability in cortical myoclonus may well be due to loss of inhibitory interneurons (Hanajima et al., 2008). Cortical reflex myoclonus usually consists of positive EMG discharges, but negative cortical reflex myoclonus also occurs (Shibasaki, 1995; Tassinari et al., 1998), where a giant somatosensory cortical potential is time-locked to EMG silence. Subcortical myoclonus is suggested when reflex myoclonus triggered by peripheral stimuli occurs after a latency that is too short to involve cortical pathways (Thompson et al., 1994; Cantello et al., 1997).
Psychogenic myoclonus is suggested if stimulus-evoked jerks are of very variable latency and longer than a voluntary reaction time (Thompson et al., 1992; Brown, 2006), and when the Bereitschaftspotential is evident prior to EMG bursts on jerk-locked back-averaging of the EEG, as in voluntary movement (Terada et al., 1995). Monday and Jankovic (1993) reported the clinical features of 18 such patients. There were 13 women and 5 men with an age range of 22–75 years. The myoclonus was present for 1–110 months; and it was segmental in 10, generalized in 7, and focal in 1. Stress precipitated or exacerbated the myoclonic movements in 15 patients; 14 had a definite increase in myoclonic activity during periods of anxiety. The following findings helped to establish the psychogenic nature of the myoclonus: clinical features incongruous with “organic” myoclonus, evidence of underlying psychopathology, an improvement with distraction or placebo, and the presence of incongruous sensory loss or false weakness. Over half of all patients with adequate follow-up improved after gaining insight into the psychogenic mechanisms of their movement disorder.
Focal myoclonus
Jerking of one body part may arise anywhere from the peripheral nerve to the motor cortex (Table 20.3). With peripheral nerve lesions, the myoclonus may well arise because of secondary central nervous system changes (Shin et al., 2007). Another possibility is that there is a peripheral ectopic generator that triggers the myoclonus (Tyvaert et al., 2009). Spontaneous rhythmic focal myoclonus is likely to be epilepsia partialis continua or spinal segmental myoclonus. Stimulus-sensitive myoclonus, particularly affecting the distal limbs, is most likely to arise in the cerebral cortex. Polymyography, somatosensory evoked potentials, and back-averaging from spontaneous jerks will usually suffice to define the site of origin.
Table 20.3 Causes of focal myoclonus
| Category | Source | Etiology |
|---|---|---|
| Peripheral lesions | Peripheral nerve | Trauma |
| Plexus | Tumor | |
| Nerve roots | Electrical injury | |
| Surgery | ||
| Hemifacial spasm | ||
| Spinal lesions | (a)Spinal segmental myoclonus | Trauma |
| Inflammation | ||
| Infection | ||
| Demyelination | ||
| Tumor | ||
| Arteriovenous malformation | ||
| Ischemic myelopathy | ||
| Spondylitic myelopathy | ||
| Spinal anesthesia | ||
| Idiopathic | ||
| (b)Propriospinal myoclonus | Trauma | |
| Tumor | ||
| Idiopathic | ||
| Brainstem lesions | Palatal myoclonus | See Table 20.5 |
| Cortical lesions | Sensorimotor cortex | See Table 20.6 |
| Idiopathic |
A variety of lesions of the peripheral nerve and spinal cord have been described as causing focal myoclonus (Frenken et al., 1976; Jankovic and Pardo, 1986; Massimi et al., 2009). These include peripheral nerve tumors, trauma or radiation, and spinal cord trauma, tumor, vascular lesions, multiple sclerosis and other inflammatory myelitis. Such spinal segmental myoclonus characteristically is rhythmic (0.5–3 Hz), is confined to muscles innervated by a few spinal segments, and persists during sleep (Fig. 20.2 and Video 20.3). Spinal segmental myoclonus appears to be due to loss of inhibitory interneurons in the posterior horns, which may be demonstrated physiologically (Di Lazzaro et al., 1996). As a result, there is spontaneous bursting of groups of anterior horn cells. Usually it is not stimulus-sensitive, but it can be (Davis et al., 1981). Clonazepam is most likely to help. One case was responsive to topiramate (Siniscalchi et al., 2004). ![]()
Palatal myoclonus (alternately referred to as palatal tremor) describes the syndrome of rhythmic palatal movements at about 1.5–3 Hz, sometimes synchronously affecting the eyes, face, tongue and larynx, and even the head, trunk, intercostal muscles and diaphragm (Deuschl et al., 1990, 1994a, 1994b). The movements usually are bilateral and symmetric, occurring between 100 and 150 times per minute, and, in some circumstances, persist during sleep. There are two forms, essential palatal myoclonus and symptomatic palatal myoclonus (Table 20.4 and Video 20.4). The main symptom caused by these movements, seen only in the essential form, is clicking in the ear due to rhythmic contractions of tensor veli palatini which opens the eustachian tube. The tensor veli palatini is innervated by the trigeminal nerve. ![]()
Table 20.4 Differences between essential and symptomatic palatal myoclonus
| Essential palatal myoclonus |
| Symptomatic palatal myoclonus |
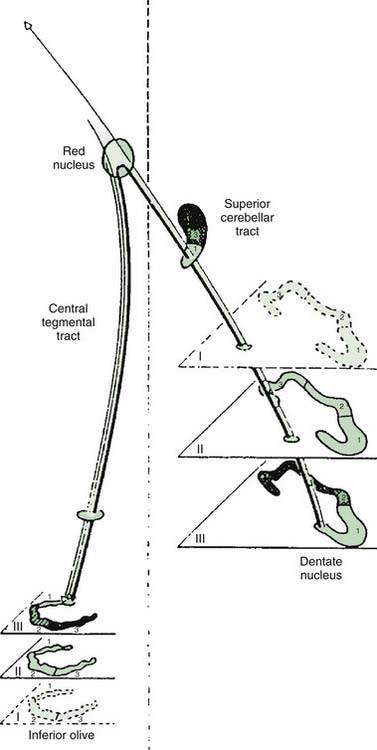
In many cases, a focal brainstem lesion can be identified (symptomatic palatal myoclonus/tremor), usually a stroke, encephalitis, multiple sclerosis, tumor, trauma, or degenerative disease (Table 20.5). Often the palatal myoclonus appears some months after the acute lesion. Such patients will have symptoms appropriate to the brainstem damage and to the underlying cause, in addition to the palatal myoclonus. They also may have pendular vertical nystagmus (ocular myoclonus), as well as facial, intercostal, and diaphragmatic jerks in synchrony with the palatal myoclonus. The pathology in symptomatic palatal myoclonus damages the dentato-olivary pathway, often in the brainstem central tegmental tract (Fig. 20.3); the resulting denervation of the inferior olive leads to hypertrophy (Fig. 20.4), which can be seen on brain MRI (Deuschl et al., 1994b). In this situation, the palatal movement is due to contractions of the levator veli palatini (innervated by the nucleus ambiguus). Only rarely can the ear clicking be caused by spontaneous contractions of the levator veli palatini (Jamieson et al., 1996).
Table 20.5 Causes of palatal myoclonus in 287 patients
| Condition | Number | |
|---|---|---|
| (A) | Primary (essential) palatal myoclonus | 77 |
| (B) | Secondary (symptomatic) palatal myoclonus | 210 |
| 1.Vascular disease | 115 | |
| 2.Trauma | 23 | |
| 3.Tumor (brainstem) | 19 | |
| 4.Multiple sclerosis | 9 | |
| 5.Degenerations | 7 | |
| 6.Encephalitis | 5 | |
| 7.Other; e.g., arteriovenous malformation, herpes zoster | 32 | |
From Deuschl G, Mischke G, Schenck E, Schulte-Monting J, Lucking CH. Symptomatic and essential rhythmic palatal myoclonus. Brain 1990;113(Pt 6):1645–1672.
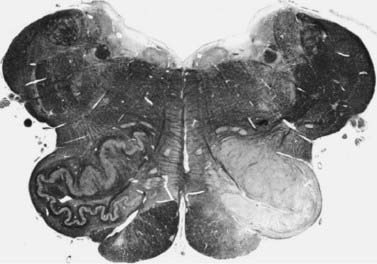
Figure 20.4 Unilateral hypertrophy of the inferior olive in a patient with unilateral symptomatic palatal myoclonus.
From Deuschl G, Toro C, Valls-Solé J, et al. Symptomatic and essential palatal tremor. 1. Clinical, physiological, and MRI analysis. Brain 1994;117:775–88, with permission.
In other cases, no cause is evident (essential palatal myoclonus/tremor). The complaint of these patients is the clicking; the eye and other structures are not involved; and there are no other symptoms or signs. These patients tend to be younger and do not appear to develop other diseases. Clonazepam, anticholinergics, or carbamazepine may help some patients with palatal myoclonus (Sakai and Murakami, 1981; Jabbari et al., 1987). Sumatriptan can be effective (Scott et al., 1996), but not in patients with symptomatic palatal myoclonus. Ear clicking can be relieved by injection of botulinum toxin into the appropriate muscles (Deuschl et al., 1991; Jamieson et al., 1996). Ear clicking occasionally may be due to simple partial seizures (Ebner and Noachtar, 1995). Some of these patients may be psychogenic (Pirio Richardson et al., 2006).
Cortical myoclonus produces spontaneous muscle jerks (spontaneous cortical myoclonus), jerks triggered by external stimuli (cortical reflex myoclonus), or jerks on movement (cortical action myoclonus) (Hallett et al., 1979; Obeso et al., 1985). Such patients have neurophysiologic evidence of an abnormal discharge in the sensory motor cortex generating the myoclonic jerks via fast conducting corticomotoneuron pathways (Figs 20.5 and 20.6). Myoclonus arising in the cerebral cortex can be focal affecting one body part, such as a hand or foot, but multiple cortical discharges can cause multifocal jerks, each jerk being due to a discrete discharge in one part of the motor cortex. In addition, cortical discharges can cause generalized muscle jerks, either by intracortical and transcallosal spread to activate both motor cortices (Brown et al., 1991a, 1996; Brown and Marsden, 1996), or by cortico-reticular pathways activating brainstem myoclonic generators. Such multifocal and generalized cortical myoclonus is discussed below. Patients with focal cortical myoclonus also exhibit epilepsia partialis continua (Juul-Jensen and Denny-Brown, 1966), partial motor seizures, and secondary generalization with tonic-clonic grand mal seizures (Cowan et al., 1986) (Fig. 20.7). Focal slow-frequency repetitive transcranial magnetic stimulation has suppressed focal cortical myoclonus in a patient with cortical dysplasia (Rossi et al., 2004).
Epilepsia partialis continua is defined clinically as a syndrome of continuous focal jerking of a body part, usually localized to a distal limb, occurring over hours, days or even years, due to a cerebral cortical abnormality (Cockerell et al., 1996) (Fig. 20.8). The most common etiologies now are Rasmussen encephalitis and cerebrovascular disease. Most, but not all patients, have epileptic or other EEG abnormalities, and over half have identifiable cortical lesions on brain MRI. A similar clinical picture can occur with subcortical lesions, in which case it is suggested that the term “myoclonia continua” be employed (Cockerell et al., 1996). Cortical myoclonus sometimes is so rhythmic as to produce a tremor (Ikeda et al., 1990; Toro and Hallett, 2004) (Video 20.5). A focal cortical lesion produces focal myoclonus in the opposite appropriate body part. Such lesions include those due to vascular disease, tumor, granulomas, and focal encephalitis (Table 20.6) (Thomas et al., 1977; Cockerell et al., 1996). Chronic, prolonged focal myoclonic jerking in children suggests the possibility of Rasmussen encephalitis. ![]()
Table 20.6 Causes of focal cortical myoclonus and epilepsia partialis continua
| Etiology | Number | |
|---|---|---|
| Thomas et al. (1977) | Cockerell et al. (1996) | |
| Infarct or cerebral hemorrhage | 8 | 9 |
| Tumor | 5 | 4 |
| Astrocytoma | ||
| Hemangioma | ||
| Lymphoma | ||
| Uncertain | ||
| Encephalitis (including Rasmussen encephalitis) | 5 | 7 |
| Trauma | 2 | 1 |
| Hepatic encephalopathy | 2 | – |
| Subarachnoid hemorrhage | 1 | – |
| Unknown | 9 | 9 |
| Total | 32 | 30 |
| Others | ||
| Subdural hematoma | ||
| Abscess | ||
| Granuloma (TB) | ||
| Multiple sclerosis | ||
| Meningitis | ||
| Nonketotic hyperglycemia | ||
| Focal gliosis | ||
| Spinocerebellar degeneration | ||
| Mitochondrial disease | ||
Rasmussen encephalitis is a disorder of childhood and adolescence in which a unilateral focal seizure disorder is accompanied by a progressive hemiplegia due to focal cortical inflammation and destruction (Hart, 2004; Freeman, 2005). The seizures are severe, often with epilepsia partialis continua, partial motor seizures, and secondary generalization. The EEG may show focal epileptiform activity, or periodic lateralized discharges (PLEDs), or both. In addition to the progressive hemiplegia, there often is cognitive decline. The cause appears due to an autoimmune process, specifically to the glutamate receptor (GluR) 3 subunit, with such antibodies acting as a GluR agonist to cause an excitotoxic cascade (Gahring et al., 2001). Immunotherapy (steroids, plasmapheresis, or intravenous human immunoglobulin (IVIg)) may help some, but hemispherectomy may be necessary.
Axial myoclonus
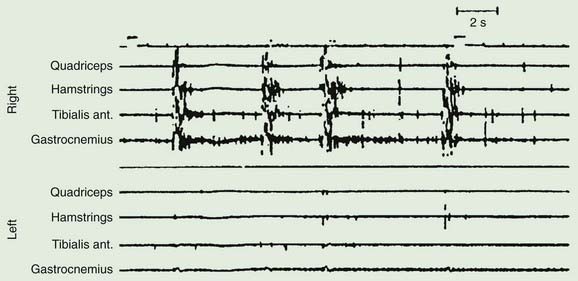
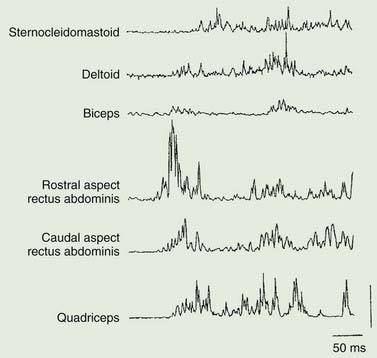
Axial myoclonic jerks may arise in the spinal cord or brainstem. Propriospinal myoclonus involves long propriospinal fibers in the spinal cord distributed to axial muscles (Brown et al., 1991c) (Fig. 20.9). The most prominent movement of propriospinal myoclonus is truncal flexion, and it can be either spontaneous or stimulus induced (Video 20.6). A review of 60 patients noted a middle-aged male predominance (Roze et al., 2009). The myoclonus tended to be worse when lying down and at wake–sleep transitions. A premonitory sensation might be present before the jerks. The etiology of many of these cases is not clear, although injury to the spinal cord from trauma, infection, tumor, and disk herniation has been described (Capelle et al., 2005; Shprecher et al., 2010). Diffusion tensor imaging with fiber tracking of the spinal cord may reveal abnormalities (Roze et al., 2009). The EMG pattern of propriospinal myoclonus can be mimicked voluntarily, indicating that psychogenic myoclonus should be in the differential diagnosis in these cases (Kang and Sohn, 2006), and several such cases have now been reported (Williams et al., 2008; Slawek et al., 2010). In a series of 35 patients referred with axial jerks, 34 of them were considered to be psychogenic (van der Salm et al., 2010). More work is needed in this area to understand this disorder better. The most effective drug for treatment has been clonazepam (Roze et al., 2009). ![]()
Brainstem reticular myoclonus (Table 20.7 and Fig. 20.10) may follow cerebral anoxia, and probably is responsible for the generalized muscle jerks that occur in a number of toxic myoclonic syndromes, as for example in uremia and other metabolic disturbances, as well as those precipitated by drugs. It is characterized by a generalized axial myoclonic jerk which starts in muscles innervated by the lower brainstem, then spreading up the brainstem and down the spinal cord (Hallett et al., 1977a). There is no time-locked cortical correlate and sensory evoked potentials are normal. Such brainstem reticular myoclonus may occur after cerebral anoxia, and can be seen in some patients with the stiff-man syndrome (Leigh et al., 1980). Brainstem lesions, such as occur in multiple sclerosis, also may be responsible (Smith and Scheinberg, 1990).
Table 20.7 Two types of brainstem reticular myoclonus