Chapter 35 Neuro-ophthalmology
Ocular Motor System
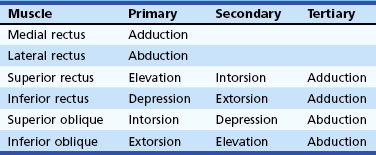
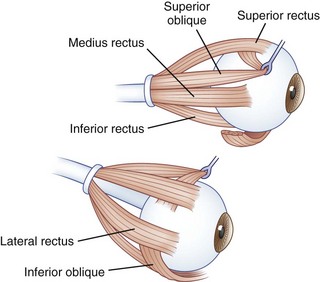
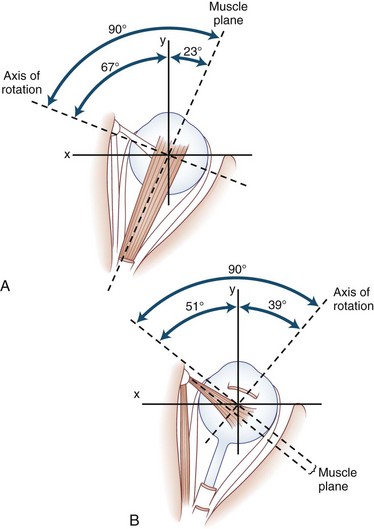
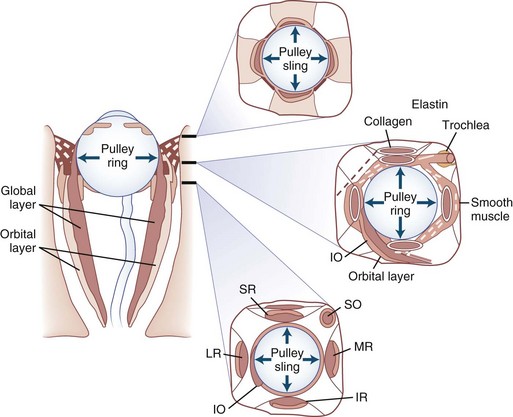
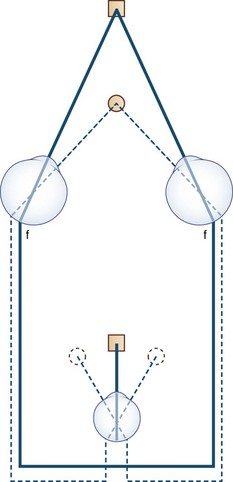
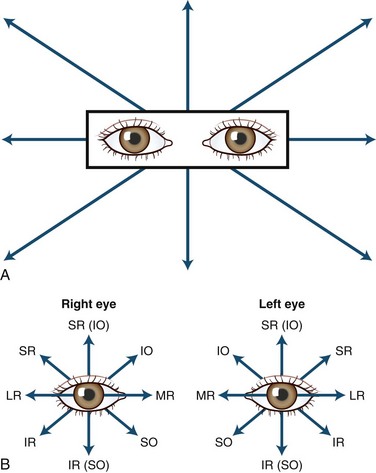
The human fovea is a highly sensitive part of the retina capable of resolving angles of less than 20 arc seconds. The ocular motor system places images of objects of regard on the fovea and maintains fixation (foveation) if the object or head moves. Each eye has six extraocular muscles (Table 35.1) yoked in pairs (Table 35.2) that move the eyes conjugately (versions) to maintain alignment of the visual axes (Fig. 35.1). The actions of the medial and lateral recti are essentially confined to the horizontal plane. The actions of the superior and inferior recti are solely vertical when the eye is abducted 23 degrees. The oblique muscles, the main cyclotortors, also act as pure vertical movers when the eye is adducted 51 degrees (Fig. 35.2). For practical purposes, the vertical actions may be tested at 30 degrees of adduction and abduction. According to the Hering law of dual innervation, yoked muscles receive equal and simultaneous innervation while their antagonists are inhibited (the Sherrington law of reciprocal inhibition), thereby allowing the eyes to move conjugately and with great precision. The pulling actions of the extraocular muscles evolved to move the eyes in the planes of the semicircular canals, which are not strictly horizontal or vertical. These pulling actions are influenced by both the conventional insertions of the global layer of each extraocular muscle directly into the eyeball and by the insertion of the orbital layer into the fibromuscular connective tissue sheath that envelopes each rectus muscle (Fig. 35.3). This arrangement forms a pulley system that is actively innervated (Demer, 2002), stabilizes rotation of the globes in three-dimensional space during complex eye movements (e.g., when a horizontal muscle contracts during upgaze), and prevents excessive retraction of the globe within the orbit during extraocular muscle contraction.
| Ipsilateral | Contralateral |
|---|---|
| Medial rectus | Lateral rectus |
| Superior rectus | Inferior oblique |
| Inferior rectus | Superior oblique |
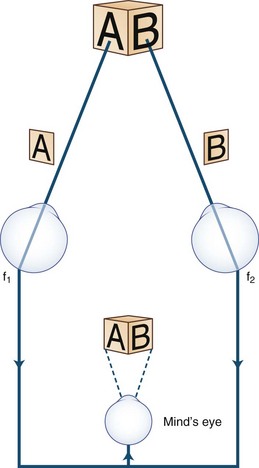
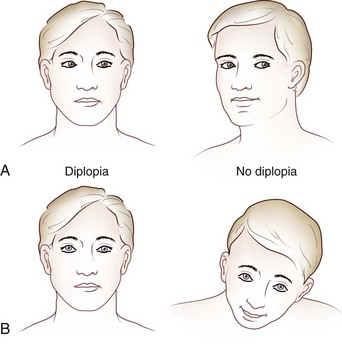
Images of the same object must fall on corresponding points of each retina to maintain binocular single vision (fusion) and stereopsis (Fig. 35.4). If the visual axes are not aligned, the object is seen by noncorresponding (disparate) points of each retina, and diplopia results (Fig. 35.5). In patients with paralytic strabismus, the image from the nonfixating paretic eye is the false image and is displaced in the direction of action of the weak muscle. Thus, a patient with esotropia has uncrossed diplopia (see Fig. 35.5, A), and a patient with exotropia has crossed diplopia (see Fig. 35.5, B). After a variable period, the patient person learns to ignore or suppress the false image. If suppression occurs before visual maturity (approximately 6 years of age) and persists, central connections in the afferent visual system fail to develop fully, leading to permanent visual impairment in that eye (developmental amblyopia). Amblyopia is more likely to develop with esotropia than with exotropia, because exotropia is commonly intermittent. After visual maturity, suppression and amblyopia do not occur; instead, the patient learns to avoid diplopia by ignoring the false image.
Heterophorias and Heterotropias
Divergent eyes are designated exotropic and convergent eyes are esotropic. Vertical misalignment of the visual axes is less common: When the nonfixating eye is higher, the patient is said to have a hypertropia, and when it is lower, a hypotonia (Donahue, 2007), irrespective of which eye is abnormal; for example, with a right hypertropia, the right eye is higher. Asymptomatic hypertropia on lateral gaze often is a congenital condition or “physiological hyperdeviation.”
Comitant Strabismus
New-onset strabismus at school age (after age 6 years) is unusual and warrants evaluation for a neurological disorder. Comitant strabismus occurs early in life; the magnitude of misalignment (deviation) is similar in all directions of gaze, and each eye has a full range of movement (i.e., full ductions). Probably, it occurs because of failure of central mechanisms in the brain that keep the eyes aligned. Infantile (congenital) esotropia may be associated with maldevelopment of the afferent visual system, including the visual cortex, and presents within the first 6 months of life; those with comitant esotropia of more than 40 prism diopters (20 degrees) do not “grow out of it” and require surgical correction (Donahue, 2007). Evidence using cortical motion visual evoked potentials indicates that early correction of strabismus (before 11 months of age) improves visual cortical development (Gerth et al., 2008). Cases of comitant esotropia that manifest between the ages of 7 months and 7 years (average 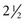 years) are caused by hyperopia (farsightedness) resulting in accommodative esotropia: children with excessive farsightedness must accommodate to have clear vision; the constant accommodation causes excessive convergence and leads to persistent esotropia. Accommodative esotropia responds well to spectacle correction alone. Evidence indicates that high-level stereopsis is restored in these children (unlike those with uncorrected infantile esotropia) if treatment is initiated within 3 months of the onset of constant esotropia (Fawcett et al., 2005).
years) are caused by hyperopia (farsightedness) resulting in accommodative esotropia: children with excessive farsightedness must accommodate to have clear vision; the constant accommodation causes excessive convergence and leads to persistent esotropia. Accommodative esotropia responds well to spectacle correction alone. Evidence indicates that high-level stereopsis is restored in these children (unlike those with uncorrected infantile esotropia) if treatment is initiated within 3 months of the onset of constant esotropia (Fawcett et al., 2005).
Occasionally, children with Chiari malformations or posterior fossa tumors present with isolated esotropia before the appearance of other symptoms or signs. Features that suggest a structural cause for the esotropia include presentation after age 6, complaints such as diplopia or headache, incomitance in horizontal gaze, esotropia greater at distance than near, and neurological findings such as abduction deficits, ataxia, optic disc edema, pathological nystagmus, and saccadic pursuit. Adults in whom isolated esotropia develops, particularly when they become presbyopic in their early 40s, should have a cycloplegic refraction to detect latent hyperopia. Other causes of adult-onset esotropia include Chiari malformations and acute thalamic hemorrhage (Box 35.1).
Box 35.1 Causes of Esotropia
 Congenital esotropia (also acquired, cyclic)
Congenital esotropia (also acquired, cyclic)
 Abducens palsy (unilateral or bilateral)
Abducens palsy (unilateral or bilateral)
 Tonic convergence spasm (part of dorsal midbrain syndrome)
Tonic convergence spasm (part of dorsal midbrain syndrome)
 Pseudo–sixth cranial nerve palsy of Fisher
Pseudo–sixth cranial nerve palsy of Fisher
 Posterior internuclear ophthalmoplegia of Lutz (pseudo-sixth)
Posterior internuclear ophthalmoplegia of Lutz (pseudo-sixth)
 Cyclical oculomotor palsy (spastic phase)
Cyclical oculomotor palsy (spastic phase)
 Nystagmus blockage syndrome (in congenital and latent nystagmus)
Nystagmus blockage syndrome (in congenital and latent nystagmus)
 Abducens palsy with contracture of antagonist (ipsilateral medial rectus) during recovery
Abducens palsy with contracture of antagonist (ipsilateral medial rectus) during recovery
 Medial rectus entrapment (blowout fracture)
Medial rectus entrapment (blowout fracture)
 Thyroid myopathy (rare at presentation)
Thyroid myopathy (rare at presentation)
 Orbital disorders (orbital varix, infiltrative lesions)
Orbital disorders (orbital varix, infiltrative lesions)
 Stiff person syndrome (associated with abduction deficits, hypometric saccades) (Economides and Horton, 2005)
Stiff person syndrome (associated with abduction deficits, hypometric saccades) (Economides and Horton, 2005)
 Wernicke encephalopathy (bilateral abducens palsies)
Wernicke encephalopathy (bilateral abducens palsies)
Noncomitant (Incomitant) Strabismus
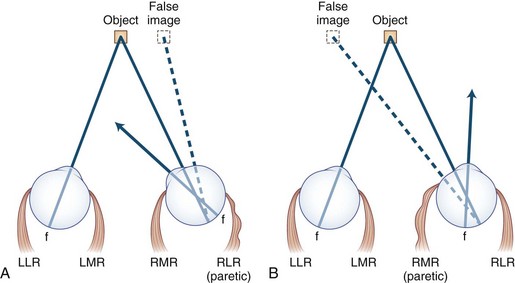
Noncomitant strabismus occurs when the degree of misalignment of the visual axes varies with the direction of gaze as a result of weakness or restriction of one or more extraocular muscles. When a patient with a noncomitant strabismus fixates on an object with the nonparetic eye, the angle of misalignment is referred to as the primary deviation. When the patient fixates with the paretic eye, the angle of misalignment is referred to as the secondary deviation. Secondary deviation is always greater than primary deviation in noncomitant strabismus because of the Hering law of dual innervation; it may mislead the examiner to believe that the eye with the greater deviation is the weak one (Fig. 35.6).
Diplopia
Theoretically, the onset of double vision should be abrupt. However, in practice the history of onset may be vague for various reasons. The patient may interpret subtle diplopia as blurring unless one eye is covered, or the onset may be uncertain because the diplopia is intermittent initially, of small amplitude, or compensated for by head position, as may be the case in disorders such as congenital superior oblique palsy, ocular myasthenia, and thyroid eye disease. Guidelines for evaluation for diplopia are presented in Box 35.2.
Box 35.2 Rules for Evaluation for Diplopia
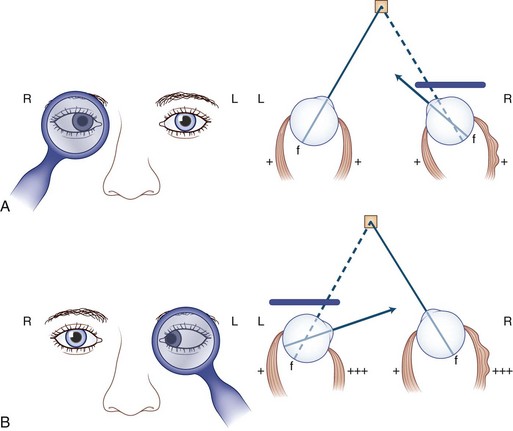
1. Head tilt: When the weak extraocular muscle is unable to move the eye, the head moves the eye. Therefore, the head tilts or turns (or both) in the direction of action of the weak muscle (see Fig. 35.9).
2. Image from the nonfixating eye is the false image and is displaced in the direction opposite the deviation; when the patient fixates with the nonparetic eye, the false image is displaced in the direction of action of the paretic muscle (see Fig. 35.5).
3. False image is the most peripheral image and is displaced in the direction of action of the weak muscle, except when the patient fixes with the paretic eye. When the lateral rectus is paralyzed, the eyes are esotropic (crossed), but the images are uncrossed (see Fig. 35.5A). Diplopia is worse at a distance and on looking to the side of the weak muscle. When the medial rectus is paralyzed, the eyes are exotropic (wall-eyed), but the images are crossed (see Fig. 35.5B). Diplopia is worse at near and on looking to the opposite side.
4. Images are most widely separated when an attempt is made to look in the direction of the paretic muscle.
5. Secondary deviation (angle of ocular misalignment when paretic eye is fixating) is always greater than primary deviation (when normal eye is fixating; see Fig. 35.6). Patients who fixate with the paretic eye may appear to have intracranial disease.
6. Comitance: With a comitant strabismus, the angle of ocular misalignment is relatively constant in all directions of gaze. With a noncomitant (paralytic) strabismus, the angle of misalignment varies with the direction of gaze.
Most adult patients with acquired heterotropia complain of frank double vision, but if the images are close together, the patient may not be aware of frank diplopia but merely perceive blurring, overlapping images (ghosting), or strain. Occasionally, visual confusion occurs because each fovea fixates a different object simultaneously, causing the perception of two objects in the same place at the same time (Fig. 35.7).
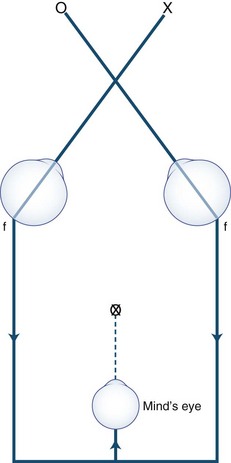
Anxious or histrionic patients may misinterpret physiological diplopia, a normal phenomenon, as a pathological symptom. Physiological diplopia occurs when a subject fixates an object in the foreground and then becomes aware of another object farther away but in the direction of gaze. The nonfixated object is seen by noncorresponding parts of each retina and is perceived by the mind’s (cyclopean) eye as double (Fig. 35.8). Conversely, when the subject fixates a distant object, a near object may appear double.
Isolated vertical diplopia (Box 35.3) most commonly is caused by superior oblique muscle palsy. If the palsy is acquired, one image is virtually always tilted—an infrequent finding when the palsy is congenital. If recently acquired diplopia is worse in downgaze, the weak muscle is a depressor. If the diplopia is worse in upgaze, it is an elevator. If one image is tilted, the weak muscle is more likely an oblique rather than a vertically acting rectus.
Box 35.3 Causes of Vertical Diplopia
Less Common Causes
 Orbital inflammation (myositis, idiopathic orbital inflammatory syndrome [previously designated “pseudotumor”])
Orbital inflammation (myositis, idiopathic orbital inflammatory syndrome [previously designated “pseudotumor”])
 Orbital infiltration (lymphoma, metastases, amyloid)
Orbital infiltration (lymphoma, metastases, amyloid)
 Entrapment of the inferior rectus (blowout fracture)
Entrapment of the inferior rectus (blowout fracture)
 Superior division third nerve palsy
Superior division third nerve palsy
 Atypical third nerve (partial nuclear lesion)
Atypical third nerve (partial nuclear lesion)
 Aberrant third nerve reinnervation
Aberrant third nerve reinnervation
 Brown syndrome (congenital, acquired)
Brown syndrome (congenital, acquired)
 Congenital extraocular muscle fibrosis or muscle absence
Congenital extraocular muscle fibrosis or muscle absence
 Double elevator palsy (monocular elevator deficiency); controversial in origin
Double elevator palsy (monocular elevator deficiency); controversial in origin
Other Causes
If double vision persists when one eye is covered, the patient has monocular diplopia, which may be bilateral. The most common cause of monocular diplopia is an optical aberration (refractive error) and warrants appropriate correction (Box 35.4). Less commonly, monocular diplopia is psychogenic, but occasionally it can be attributed to dysfunction of the retina or cerebral cortex. The pinhole test quickly settles the matter. The patient is asked to look through a pinhole; if the cause is refractive, the diplopia abates because optical distortion is eliminated as the light rays entering the eye through the pinhole are aligned along the visual axis and thus not deflected. Oscillopsia (see later discussion) may be misinterpreted as diplopia.
Box 35.4 Causes of Monocular Diplopia
 After surgery for long-standing tropia (eccentric fixation)
After surgery for long-standing tropia (eccentric fixation)
 Corneal disease (e.g., astigmatism, dry eye, keratoconus)
Corneal disease (e.g., astigmatism, dry eye, keratoconus)
 Corrected long-standing tropia (eccentric fixation)
Corrected long-standing tropia (eccentric fixation)
 Equipment failure (defective contact lens, ill-fitting bifocals in patients with dementia)
Equipment failure (defective contact lens, ill-fitting bifocals in patients with dementia)
 Foreign body in aqueous or vitreous media
Foreign body in aqueous or vitreous media
 Iris abnormalities (polycoria, trauma)
Iris abnormalities (polycoria, trauma)
 Lens: multirefractile (combined cortical and nuclear) cataracts, subluxation
Lens: multirefractile (combined cortical and nuclear) cataracts, subluxation
 Monocular oscillopsia (nystagmus, superior oblique myokymia, eyelid twitching)
Monocular oscillopsia (nystagmus, superior oblique myokymia, eyelid twitching)
 Occipital cortex: migraine, epilepsy, stroke, tumor, trauma (palinopsia, polyopia)
Occipital cortex: migraine, epilepsy, stroke, tumor, trauma (palinopsia, polyopia)
Occasionally, disorders that displace the fovea, such as a subretinal neovascular membrane, can cause binocular diplopia by disrupting the alignment of the photoreceptors (foveal displacement syndrome). The diplopia probably results from rivalry between central and peripheral fusional mechanisms (Brazis and Lee, 1998). Central disruption of fusion (see later) and horror fusionis (in patients with asymmetrical retinal disease) causes intractible diplopia.
Clinical Assessment
History
Box 35.5 shows the procedure for assessing patients with diplopia. The following points should be clarified if the patient has not volunteered the information: Is the diplopia relieved by covering either eye? (If not, it is monocular diplopia; see Box 35.4.) Is it worse in the morning or in the evening? Is it affected by fatigue? Are the images separated horizontally, vertically, or obliquely? If obliquely, is the horizontal or vertical component more obvious? Is the distance between images constant despite the direction of gaze, or does it vary? Is the diplopia worse for near vision or for distance? Is one image tilted? Do the eyelids droop? Is the diplopia influenced by head posture? Has this condition remained stable, improved, or deteriorated? Are there any general health problems? Are there associated symptoms such as headache, dizziness, vertigo, or weakness? What medications are taken? Is there a family history of ocular, neurological, autoimmune, or endocrine disease? Has the patient had a “lazy” eye, worn a patch, or had strabismus surgery?
Box 35.5 Assessment of the Patient with Diplopia
Eye Examination
 Visual acuity (each eye separately, and binocularly if primary position nystagmus present)
Visual acuity (each eye separately, and binocularly if primary position nystagmus present)
 Versions (pursuit, saccades, and muscle overaction)
Versions (pursuit, saccades, and muscle overaction)
 Convergence (does miosis occur?)
Convergence (does miosis occur?)
 Ocular alignment (muscle balance) in the “forced primary position”
Ocular alignment (muscle balance) in the “forced primary position”
 Lids (examine palpebral fissures, levator function, fatigue)
Lids (examine palpebral fissures, levator function, fatigue)
 Vestibulo-ocular reflexes (doll’s eye reflex)
Vestibulo-ocular reflexes (doll’s eye reflex)
 Stereopsis (Titmus stereo test)
Stereopsis (Titmus stereo test)
Head Posture
Because the weak extraocular muscle cannot move the eye fully, patients compensate by tilting or turning the head in the direction of action of the weak muscle (Fig. 35.9). For example, with right lateral rectus palsy, the head is slightly turned to the right; then on attempted right gaze, the patient turns the head farther to the right (see Fig. 35.9, A). With a right superior oblique palsy, the head tilts forward and to the left (see Fig. 35.9, B). The rule is as follows: The head turns or tilts in the direction of action of the weak muscle.
Versions (Pursuit, Saccades, and Ocular Muscle Overaction)
Pursuit movements are tested by asking the patient to fixate and follow (track) a moving target in all directions (Fig. 35.10, A). This test determines the range of eye movement and provides an opportunity to observe for gaze-evoked nystagmus. If spontaneous primary-position nystagmus is present, the effects of the direction of gaze and convergence on the nystagmus may be determined. Pursuit movements should be smooth and full. Cogwheel (saccadic) pursuit is a nonspecific finding and is normal in infants. When present in only one direction, however, it suggests a defect of the ipsilateral pursuit system.
Saccades (fast eye movements) are tested by asking the patient to look rapidly from one target to another (e.g., from the examiner’s nose to a pen) while observing for a delay in initiating the movement (latency) as well as the movement’s speed, accuracy, and conjugacy. An internuclear ophthalmoplegia is best detected by this method (![]() Video 35.1, available at www.expertconsult.com). If a specific muscle (particularly an oblique) underacts or overacts, this can be observed in eccentric gaze before testing ductions in each eye separately, as shown in Fig. 35.10, B. Assessment of disorders of conjugate (supranuclear) gaze is discussed later.
Video 35.1, available at www.expertconsult.com). If a specific muscle (particularly an oblique) underacts or overacts, this can be observed in eccentric gaze before testing ductions in each eye separately, as shown in Fig. 35.10, B. Assessment of disorders of conjugate (supranuclear) gaze is discussed later.
Ductions
Ductions are tested monocularly by having the patient cover one eye and checking the range of movements of the other eye (see Fig. 35.10, B). If ductions are not full, the physician should check for restrictive limitation by moving the eye forcibly (see Forced Ductions, later).
Ocular Alignment and Muscle Balance
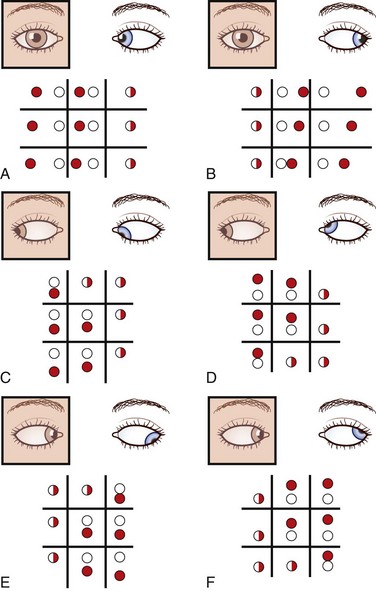
With the red glass test, the patient views a penlight while a red filter or glass is placed, by convention, over the right eye. This allows easier identification of each image; the right eye views a red light and the left a white light. The addition of a green filter over the left eye, using red-green glasses, further simplifies the test for younger or less reliable patients. The target light is shown to the patient in the nine diagnostic positions of gaze (see Fig. 35.10, A). As the light moves into the field of action of a paretic muscle, the images separate. The patient is asked to signify where the images are most widely separated and to describe their relative positions. Interpretation of the results is summarized in Fig. 35.11.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree