Nasopharyngeal, Anterotemporal, and Sphenoidal Electrodes
Andres M. Kanner
Travis Stoub
Steve Bild
INTRODUCTION
Temporal lobe epilepsy (TLE) is the most frequent type of epilepsy of focal origin affecting adults. It is a heterogeneous type of epilepsy as the epileptogenic zone can be restricted to mesial temporal structures and/or involve temporal lateral neocortex. Accordingly, the yield of detection of epileptiform activity with scalp electrodes depends on the following variables: (i) the source and extent of the epileptogenic area relative to the position of the scalp electrodes, (ii) the electric field generated by the epileptiform activity and the orientation of the electric vectors, and (iii) the extent of propagation of the epileptiform activity from mesial to temporal lateral regions. For example, epileptiform discharges originating in temporal lateral neocortex would be readily recorded with the standard 10-20 midtemporal scalp electrodes. On the other hand, such electrodes may often fail to detect epileptiform activity of mesial temporal origin. For one, the amygdala nucleus is not a laminar structure and, therefore, it generates very restricted electrical field potentials. Likewise, the hippocampus, with its “double C shape,” appears as a closed loop electrically and generates epileptiform discharges with a vertical dipole that could only be detected by frontotemporal and temporal lateral electrodes if the epileptiform activity were to propagate to those regions.
Several studies have shown that the standard 10-20 international system of scalp electrode placement may often fail to identify epileptiform activity of mesial-temporal lobe origin (1, 2, 3 and 4). Indeed, the overall findings of these studies suggest that the lateral and anterior portion of the temporal lobe is not adequately covered using the standard 10-20 system. In the 10-20 system, electrodes F7, F8, T7, T8 (also known as T3-T4), and A1-A2 have been used to record anterior temporal electrical activity. Yet, electrodes F7 and F8 are positioned over the inferior frontal gyrus and the rostral pars triangularis (1), while electrodes T7 and T8 are positioned over the middle and superior temporal gyri posterior to the rolandic fissure (1). Hence, the angle subtended by these electrodes relative to the mesial-temporal source of the epileptiform discharges may often be inadequate (lest it propagates to temporal lateral regions).
This problem was recognized in the first half of the 20th century, which led to the development of sphenoidal electrodes (SE) and nasopharyngeal electrodes (NPE). Later on, anterotemporal (ATE), minisphenoidal (MSE), and basal-temporal (scalp) electrodes (BTE) started to be used in an attempt to avert the inconvenience and discomfort associated with the insertion of SE.
Studies carried out in the 1980s and 1990s reported that SE and ATE identified a significantly greater number of epileptiform discharges that were not recorded with the standard temporal electrodes included in the 10-20 system. The noninvasive and user-friendly placement of ATE led to their use in most epilepsy centers. Yet, in the last two decades an ongoing debate has been taking place on whether SE and NPE add any additional data to those yielded by ATE. The purpose of this chapter is to review the conditions in which SE, NPE, and ATE should be used in patients suspected of suffering from TLE of mesial-temporal origin.
TECHNICAL ASPECTS
Sphenoidal Electrodes
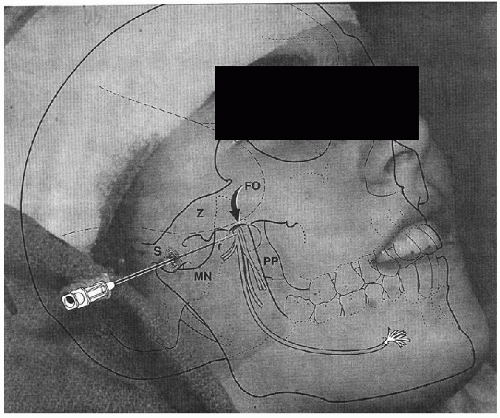
Jones was the first to report the use of SE in 1950 (5). At that time, the electrodes consisted of a fine insulated needle electrode inserted under local anesthesia. The point of entry was under the zygomatic arch through the mandibular notch and targeted a position under the base of the skull lateral to the foramen ovale (FO). In 1957, Marshall introduced SE made of flexible wire initially made of stainless steal to allow for longer periods of recording (6), particularly during presurgical EEG monitoring studies, which over the following two decades underwent further modifications. More recently, SE consisted of multistranded silver and platinum wires, the latter to avert its lateral displacement (7).
Typically, the insertion of SE is carried out at the bedside, under local anesthesia with lidocaine. The SE wire is mounted on a #22-gauge lumbar puncture needle and the recording end is inserted into the lumen of the needle. The needle is inserted through the skin at a point 3 cm anterior to the external auditory canal, beneath the zygomatic arch, and through the mandibular notch oriented in a 10° posterior direction targeting the lateral border of the FO (Fig. 32.1). The tip of the needle is advanced to a depth of 4 to 5 cm beneath the skin’s surface or until the patient reports mandibular pain (8).
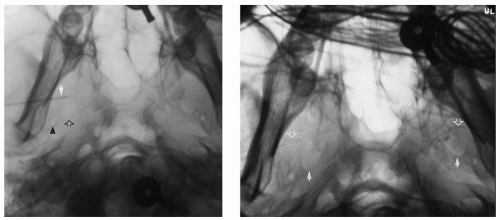
Yet, the blind insertion of SE can have several problems that may negate any advantage of their use. First, when SE are inserted blindly, the recording tips may end up in a position at a distance from the FO, either anterior or posterior to it. Recordings from SE positioned posterior to the FO are hampered by recording through the thick (2 cm) petrous pyramid, while a position anterior or lateral to the foramen has the double disadvantage of recording through bone and at an increased distance from target mesial-temporal structures. To overcome this potential problem, Kanner et al. suggested the insertion of SE under fluoroscopic guidance (Fig. 32.2, left and right panels) (9,10). This technique ensured that the recording tip is
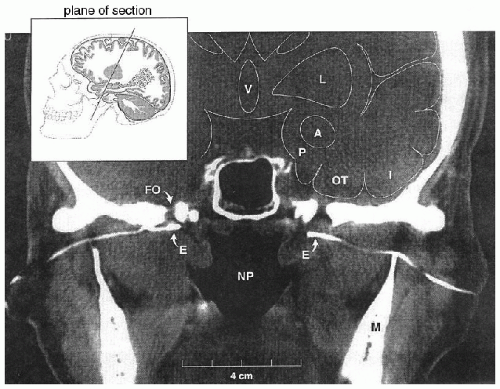
positioned inferior to FO (Fig. 32.3). Furthermore, the variable shape of the sphenoid wing among patients can result in an obstacle to the insertion of the needle when done blindly, which in turn results in additional discomfort to patients. The insertion under fluoroscopic guidance averts this problem. On the other hand, the higher costs associated with the insertion of SE under fluoroscopic guidance is a disadvantage of this insertion technique.
positioned inferior to FO (Fig. 32.3). Furthermore, the variable shape of the sphenoid wing among patients can result in an obstacle to the insertion of the needle when done blindly, which in turn results in additional discomfort to patients. The insertion under fluoroscopic guidance averts this problem. On the other hand, the higher costs associated with the insertion of SE under fluoroscopic guidance is a disadvantage of this insertion technique.
Minisphenoidal Electrodes
MSE were introduced by Laxer in 1984 as an alternative to SE (11) in a study of 100 patients, 34 of whom had epileptiform activity of temporal lobe origin. In 21 of these patients, the epileptiform activity was only detected by MSE. MSE consist of stainless steel 0.5 cm needles inserted under the zygomatic arch, yet the insertion of these electrodes is associated with less discomfort than that of SE (12). In a study of 100 patients, Buchhalter et al. found that
MSE and SE yielded comparative data in nine patients; however, SE identified interictal epileptiform activity not detected by MSE in six patients (12). On the other hand, other investigators, however, failed to find any advantage of MSE to ATE (3,13).
MSE and SE yielded comparative data in nine patients; however, SE identified interictal epileptiform activity not detected by MSE in six patients (12). On the other hand, other investigators, however, failed to find any advantage of MSE to ATE (3,13).
Nasopharyngeal Electrodes
In 1948, Roubicek and Hill (14) introduced NPE, and their increased yield of localization of epileptiform activity of mesial temporal origin was confirmed by several authors (3,14). These electrodes consist of a solid silver rod with a small silver ball at the tip (Fig. 32.4) of 0.67 and 0.055 inches in diameter for use in adults and children, respectively (15, 16). The shaft is flexible and malleable and is insulated up to the ball tip with six or seven coats of insulex varnish. The distal 1 inch is angled about 20°, but such angle can be changed to fit any nasal passage. Unlike SE, NPE can be inserted by EEG technologists through the nasal cavity to target the posterior pharyngeal wall. When positioned optimally, the ball tip is expected to lie in an area beneath the foramen lacerum.
Scalp Anterotemporal and Basal-Temporal Electrodes
In 1960, Silverman suggested a position for scalp electrodes outside of the 10-20 international system aimed at recording electrical activity of anterotemporal origin (17). These ATE, labeled T1 and T2, are positioned “one centimeter above a point one third of the distance along a line from the external auditory meatus to the outer canthus of the ipsilateral eye.” A disadvantage of T1 and T2 is that their locations are not proportional to 10-20 or 10-10 system positions and thus display disproportionate voltages when utilized in bipolar derivations (Fig. 32.5). Accordingly, referential derivations may be preferable when using these electrodes (Figs. 32.6 and 32.7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree