11 Neuro-Developmental Treatment Assumptions of Motor Dysfunction: Stroke and Adult-Onset Hemiplegia This chapter provides core knowledge for the examination, evaluation, and intervention planning for individuals who have suffered a stroke or traumatic brain injury (TBI). Common clinical presentations seen in individuals poststroke are explored. The underlying single- and multisystem impairments contributing to these clinical presentations are discussed as well as their influence on the International Classification of Functioning, Disability and Health (ICF) activity and participation domains of functioning. Learning Objectives Upon completing this chapter the reader will be able to do the following: • Recognize four common clinical presentations and subcategorizations within these presentations that are frequently • seen in individuals following stroke. • List primary and secondary impairments that contribute to these common clinical presentations. • Identify intervention principles specific to each clinical presentation that would enhance clinical outcomes. Within the Neuro-Developmental Treatment (NDT) Practice Model, it is accepted that individuals are unique in their participation roles and thus perform activities to fulfill these personal and societal roles. This occurs because of a complexity of interacting contextual factors (environmental, cultural, genetic, etc.), which leads to a unique combination of abilities and then disabilities postinjury, after stroke or TBI. In an effort to assist clinicians who work with this population of individuals, a categorization based on these common clinical presentations is proposed. Within each of these categorizations, the collection of system impairments (i.e., impairment groupings) is discussed. Intervention strategies to effectively manage these impairment groupings and, thus, ultimately influence activity and participation on an individual basis are also briefly discussed. Stroke is the leading cause of disability in adults in Canada and the United States. In 2014 alone, 795,000 individuals in the United States and 50,000 individuals in Canada will have suffered a stroke.1,2,3 Statistics show 20 to 32% of these individuals will die from their stroke, and 43% of the survivors will be left with some form of permanent disability.1,2,3 A stroke interrupts the blood flow to the brain, often leading to damage in some brain functions. This brain damage can cause paralysis of some parts of the body and/or difficulties with affected body structures and functions, for example, somatosensory, visual, cognitive, perceptual, and language function. There are two mechanisms for stroke: ischemic infarction and hemorrhage. Statistics vary but ~ 70 to 85% of strokes are from ischemic events and 10 to 30% are from hemorrhages.2,3,4 An ischemic stroke occurs when a blood clot stops the blood flow to an area of the brain. A hemorrhagic stroke occurs when a weakened or diseased blood vessel ruptures, causing blood to leak into brain tissue. Hemorrhagic strokes typically occur from aneurysms and arteriovenous malformations (AVMs). There may be other causes of strokes, such as tumors or infections.2,4 Strokes are most often classified according to mechanism (ischemic or hemorrhagic) and the location of the lesion (e.g., right brain, left brain, cortical, cerebellar, thalamic, brainstem, middle cerebral artery [MCA] involvement, etc.).4 It is not the intent of this chapter to repeat what is well documented in the neurology texts. The reader is encouraged to refer to well researched resources and expert knowledge for this type of information.3,4,5,6 The clinical presentations, the multisystem posture and movement impairments, and the single-system impairments observed in the population of individuals with traumatic brain injury (TBI) can be similar in many ways to those found in the population of individuals poststroke. The more global term of acquired brain injury includes the subcategories of stroke and TBI. Thus the diagnosis and clinical presentation of brain injury are not specifically and separately addressed in this chapter. It is true that damage to a certain area of the brain may result in certain functional losses, at least temporarily. For example, individuals with damage to the posterior inferior frontal gyrus (Broca’s area) often demonstrate expressive aphasia.4 However, what is also true is that the brain is a highly integrated organ with complex interconnections among its parts (structures) and with the rest of the body systems. Older beliefs and knowledge about the brain structures and functions led health care professionals and researchers to have a somewhat narrow and limited perspective of the clinical pictures seen as a result of damage to a particular area of the brain; it was thought that all individuals with damage to the same area of the brain would present clinically with discreet impairments depending on the central nervous system (CNS) site of the lesion, as described previously in the example of Broca’s area and expressive aphasia. However, it is now known that the brain is not rigid in its functional organization or in its response to injury and subsequent recovery process. The brain’s organization and its response to injury (and its ability to recover) is variable and based on personal factors, contextual factors, environmental factors, age, degree of damage/impairment, and so on. Thus the clinical picture from one individual to another with damage in the same brain location may be quite different.6 As discussed in Chapter 12 about motor control and Chapter 15 about neuroplasticity and recovery, each individual’s brain organization is unique. Clinicians may limit themselves in evaluation and intervention, as well as prognostic expectations for their clients who have had a stroke, when they hold preconceived beliefs and expectations of an individual’s clinical presentation based solely upon damage to a particular area of the brain. There is some benefit to knowing what impairments to expect with an individual who has had damage in a particular area of the brain (right cortex, cerebellum, thalamus, brain stem, MCA distribution, etc.). This knowledge provides useful general information to clinicians to predict the systems likely to be impaired. Table 11.1 outlines these global functional areas for the various brain structures/regions. However, as Berta Bobath said, “It is important to see what you see, not what you think you see.”7 Damage from a stroke typically occurs in focal areas of the brain—the infarcted area. Even in most individuals with TBI, there is often greater damage in one area of the brain. This focal damage explains why individuals poststroke typically present with more profound impairments on one side of the body leading to asymmetry in postural alignments and sensory and movement abilities. However, because of the brain’s integrated organization, damage to an area in the right cortex of the brain, for example, will influence functional performance on the left side of the trunk and in the left limbs, and also on the right side of the body and bilaterally in the trunk. Similarly, focal damage in other areas of the cortex, cerebellum, and brain stem can contribute to posture and movement dysfunction on both sides of the body and bilaterally in the trunk. Thus it is best to think of a more affected side and a less affected side of the body (in the trunk and limbs) after a stroke versus there being a good or spared side and a bad or damaged side. Commonly used terms to describe the more affected side poststroke are hemiplegia and hemiparesis, meaning total paralysis of one side of the body (i.e., more severe) and weakness of one side of the body (less severe), respectively.4 The possible impairments resulting from a stroke, as listed in Table 11.1, can be numerous, and the influence on activity and participation domains of function can be wide ranging.1 An understanding of the posture and movement impairments caused by stroke is particularly relevant to therapists who practice Neuro-Developmental Treatment (NDT). The NDT Practice Model emphasizes the careful examination of all body systems, the interactions of systems in posture and movement, and the influence of these systems and interactions on the activity and participation domains of individuals after CNS damage. Based on observing and analyzing the posture and movement dysfunction, the specific system impairments (primary and secondary) that influence the individual’s activity and participation domains can be better identified, understood, and predicted. Intervention strategies are then developed to address the specific impairments within relevant activities and contexts. Section A in Unit V provides specific clinical examples of this evaluation → intervention process. The NDT Practice Model offers clinicians a framework within which to examine, evaluate, and develop intervention strategies for individuals with CNS pathology. In particular, clinicians working within an NDT framework develop a focused understanding of how the neuromuscular, sensory, perceptual, musculoskeletal, cardiovascular, and respiratory systems’ roles in effective and ineffective postures and movements may contribute to either efficient or inefficient function, respectively, at both the Activity and Participation domains of the International Classification of Functioning, Disability and Health (ICF). Clinicians have used the NDT Practice Model to evaluate many clients poststroke or with TBI. Their observations have led to four categories of commonly seen clinical presentations in individuals poststroke and with TBI: • Absent or decreased force production: Individuals who demonstrate an overall low tone base. • Hypertonicity or motor overactivity: Individuals who demonstrate an overall high tone base. • Ataxic: Individuals who demonstrate impairments with the grading/scaling of muscle recruitment. • Pushing behavior or contraversive pushing: Individuals who demonstrate contraversive pushing behavior. Scheets et al8 documented the use of movement system diagnoses in the management of individuals with neuromuscular conditions poststroke. The authors state that the medical diagnosis of stroke results in several different clinical presentations and is not sufficient to direct the therapeutic intervention. Scheets et al9 initially proposed their movement diagnoses or classification based “largely on systematic clinical observations for many years.” They propose that movement system diagnoses, at the level of the impairment, provide a rational basis for treatment selection. Thus, similarly, this chapter proposes that the four commonly seen clinical presentations listed will provide clinicians with a useful categorization for evaluation and intervention management of these individuals. Table 11.1 Clinical presentations related to involvement of arterial supply and/or damage to brain structures
11.1 Introduction
11.2 What Is Stroke?
11.2.1 Classification of Stroke
Artery involved | Brain structure potentially affected | Potential impairments/effects |
| Right hemisphere | • Left sensorimotor deficits |
• Visual deficits leading to inattention | ||
• Perceptual (visual, spatial) deficits | ||
• Motor perseveration | ||
• Cognitive deficits—memory loss | ||
• Behavioral changes—quick, impulsive behavioral style | ||
| Left hemisphere | • Right sensorimotor deficits |
• Communication deficits (speech and language) | ||
• Apraxia (difficulty initiating, sequencing, processing a task) | ||
• Cognitive deficits—memory loss | ||
• Behavioral changes—slow, cautious behavioral style | ||
Anterior cerebral artery | Superior border of the frontal and parietal lobes | • Contralateral sensory and motor deficits affecting lower extremity more than upper |
• Speech/language deficits | ||
• Apraxia | ||
• Neglect/inattention | ||
• Memory and behavioral deficits | ||
• Incontinence | ||
Middle cerebral artery | Surface of the cerebral hemispheres and deep frontal and parietal lobes | • Contralateral sensory and motor deficits of the face, upper and lower extremities |
• Speech/language deficits (Broca’s or Wernicke’s aphasia) | ||
• Perceptual deficits (spatial) | ||
• Visual deficits—homonymous hemianopsia (HH) | ||
Vertebrobasilar artery | Brainstem and cerebellum | • Diplopia, nystagmus, dysphagia, dysarthria |
• Ataxia | ||
• Incoordination and equilibrium deficits | ||
• Headaches | ||
• Dizziness | ||
Posterior cerebral artery | Occipital and temporal lobes, thalamus, upper brainstem | • Contralateral sensory loss |
• Thalamic pain syndrome | ||
• Visual deficit—homonymous hemianopsia, visual agnosia, cortical blindness |
Note: This table summarizes information from the cited references.3–6
Absent or Decreased Force Production
A common clinical presentation seen following stroke and TBI is that of the individual who presents with little or no muscle activation. Commonly, this individual may be referred to clinically as flaccid, hypotonic, or weak. These individuals typically possess a common grouping of impairments. The complexity of dealing with this clinical presentation of absent or decreased muscle activation becomes more manageable if the underlying system impairments are determined and understood. From here, clinicians are able to make appropriate intervention choices.
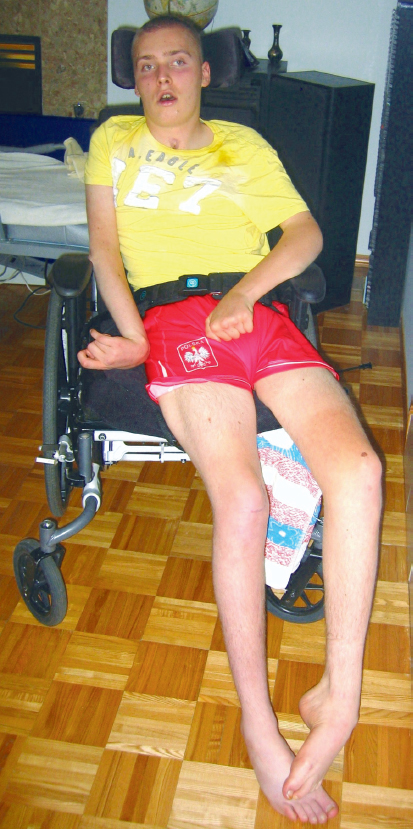
This clinical presentation is observable in all postures but becomes particularly evident when the individual is first brought to a sitting position at the bedside. This individual often sits with overall flexion in the trunk and asymmetry in the frontal plane, which can fluctuate between a passive elongation or shortening of the more affected side of the trunk, depending on where the weight is concentrated. The more affected limbs fall into alignments that gravity and the location of the center of mass (COM) will dictate—abduction or adduction at the hip/lower extremity (LE) and into glenohumeral (GH) flexion, internal rotation, and adduction (Fig. 11.1).
In supine lying, this asymmetry may also be observable, but because of the large supportive base in supine, asymmetrical alignments are not always as obvious as when the individual is brought vertical to gravity. The individual’s balance into all vertical postures is poor. This poor posture can be accompanied by the additional issue of lack of or impaired head control. Many impairments may contribute to this poor postural control and activity level, including visual, perceptual, and sensory impairments. However, research demonstrates that reduced ability to recruit, modulate, and control muscles of the trunk and affected limbs poststroke is a major contributing factor to impaired postural control in sitting and standing. As they relate to impaired postural control and instability, these studies have revealed that individuals poststroke demonstrate greater movement of the upper trunk, with little anterior tilt of the pelvis during performance of sitting activities10 with the following:
• Reduced force production as well as delayed recruitment of trunk muscle activity on the paretic side compared with the less affected side.11
• Deficits in strength, control, and the coordination of limb segments as the primary motor control impairments post stroke.12,13,14
Fig. 11.1 Carol, a client poststroke, through her sitting posture and limb alignments, demonstrates a clinical presentation frequently observed in individuals with absent or decreased force production.
• Lack of activation of leg muscles for support and balance during reaching activities in comparison to healthy counterparts.15,16
Historically, NDT education has taught about the importance of the role of trunk motor control and range of motion to enhance an individual’s ability to control the center of mass over the base of support (BOS) and superimpose head and limb function on this stable base.17,18 Di Monaco et al19 in 2010 and Hsieh et al in 200220 demonstrated the importance of trunk and proximal limb control and independent sitting to the performance of activities of daily living and gross motor functions. As identified previously, many individuals poststroke will move their upper trunk (head, neck, and thoracic spine) but have limited ability to move in the lower trunk (lumbar spine and pelvis). There may also be asymmetry in the trunk movement (right and left sides) if the stroke is moderate to severe. Examination of trunk and limb control through functional activities relevant for that individual, initially emphasizing anterior pelvic tilt with lumbar extension, posterior tilt with lumbar flexion, as well as lumbar lateral flexion to both the more affected and the less affected side, allows the clinician to hypothesize if the underlying impairment is a motor recruitment issue or whether an underlying range of motion restriction contributes to movement inefficiency.
Other secondary impairments can develop over time depending on the individual’s initial clinical presentation and impairments, personal and contextual factors, and the interventions received since the infarct. Examples include respiratory system compromise from range of motion restrictions and muscle activation impairments of the thorax/rib cage, impaired cardiovascular endurance from inactivity, further weakness from inactivity in the more involved and less involved sides of the body, cognitive inattention, and lethargy from decreased social interactions and stimulation.
Visual, sensory, perceptual, and other system impairments are definitely important and will influence the specific clinical picture seen in an individual poststroke, but it is paramount to understand the role motor impairments play in interfering with postural control.
Smidt and Rogers,21 in 1982, defined strength as the ability to generate sufficient tension in a muscle for the purposes of posture and movement. Gracies,22 in 2005, defined weakness as the inability to recruit or modulate motor neurons secondary to a lesion of descending motor pathways. Thus, force production in muscle can be influenced by both musculoskeletal and neural properties.
The impaired neural aspects influencing force production are related to the following:
• A delay in initiation and termination of muscle contraction, which may be due to the lesion and results in impairments in motor processing and efferent mechanisms.23
• The number of motor units recruited, the frequency of firing of these units, or a combination of these factors.24,25,26
• The number and type of motor units recruited, the frequency of firing of these units, or a combination of these factors.24,25,26
Weakness can be further characterized by the inability to (1) initiate a muscle contraction, (2) increase muscle force to meet the requirements of the task (strength), or (3) sustain muscle force for the time necessary to complete the task (endurance). Following an upper motor neuron lesion, weakness may vary in severity from paralysis to paresis.
Muscle atrophy (typically from disuse) refers to a reduction in muscle fiber size. Muscle strength, or the ability of the muscle to generate force, is directly proportional to the cross-sectional area of the muscle.27
Many physiological changes occur in muscles poststroke.6 Some of the changes result from the stroke itself, but changes also occur as a result of immobility and disuse and the natural process of aging. These changes occur on both sides of the body (greater on the more involved side) and include the following:
• A conversion of fiber types from type IIB (very fast twitch, high power, and fast fatigable) to type IIA (moderately fast, medium power, and fatigue resistant) and type I (slow, fatigue resistant) with disuse and aging changes.28,29
• A loss of both types of fibers (type I and type II) poststroke24,30 and with aging.29,31
• A reduction in muscle mass (sarcopenia) occurs as a result of both aging (30–40%), affecting the LE muscles more than the upper extremity (UE) muscles32,33 and as a result of stroke.34
• A loss of 10% strength per week (a loss of sarcomeres, not just muscle fibers) from disuse. This is referred to as disuse atrophy.24,35,36,37
• Decreased endurance from atrophy of fatigue-resistant muscles.31
• Decreased metabolic activity secondary to decreased oxygen uptake when no demand is placed on the muscle(s).31,38
• Some muscles (primarily antigravity muscles) atrophy more than their antagonists. This atrophy is related to the glycolytic response.38,39
Thus, skeletal muscle response to stroke and decreased use is due to the following:
• Muscle fiber atrophy.
• Decreased capacity to generate muscle force.
• Conversion of fast to slow muscle fiber type.
The changes in muscle physiology poststroke have been shown to correlate with the degree of severity of the stroke and are also correlated with the degree of functional impairment as measured by the Fugl–Meyer UE assessment poststroke; that is, the more severe the stroke, the greater the changes (losses) in muscle physiology and the greater the functional losses for the individual.12,24,25 Similar losses occur in connective tissue and bone from immobility or lack of a load. These include adhesions, disorganization, and disuse osteoporosis. The disuse osteoporosis is greater in weight-bearing bones, such as the vertebral bodies and femurs.40,41
Muscle weakness and atrophy, tissue shortening, and bone demineralization can occur within hours of immobility. Individuals poststroke from a moderate to severe event are often immobile or at least less mobile for the first few days. Thus secondary impairments (e.g., range of motion restrictions, disuse weakness, and muscle atrophy) are often developing or present during the first clinical examination of the client. If the individual remains immobile or less mobile for any length of time, weight gain may occur, making mobilizing harder in general. With immobility, impairments in the cardiovascular and respiratory capacities (endurance) of the individual develop.42,43 Thus poststroke, even in the short term, there is weakness, shortening of tissues, a risk of weight gain, and decreased cardiovascular and respiratory endurance/capacity, which may all increase in magnitude through a self-perpetuating cycle. When these primary and secondary impairments from the stroke and immobility are combined with the effects of aging, individuals poststroke (especially those over the age of 40 when aging-related changes begin to occur in muscles), face an uphill challenge to regain their function.44 Therapists planning interventions with the client aimed at targeting the primary and secondary impairments of the stroke itself must also consider these secondary impairments from immobility and, in many cases, aging affecting the musculoskeletal system on both sides of the body, the cardiovascular system, and the respiratory system. It is challenging for an individual to work in therapy poststroke, and the effects of deconditioning render the task even more difficult.
Studies have demonstrated that exercise, aerobic and resistance, even when performed by individuals at an advanced age or many months or years poststroke, is effective at reversing many of these changes.31,38,42,43,45,46,47,48 Studies have also confirmed that the sooner exercise is initiated poststroke, the better the outcomes.49 In a meta-analysis of 21 studies, findings demonstrated that, for individuals in the acute phase (< 6 months poststroke) who engaged in resistance exercises, the strength and functional outcomes were greater than those who began their exercise after this period.49 However, even in individuals who were considered more chronic (> 6 months poststroke), resistance exercise was beneficial in improving strength and functional performance.50 The difference between the two groups was hypothesized to be because the individuals considered chronic stroke survivors (> 6 months poststroke) were suspected of having greater loss of muscle strength and motor unit activity from disuse weakness.50
Many strokes occur as a result of cardiovascular dysfunction. This leads to a hesitation on both the client’s and the therapist’s part to increase the demand on the cardiovascular system through aerobic exercise. Traditional physical therapy poststroke often does not provide enough cardiovascular challenge to the individual to address the deconditioning changes.51,52 Studies have shown that individuals poststroke often have exercise capacities at ~ 40% below age- and gender-matched norms for sedentary individuals.53 A balance must be found that does not overstress the individual’s cardiovascular and respiratory systems but is challenging enough to ensure that the deconditioning does not become the limiting factor to recovery from the stroke.
When an individual is recovering from a heart attack (another result of cardiovascular dysfunction), standard care includes cardiac rehabilitation—exercise.54
If we recognize that regaining cardiovascular fitness is important to recovery from a heart attack, why would we not think the same for recovery from a stroke? A further convincing factor for increasing activity levels for individuals poststroke may be recent evidence demonstrating that aerobic and resistance exercise are believed to create an environment that promotes positive brain plasticity.55,56,57 Exercise improves cerebral vascular perfusion, metabolism, and growth factor regulation.57 In 2007, Cotman et al57 reviewed the evidence for the beneficial effects of exercise on many aspects of brain functioning—learning, memory, depression, and neurogenesis. Aerobic and resistance exercise training poststroke is an important and emerging area of practice, and recent publications58,59 provide health care professionals with current recommendations and guidelines.
Typical Primary and Secondary Impairments in the Neuromuscular and Musculoskeletal Systems for the Individual with Absent or Decreased Force Production
The following impairments can be present on both sides of the body but are greater on the more affected side of the trunk and limbs:
• Weakness (including and varying between the inability to initiate muscle contractions, the inability to generate sufficient force in muscles, and the inability to sustain muscle(s) contractions of the following:
 Trunk extensors (lumbar, thoracic, cervical)—evident early.
Trunk extensors (lumbar, thoracic, cervical)—evident early.
 Trunk flexors (external and internal obliques, transversus abdominis, rectus abdominis)—more evident once the trunk extensors improve and the individual is able to move out of an overall flexed alignment.
Trunk flexors (external and internal obliques, transversus abdominis, rectus abdominis)—more evident once the trunk extensors improve and the individual is able to move out of an overall flexed alignment.
 Lateral trunk flexors (quadratus lumborum).
Lateral trunk flexors (quadratus lumborum).
 Hip extensors or abductors (gluteus maximus and minimus, hamstrings).
Hip extensors or abductors (gluteus maximus and minimus, hamstrings).
 Knee extensors—evident early.
Knee extensors—evident early.
 Knee flexors—more evident once the individual is able to support his body weight against gravity in activities such as walking and stair climbing.
Knee flexors—more evident once the individual is able to support his body weight against gravity in activities such as walking and stair climbing.
 Ankle dorsiflexors and evertors.
Ankle dorsiflexors and evertors.
 Scapular stabilizers (serratus anterior), adductors, depressors, and upward rotators.
Scapular stabilizers (serratus anterior), adductors, depressors, and upward rotators.
 Glenohumeral flexors, external rotators, extensors, and abductors.
Glenohumeral flexors, external rotators, extensors, and abductors.
 Elbow extensors.
Elbow extensors.
 Wrist and finger extensors.
Wrist and finger extensors.
• Overlengthened tissues.
 Lumbar and thoracic extensors.
Lumbar and thoracic extensors.
 Scapular adductors and depressors.
Scapular adductors and depressors.
• Shortened tissues.
 Lateral trunk flexors.
Lateral trunk flexors.
 Short cervical extensors.
Short cervical extensors.
 Scapular elevators (upper trapezius and levator scapulae muscles) and abductors.
Scapular elevators (upper trapezius and levator scapulae muscles) and abductors.
 Glenohumeral adductors, flexors and internal rotators.
Glenohumeral adductors, flexors and internal rotators.
 Elbow flexors.
Elbow flexors.
 Wrist and long finger flexors.
Wrist and long finger flexors.
 Thumb flexors and adductors.
Thumb flexors and adductors.
 Hip flexors and adductors.
Hip flexors and adductors.
 Knee flexors.
Knee flexors.
 Ankle plantar flexors, toe flexors.
Ankle plantar flexors, toe flexors.
• Joint limitations.
 Decreased extension range of the thoracic spine joints.
Decreased extension range of the thoracic spine joints.
Impairments in other body systems are also frequently present in individuals with this clinical presentation. Examples of these may include but are not limited to the following:
• Absent or impaired light touch sensation or proprioception.
• Impaired vision (e.g., hemianopsia).
• Visual–spatial perception impairments (e.g., inattention).
• Impaired cognition (e.g., impaired recent or short-term memory).
The reader is referred to Chapter 16 on occupational therapy for a more detailed discussion of impairments in these systems.
The foregoing list appears long, and at first glance, it seems to list all muscle groups. However, after a more detailed review, the reader may notice that the list contains primarily muscles and muscle groups on one side of a joint or limb (e.g., weakness of the glenohumeral external rotator muscles vs. weakness of the internal rotator muscles and weakness of the hip extensors and abductors vs. weakness of the hip flexors and adductors). There are always exceptions to every rule, but, for example, around the hip joint, clinicians will be wise to expect greater impairments in weakness of one side of a limb or joint versus in their antagonists. In the example of the hip joint, weakness of the hip extensor and abductor muscles is more common than weakness of the hip flexor and adductor muscles. This difference in strength is not to say that the hip flexor and adductor muscles will be normal from a motor recruitment perspective (this is rarely the case), but if the motor recruitment impairments in the hip extensor and abductor muscles (and the others in the list) are focused on early in evaluation and intervention, the impairments in these muscles’ antagonists (the hip flexors and adductors in this example) are often less significant. In individuals after a moderate to severe stroke, almost all muscle groups in the trunk and on both sides of the body will demonstrate impairments in their ability to recruit force efficiently and effectively. However, the groups listed should be considered at the top of the clinicians’ lists of hypothesized impairments for individuals in this category of absent or decreased force production. Also, whenever there is significant weakness in a muscle or muscle groups, clinicians are encouraged to examine for tissue shortening of their antagonists. Examples of these include the glenohumeral internal rotator muscles, the wrist and finger flexors, the hip flexors and adductors, and the ankle plantar flexors.
The NDT Clinical Practice Model recognizes that each individual is unique in his or her participation and activity domains of function. This uniqueness, combined with the severity of the stroke and the personal and contextual factors that each person possesses, leads to variations in the influence and, at times, severity of the list of impairments an individual will have after CNS injury. However, despite this uniqueness, common groupings of impairments leading to common clinical presentations are frequently seen in individuals poststroke. Within this clinical presentation of absent or decreased force production, the development of three frequently seen conditions, the hyperextended knee, the subluxed shoulder, and the edematous hand, will be presented. The evolution and combination of the primary impairments into secondary impairments that result in these clinical sequelae will be described. In each of the three descriptions, the importance of determining the underlying impairments to effectively manage these clinical symptoms in intervention will be discussed.
Knee Hyperextension
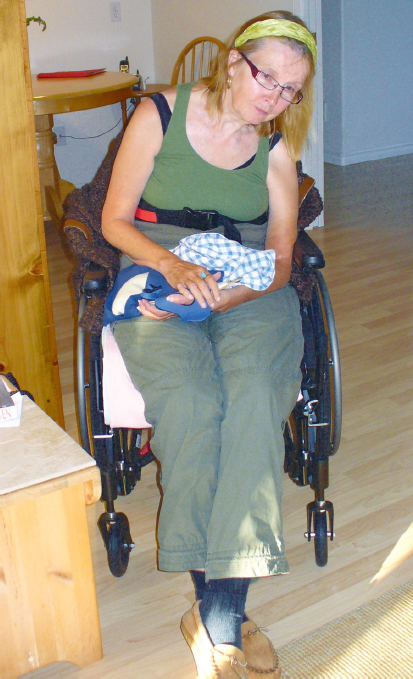
In standing and in gait, there is often a tendency for individuals following stroke and brain injury to be inactive in their postural trunk and hip muscles, specifically the thoracic spine extensors, the abdominal muscles, and the hip extensors and abductors, and to stand in slight hip flexion, possibly also in relative hip adduction. This alignment may cause a posterior and/or lateral deviation of the pelvis, resulting in a pelvis on the more affected side that is often rotated back and/or shifted laterally. These alignments change the ground reaction forces through the lower extremity. Ground reaction forces now fall in front of and/or medial to the knee joint, mechanically forcing the knee into hyperextension. With the proximal tibia forced back, the ankle is positioned in relative plantar flexion and inversion (if the lateral hip muscles are not stabilizing the pelvis). The alignment results of this biomechanical malalignment, stemming from an inability to coactivate the abdominals and hip extensors or hip abductors to maintain pelvic stability in this upright posture and throughout gait, is often a hyperextended knee. Without intervention to address these proximal muscle weaknesses and shortened tissues, little change will be made in the individual’s gait deviations.
Often, a brace is prescribed for the ankle to attempt to address the knee hyperextension and ankle plantar flexion. However, the underlying impairments and the rerouting of the ground reaction forces remain un-checked. Lack of movement through range over time may result in secondary changes in mechanical and elastic properties of muscle (e.g. plantar flexors) further contributing to a hyperextended knee. In the case report for Carol in Unit V (A3), the client had significant weakness of her hip abductors and extensors as well as the thoracic extensors and lateral trunk flexors on her left side. In the early and subacute phase poststroke, she stood only long enough for a quick dependent transfer between surfaces and often with less than optimal trunk and lower extremity alignments. Her gastrocnemius–soleus muscle group became significantly shortened. The combination of these impairments resulted in knee hyperextension with a foot and ankle that tended to be in plantar flexion and inversion (Fig. 11.2).
Shoulder Subluxation
Shoulder subluxation is a common clinical picture poststroke. Many authors, including O’Sullivan,6 Neumann,60 Donatelli,61 and Caillet,62 cite the importance of capsular and ligamentous structures, the glenoid and glenoid labrum, as well as the activity of the deltoid and rotator cuff muscles that seat the head of the humerus in the glenoid fossa, as key to providing stability at the glenohumeral joint. Lo et al,63 in 2003, identified the lack of muscle tone and muscle paralysis hindering the dynamic control and supportive function of the rotator cuff as substantial contributors to shoulder subluxation following stroke.
Neumann60 and Caillet62 identify the forward- and upward-oriented angulation of the glenoid fossa and the proper alignment and muscular support of the scapula on the rib cage as well as the support of the superior portion of the joint capsule as mechanical contributors to glenohumeral joint stability. Caillet62 postulated that, in the absence of muscle activity, as in hemiplegia poststroke, the alignment of the scapula and orientation of the glenoid fossa may well become more significant factors in subluxation.
Neumann60 and Donatelli,61 in their discussion of functional anatomy and mechanics of the shoulder, both cite the scapulothoracic joint as key to the stability and mobility of the glenohumeral joint. Neumann60 states that a chronically downwardly rotated scapula may be associated with poor posture secondary to paralysis or weakness of certain muscles. He states that regardless of the cause, the loss of the upwardly rotated position and gravity can pull the humerus down the face of the glenoid. Eventually, he states, the glenohumeral joint becomes mechanically unstable and will sublux.
Thus shoulder subluxations seen in individuals post-stroke are a direct consequence of biomechanical malalignments and muscle inactivity. The role of the more proximal body structures to the development of shoulder subluxation, such as the thorax, lumbar spine, pelvis, and LEs, as critical components as bases of support for the shoulder girdle must also be considered. Adequate trunk muscle activity to support the alignment of the rib cage and influence alignment of the scapula and orientation of the glenoid fossa is paramount.60,64
Fig. 11.2 A client poststroke demonstrates alignments through the more affected lower extremity that frequently result in knee hyperextension.
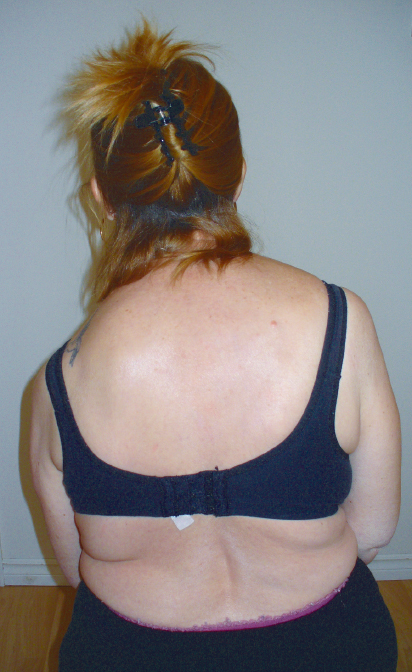
If an individual has asymmetry in the trunk from one or a combination of a sensory, motor, visual, or musculoskeletal system impairments, the result can be a malalignment in the shoulder girdle structures and optimal conditions for a subluxation. Commonly, the lumbar and thoracic spine remains in flexion with the pelvis in a posteriorly rotated position. The rib cage on the more affected side is also frequently rotated posteriorly. This position is perpetuated by long periods of sitting in wheelchairs or standing with more weight on the less affected LE. With a flexed thorax and asymmetrical rib cage alignment, the scapula typically sits on the thorax in more of an abducted, elevated, and often downwardly rotated orientation. Biomechanically, these alignments allow the humeral head to be unsupported from a structural perspective because the glenoid labrum is no longer underneath it. When the individual is also unable to independently move into and sustain thoracic extension and posteriorly extend and tuck the rib cage and is unable to use the dynamic muscle stabilizer system of the scapular stabilizers and the muscles of the rotator cuff to hold the humeral head into the glenoid fossa, the capsule of the glenohumeral joint becomes stretched and ineffective. Over time, this biomechanical alignment, muscle inactivity, and prolonged stretch on ligaments and muscles can lead to a shoulder subluxation (Fig. 11.3).
Similarly, when an individual has a bias toward an asymmetrical alignment in the ability to use his or her legs, the consequences from this BOS up to the shoulder girdle can result in the conditions for a subluxation to occur. For example, if there is weakness of the hip extensors or abductor muscle groups and/or tightness limiting ankle dorsiflexion from a joint or muscle length impairment, such as shortness of the gastrocnemius–soleus group, the individual’s COM is no longer aligned evenly over both feet in standing (during transitions and in standing/gait activities). As previously described, the pelvis and hip are often shifted laterally or posteriorly on the more affected side. This shift tends to rotate the trunk posteriorly on the more affected side as well. The individual often sits and stands with the COM shifted over to the less affected side of the body, contributing to a counterrotation forward in the upper trunk for balance (i.e., a zig-zag). This weight shift may be further increased with the use of an assistive device, such as a cane. The result is a thorax that is flexed with an asymmetrical rib cage (posterior on the more affected side) leading to the scapular and humeral alignment that was described earlier. This alignment, if sustained over time and combined with impairments in the visual, neuromuscular, musculoskeletal, and/or sensory/perceptual systems, for example, once again sets up the conditions for a shoulder subluxation.
Hand and Finger Edema
Frequently, but not always, the individual who has profoundly impaired force production in muscles of the trunk and limbs may also experience increased edema of the hand and fingers.65,66,67 The overall and extreme flexion of the thoracic spine as described earlier decreases the space between the clavicle and upper ribs and compromises neurovascular structures that lie in this subclavicular space. This decreased space, in conjunction with the absence of the natural venous pump action of muscle activity in the arm, further compromises the situation. From the previous descriptions of the postural malalignments in the subluxed shoulder that may occur from asymmetrical postures sustained over time from impairments in multiple systems, the conditions for increased edema of the hand and fingers can be better understood.
Fig. 11.3 Scapula malalignment and muscle inactivity poststroke contributing to shoulder subluxation on the left.
Intervention Principles for the Individual with Absent or Decreased Force Production
The following list shows intervention principles for individuals with absent or decreased force production. As has been discussed, each individual is unique premorbidly and poststroke. However, these underlying principles may provide clinicians with guidance in choosing intervention strategies to achieve optimal outcomes for individuals within this clinical presentation. These principles are based on an understanding of the underlying collection of impairments contributing to this clinical presentation. They are not intended to be used in a particular order, as listed here, but are to be integrated within and throughout any given intervention session.
• Emphasize dynamic control in the trunk, primarily the lower trunk initially, with an emphasis on movements of anterior pelvic tilt with lumbar extension, posterior tilt with lumbar flexion, and lumbar lateral flexion to both sides. Even in individuals with poor head and oral motor control, as the control in the trunk improves, these functions begin to improve as well, as demonstrated in the case report about Ernie in Unit V (A4).
• Work in functional activities in a variety of postures and transitions.
 Postures: Choose postures that emphasize movement from the individual’s overall flexed position (into gravity) to a more upright extended posture (out of gravity) in multiple planes. Examples of postures to work in and sequence through while engaging in functional tasks are as follows:
Postures: Choose postures that emphasize movement from the individual’s overall flexed position (into gravity) to a more upright extended posture (out of gravity) in multiple planes. Examples of postures to work in and sequence through while engaging in functional tasks are as follows:
 High sitting (hips above knees).
High sitting (hips above knees).
 Higher sitting.
Higher sitting.
 Standing.
Standing.
 Squatting (various ranges).
Squatting (various ranges).
Once the client is in a dynamically sustained, coactivated trunk alignment (with the trunk muscles working primarily isometrically), engage the individual in activities while maintaining this stable BOS. Even in sitting (with the hips higher than the knees), limb muscle activation is required to assist with maintaining this posture or to engage in the task. Examples of activities in these postures could include visually scanning the environment for family members, having a conversation with the therapist, using the less involved arm to reach for contextual objects for the task (golf ball, paintbrush, hammer, toothbrush, etc.), sliding or stepping the less involved foot toward an object or in the direction of reach, and so on.
 Transitions: Choose transitions that bias the more involved trunk muscles and limb extensor and abductor muscles to actively recruit to support and move in and through the transition. Transitions often require a more sustained activation of the trunk muscles while superimposing muscle contractions in the limbs in eccentric and concentric contractions. Examples of transitions to sequence through while engaging in functional tasks are as follows:
Transitions: Choose transitions that bias the more involved trunk muscles and limb extensor and abductor muscles to actively recruit to support and move in and through the transition. Transitions often require a more sustained activation of the trunk muscles while superimposing muscle contractions in the limbs in eccentric and concentric contractions. Examples of transitions to sequence through while engaging in functional tasks are as follows:
 Lateral weight shifts in high and higher sitting in both directions but initially toward the less involved side to bias the more involved lateral trunk muscles and limb extensor muscles to actively recruit to “drive” the lateral shift.
Lateral weight shifts in high and higher sitting in both directions but initially toward the less involved side to bias the more involved lateral trunk muscles and limb extensor muscles to actively recruit to “drive” the lateral shift.
 High sit ↔ liftoff.
High sit ↔ liftoff.
 Scooting in all directions but initially toward the less involved side so the more involved limbs are more likely to actively recruit to move the body weight toward the intended direction.
Scooting in all directions but initially toward the less involved side so the more involved limbs are more likely to actively recruit to move the body weight toward the intended direction.
 Transfers on and off various surfaces in both directions but initially toward the less involved side as explained above for scooting.
Transfers on and off various surfaces in both directions but initially toward the less involved side as explained above for scooting.
 Standing and stepping with the less involved leg in all directions, but choose the planes of movement based on the most significant impairments (the next paragraph discusses further).
Standing and stepping with the less involved leg in all directions, but choose the planes of movement based on the most significant impairments (the next paragraph discusses further).
 Early gait activities.
Early gait activities.
In both postures and transitions, individuals need to gain control in all directions, but, initially, choose the plane(s) of movement based on the individual’s most significant impairments. For example, if hip extensor weakness is the most significant weakness at the hip joint, initially choose movements in the sagittal plane. If hip abductor weakness is the most significant weakness at the hip joint, choose movements in the frontal and transverse planes initially.
• Work in functional activities and contexts that are familiar and meaningful to that individual. Functional tasks and subtasks are chosen based on two factors.
 They are tasks that are meaningful for the individual, and he or she wants to resume these activities.
They are tasks that are meaningful for the individual, and he or she wants to resume these activities.
 They are tasks or subtasks that allow the individual’s impairments to be addressed.
They are tasks or subtasks that allow the individual’s impairments to be addressed.
Other considerations for the functional activities include the following:
 The tasks or subtasks engaged in need to be specifically and intentionally chosen to facilitate the desired movement/activation.
The tasks or subtasks engaged in need to be specifically and intentionally chosen to facilitate the desired movement/activation.
 The environmental setup of the individual (e.g., whether the client is sitting or standing, which foot is forward or back in the BOS, whether the less involved limbs are part of the BOS or not), and the location of the objects and tools of the tasks also need to be specifically and intentionally chosen to facilitate the desired movement/activation.
The environmental setup of the individual (e.g., whether the client is sitting or standing, which foot is forward or back in the BOS, whether the less involved limbs are part of the BOS or not), and the location of the objects and tools of the tasks also need to be specifically and intentionally chosen to facilitate the desired movement/activation.
Examples to illustrate the foregoing choices include the following:
 If sustained trunk extension in an upright extended posture is the movement or alignment that is desired, the objects to reach for need to be high enough to require trunk extension, and the duration of the activity needs to ensure sustained activation is achieved.
If sustained trunk extension in an upright extended posture is the movement or alignment that is desired, the objects to reach for need to be high enough to require trunk extension, and the duration of the activity needs to ensure sustained activation is achieved.
 With an individual who is a seamstress and wants to resume this activity, and whose most significant trunk impairment is an inability to sustain thoracic extensor activity, it would not be a wise choice to have her in sitting on a typical height surface hunched over the sewing machine to thread the needle. More success would likely be achieved to target this impairment by choosing the subtask of reaching up into a higher cupboard for sewing patterns or pieces of material from a higher sitting position. Often, therapists will work on basic activities of daily living (BADLs) tasks with such an individual. This individual may have activity limitations in BADLs but she may not be interested in or as motivated to practice these activities in therapy compared with working on the subtasks and task of sewing. The underlying impairments limiting both of these activities (BADLs and sewing) are the same because it is the same individual.
With an individual who is a seamstress and wants to resume this activity, and whose most significant trunk impairment is an inability to sustain thoracic extensor activity, it would not be a wise choice to have her in sitting on a typical height surface hunched over the sewing machine to thread the needle. More success would likely be achieved to target this impairment by choosing the subtask of reaching up into a higher cupboard for sewing patterns or pieces of material from a higher sitting position. Often, therapists will work on basic activities of daily living (BADLs) tasks with such an individual. This individual may have activity limitations in BADLs but she may not be interested in or as motivated to practice these activities in therapy compared with working on the subtasks and task of sewing. The underlying impairments limiting both of these activities (BADLs and sewing) are the same because it is the same individual.
 Through working in tasks and subtasks that are meaningful and motivating to individuals, clinicians are able to help clients improve the impairments that limit them in a variety of activities, including the activities on a typical checklist of desired therapy outcomes in acute and outpatient care (BADLs, bed mobility, basic transfers between wheelchair and bed, wheel-chair and toilet, etc.).
Through working in tasks and subtasks that are meaningful and motivating to individuals, clinicians are able to help clients improve the impairments that limit them in a variety of activities, including the activities on a typical checklist of desired therapy outcomes in acute and outpatient care (BADLs, bed mobility, basic transfers between wheelchair and bed, wheel-chair and toilet, etc.).
• Within each posture and transition, the more involved limbs are kept in closed kinetic chain functions as part of the active BOS. (As motor function improves in the more involved limbs, they can be challenged from closed kinetic chain activity to modified chain, and eventually open kinetic chain activity.)
 The less involved limbs are typically chosen to be the action limbs for the activity(s) and are progressively challenged to gradually move out of the BOS, first with modified chain → open kinetic chain movements, with small → large ranges and for short → longer periods of time.
The less involved limbs are typically chosen to be the action limbs for the activity(s) and are progressively challenged to gradually move out of the BOS, first with modified chain → open kinetic chain movements, with small → large ranges and for short → longer periods of time.
For example, in standing, initially both UEs (hands) may be on a stable surface while the individual is reading her favorite cookie recipe. A progression from this posture may be asking her to slide the less involved hand over the counter (a stable surface) to reach for a cup of sugar to make the cookies. Both legs are still part of a narrow BOS. The individual is then asked to lift the cup of sugar and pour it into the mixing bowl. Next, the individual is asked to slide the less involved foot to the side to reach for the bag of flour on the counter on the less involved side. This can be progressed to taking a small lateral step with the less involved leg for the next ingredient on the counter.
Hypertonicity or Motor Overactivity
Another clinical presentation commonly seen is that of the individual presenting with hypertonicity of muscles following a CNS lesion. Kandel and colleagues,68 in 2000, defined normal tone as a slight constant tension of healthy muscle so that when limbs are handled, they offer only modest resistance to displacement. This normal muscle tone is influenced by the physical inertia of the limb, mechanical and elastic properties of muscle and connective tissue, and reflex muscle contraction (tonic stretch reflexes). Following a CNS lesion, the physical inertia of the limb is unchanged. The hypertonia or excessive resistance to passive movement that we see clinically must then have a mechanical and/or a neurological origin. The resistance from neurological origin can manifest as spasticity, rigidity, and/or abnormal cocontraction of muscle.
Much controversy still exists surrounding the definition and measurement of spasticity.69,70,71,72 Lance,73 in 1980, defined spasticity as a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex. Young74 defines spasticity as a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes that result from abnormal intraspinal processing of primary afferent input. A more recent 2005 definition describes spasticity as a disordered sensorimotor control, resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles.75 Thus there is a lack of clarity among medical and rehabilitation professionals as to the use of the terms hypertonicity and spasticity.
The mechanisms believed to contribute to hypertonia, of which one type is spasticity, are varied.69,70,76 Research has determined that stroke results in an imbalance of excitatory and inhibitory input at the motor neuron level leading to upper motor neuron symptoms.71 Sheean and McGuire,76 in 2009, and Ward,71 in 2012, wrote comprehensive review papers on hypertonia and spasticity. Sheean and McGuire76 state that certain clinical deficits are present immediately after a lesion. These are referred to as the negative features and include weakness and loss of dexterity. In an earlier paper, Sheean77 describes a period of shock he calls a “transitional inter-lude of non-hyperactive reflex responses.” This period of flaccidity or hypotonia is variable in length, ranging from 5 days to more than 1 year poststroke.77,78,79,80 This period of the negative signs, including weakness and lack of sensory input, is typically accompanied by inactivity (bed rest or minimal activity at best). This reduced activity or inactivity further contributes to weakness from disuse. As was discussed previously in this chapter, the secondary impairments from disuse weakness of shortened muscles, atrophy of muscle fibers, and conversion of muscle fiber types, can develop in a very short time frame.
There is still no consensus among researchers on all aspects of hypertonicity, including spasticity and its causes, triggers, severity, location, timing of its appearance, or how to measure it.70,71,76,78 There is consistency in the literature, however, that the immediate of CNS damage are losses; for example, weakness, loss of sensory input, and loss of sarcomere length. Thus it seems that these losses, rather than hypertonicity and spasticity, should be considered the primary impairments of the stroke. Sheean and McGuire,76 in 2009, wrote that a “delayed consequence of damage to upper motor neuron (UMN) pathways is the appearance of some form of motor overactivity, including spasticity.” The word delayed may suggest a more secondary rather than a primary role in the development of the impairments of hypertonicity (including spasticity). These impairments should perhaps be considered secondary impairments of the stroke.
Hyperstiffness is a term used to describe the increased resistance in hemiparetic muscle from a mechanical origin.81 It results from changes in the mechanical-elastic properties of muscle and connective tissue. The tissue changes that occur are related to immobility (lack of movement of the muscle and joint) and can result in the following:
• Remodeling of muscle structural components, such as collagen, with an increase in the proportion of collagen to muscle fiber noted.82
• Shorter actual muscle length attributed in some cases to a loss of serial sarcomeres (i.e., reduced number and/or in others a decreased resting sarcomere length).36,82
• Associated decreased extensibility of other tissues, such as joint capsule and ligaments.
Carey and Burghardt83 also suggest a higher proportion of skeletal muscle cross-bridge binding that results in this abnormal stiffness.
An underlying impairment that contributes to these mechanical changes in the properties of muscle is muscle weakness. This weakness results in clients having a decreased ability to generate sufficient force to move their body and limbs, especially their trunk and more involved limbs, through joint ranges. When the antagonist of the weak muscle is also overactive (i.e., hypertonic), the weak/paretic muscle has an even greater challenge to overcome this resistance.
As clinicians, we observe hypertonicity repeatedly in certain muscle groups and we oversimplify its origins. We classify someone as having a high tone flexed upper extremity or a stiff high tone extended lower extremity. Hypertonicity and spasticity are often assumed to be primary impairments after an individual has CNS damage. However, hypertonicity is a multisystem entity with an underlying cluster of single-system impairments that require further investigation. The literature hypothesizes changes in descending commands, changes in excitatory or inhibitory inputs to the spinal interneurons and α motor neurons as mechanisms resulting in the following:
• Delay in initiation of muscle contraction.23,84
• Delay in termination of muscle activity, often expressed in the literature as an abnormal cocontraction of muscles.23,85
Again, reading the foregoing points, the descriptors for the hypothesized neurological changes that occur after CNS damage are negative signs versus positive signs—delay in initiation, reduced muscle firing, and delay in termination. Is it possible that the negative signs are the true primary impairments that then lead to the secondary positive signs of hypertonicity and spasticity? Is the hypertonicity seen in individuals with CNS damage a consequence of what is missing, decreased, or slowed in the messaging to the muscles throughout the body? Weakness of central or peripheral origin can occur within hours of disuse. Shortening of tissues can similarly occur almost immediately. We know from research that diminished or absent input to muscles itself can be the cause of the spasticity and subsequent hypertonicity.76 Do clinicians and researchers often fail to fully acknowledge and appreciate the role of these negative signs as the key to understanding hypertonicity and spasticity?
A detailed clinical examination will often reveal that these issues are expressed clinically as body segments or muscles that are weaker, often in the postural and proximal limb muscles, with overrecruitment developing in distal limb muscles. It is important to identify the neurological and mechanical components of hypertonicity before intervention strategies can be chosen. The weak or inactive muscles may be the antagonists to the hypertonic muscles or muscle group distant to the hypertonic muscle that typically provides stability.
Typical Primary and Secondary Impairments in the Neuromuscular and Musculoskeletal Systems for the Individual with Hypertonicity or Motor Overactivity
The following impairments can be present on both sides of the body but are greater on the more affected side of the trunk and limbs.
• Weakness (including and varying between the inability to initiate muscle contractions, the inability to generate sufficient force in muscle(s), and the inability to sustain muscle contractions of the following:
 Trunk extensors (lumbar, thoracic, cervical)—evident early.
Trunk extensors (lumbar, thoracic, cervical)—evident early.
 Trunk flexors (external and internal obliques, transversus abdominis, rectus abdominis)—more evident once the trunk extensors improve and the individual is able to move out of an overall flexed alignment.
Trunk flexors (external and internal obliques, transversus abdominis, rectus abdominis)—more evident once the trunk extensors improve and the individual is able to move out of an overall flexed alignment.
 Lateral trunk flexors (quadratus lumborum).
Lateral trunk flexors (quadratus lumborum).
 Hip extensors and/or abductors (gluteus maximus and minimus, hamstrings).
Hip extensors and/or abductors (gluteus maximus and minimus, hamstrings).
 Knee extensors.
Knee extensors.
 Knee flexors—more evident in the ambulatory individual.
Knee flexors—more evident in the ambulatory individual.
 Ankle dorsiflexors and evertors.
Ankle dorsiflexors and evertors.
 Scapular stabilizers (serratus anterior), adductors, depressors, and upward rotators.
Scapular stabilizers (serratus anterior), adductors, depressors, and upward rotators.
 Glenohumeral flexors, external rotators, extensors, and abductors.
Glenohumeral flexors, external rotators, extensors, and abductors.
 Elbow extensors.
Elbow extensors.
 Wrist and finger extensors.
Wrist and finger extensors.
• Shortened tissues (including those secondary to neurological and mechanical factors, such as hyperstiffness, hypertonicity, spasticity).
 Lateral trunk flexors.
Lateral trunk flexors.
 Short cervical extensors.
Short cervical extensors.
 Scapular elevators (upper trapezius and levator scapulae muscles) and abductors.
Scapular elevators (upper trapezius and levator scapulae muscles) and abductors.
 Glenohumeral adductors, flexors, and internal rotators.
Glenohumeral adductors, flexors, and internal rotators.
 Elbow flexors.
Elbow flexors.
 Wrist and long finger flexors.
Wrist and long finger flexors.
 Thumb flexors and adductors.
Thumb flexors and adductors.
 Hip flexors and adductors.
Hip flexors and adductors.
 Knee flexors.
Knee flexors.
 Ankle plantar flexors and toe flexors.
Ankle plantar flexors and toe flexors.
• Joint limitations.
 Decreased extension range of the lumbar and thoracic spine joints.
Decreased extension range of the lumbar and thoracic spine joints.
 Decreased range at various joints of the upper and lower extremity due to joint capsule and ligamentous changes (which joints demonstrate limitations will vary from individual to individual).
Decreased range at various joints of the upper and lower extremity due to joint capsule and ligamentous changes (which joints demonstrate limitations will vary from individual to individual).
• Overlengthened tissues.
 Lumbar and thoracic extensors.
Lumbar and thoracic extensors.
 Scapular adductors and depressors.
Scapular adductors and depressors.
As with the individuals who present within the clinical presentation of absent or decreased force production, these impairments of neuromuscular and musculoskeletal systems usually coexist with impairments in other body systems, such as the following:
• Absent or impaired light touch sensation and/or proprioception.
• Impaired vision (i.e., hemianopsia).
• Visual–spatial perception impairments (i.e., inattention).
• Impaired cognition (i.e., impaired recent and/or short-term memory).
As before, the reader is referred to Chapter 16 on occupational therapy from an NDT perspective for a more detailed discussion of impairments in these systems.
This list of impairments does not appear significantly different from that listed earlier for the absent or decreased force production. However, this difference should not be surprising, considering that the research findings presented previously support the hypothesis that hypertonicity develops secondary to the negative signs following a CNS lesion.
As with the individuals who present within the clinical presentation/category of absent or decreased force production, the exact clinical picture of individuals who present with hypertonicity will differ based on their personal and contextual factors, their participation/restrictions, activity/limitations, and their body structure and function integrities/impairments. However, despite these individual variances, common clinical conditions often develop over time from the interaction of the common grouping of impairments. Five of these frequently seen clinical conditions are presented with a discussion of the hypothesized impairments leading to the posture and movement dysfunction seen in each—increased extensor tone in the more affected LE in sitting, increased flexor tone in the more affected UE with standing and walking activities, the subluxed shoulder, the hyperextended knee in stance, and the stiff leg in the swing phase of gait. These commonly seen conditions are often attributed to hypertonicity.
Increased Extensor Tone in the More Affected Lower Extremity in Sitting
When sitting unsupported, individuals with CNS lesions frequently sit with a posterior pelvic tilt in trunk flexion and with the hips in relative extension. This posture places the shoulders and COM behind the hip joints. Often, impairments of musculoskeletal restrictions and muscle weaknesses in the trunk and hip muscles interfere with the individual’s ability to obtain a more optimal sitting alignment (Fig. 11.4). Landel and Fisher86 clearly outline this postural alignment and hypothesize that the stress of maintaining this posture biases the lower limbs to assume what is classically described as an abnormal lower extremity extensor pattern with equinovarus posturing of the ankles and feet (see Case Report A4 in Unit V).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 High sit ↔ stand.
High sit ↔ stand.