Chapter 37 Neuro-otology
Diagnosis and Management of Neuro-otological Disorders
Epidemiology of Vertigo, Dizziness, and Hearing Loss
Specific Disorders Causing Vertigo
Familial Hearing Loss and Vertigo
Common Causes of Nonspecific Dizziness
Common Presentations of Vertigo
Specific Disorders Causing Hearing Loss
Common Presentations of Hearing Loss
Laboratory Investigations in Diagnosis and Management
Management of Patients with Vertigo
Historical Background
Accounts of dizziness and vertigo can be found in the writings of ancient Egyptian and Greek physicians. However, prior to the late 19th century, not much was known about the causes of dizziness or hearing loss, and as a result quackery was commonplace. Patients complaining of dizziness or vertigo were usually grouped together with epileptic seizures and stroke under the rubric of “apoplectiform cerebral congestion,” meaning too much blood to the brain. As a result, common treatments included bleeding, leeching, cupping, and purging. In 1861, Prosper Meniere was the first to recognize the association of vertigo with hearing loss and thus to localize the symptom to the inner ear (Baloh, 2001). Although not well received initially, his discovery provided the basis for later studies on the physiology and pathology of the vestibular system.
The advent of modern neuroimaging in the late 1970s and 1980s greatly expanded our understanding of causes of dizziness and vertigo. Prior to this time, stroke was considered an exceedingly rare cause of vertigo (Fisher, 1967). Though it remains a controversial topic even today, infarctions within the cerebellum and brainstem have been identified on imaging studies in patients with isolated vertigo. Imaging studies continue to lead to new discoveries of causes of vertigo, as demonstrated by the recently described disorder of superior canal dehiscence (SCD). But the most common causes of vertigo—Meniere disease, BPPV, and vestibular neuritis—still have no identifiable imaging characteristics.
Over the last 25 years, our understanding of the mechanisms for the common neuro-otological disorders has been greatly enhanced. BPPV can now be readily identified and cured at the bedside with a simple positional maneuver, and variants have also been described (Aw et al., 2005; Fife et al., 2008). The head-thrust test can be used at the bedside to identify a vestibular nerve lesion, and because of this it has particular utility in helping distinguish vestibular neuritis from a posterior fossa stroke (Halmagyi and Curthoys, 1988; Kattah et al., 2009; Newman-Toker et al., 2008; Nuti et al., 2005). Controversies regarding Meniere disease have been clarified, and medical and surgical treatments have improved (Minor et al., 2004). It is now clear that patients with recurrent episodes of vertigo without hearing loss, a condition once called vestibular Meniere disease, do not actually have Meniere disease.
Migraine is now recognized as an important cause of dizziness, even in patients without simultaneous headaches. In fact, benign recurrent vertigo (patients with recurrent episodes of vertigo without accompanying auditory symptoms or other neurological features) is usually a migraine equivalent (Oh et al., 2001b). The disorder of SCD was only recently described and provides important insight into the physiology of the vestibular system (Minor, 2005). A more detailed description of the rotational vertebral artery syndrome has led to appreciation of the high metabolic demands of the inner ear and its susceptibility to ischemia (Choi et al., 2005). Genetic research has identified ion channel dysfunction in disorders such as episodic ataxia and familial hemiplegic migraine, and patients with these disorders also commonly report vertigo (Jen et al., 2004a). It is hoped that identifying specific genes causing vertigo syndromes will lead to a better understanding of the mechanisms and also create the opportunity to develop specific treatments in the future.
Epidemiology of Vertigo, Dizziness, and Hearing Loss
A recent population-based telephone survey in Germany showed nearly 30% of the population had experienced moderate to severe dizziness (Neuhauser et al., 2005). Though most subjects reported nonspecific forms of dizziness, nearly a quarter had true vertigo. Dizziness is more common among females and older people and has important healthcare utilization implications; up to 80% of patients with dizziness seek medical care at some point. In the United States, the National Centers for Health Statistics report 7.5 million annual ambulatory visits to physician offices, hospital outpatient departments, and emergency departments (EDs) for dizziness, making it one of the most common principal complaints (Burt and Schappert, 2004).
Hearing loss affects approximately 16% of adults (age >18 years) in the United States (Lethbridge-Cejku et al., 2006). Men are more commonly affected than women, and the prevalence of hearing loss increases dramatically with age, so that by age 75, nearly 50% of the population reports hearing loss. Hearing loss is an important cause of disability. The most common type of hearing loss is sensorineural, and both idiopathic presbycusis and noise-induced forms are common etiologies. Bothersome tinnitus is less frequent in the U.S. population, with about 3% reporting it, although this increases to about 9% for subjects older than 65 (Adams et al., 1999). The most common type of tinnitus is a high-pitched ringing in both ears.
Normal Anatomy and Physiology
The inner ear is composed of a fluid-filled sac enclosed by a bony capsule with an anterior cochlear part, central chamber (vestibule), and a posterior vestibular part (Fig. 37.1). Endolymph fills up the fluid-filled sac and is separated by a membrane from the perilymph. These fluids primarily differ in their composition of potassium and sodium, with the endolymph resembling intracellular fluid with a high potassium and low sodium content, and perilymph resembling extracellular fluids with a low potassium and high sodium content. Perilymph communicates with the cerebrospinal fluid (CSF) through the cochlear aqueduct.
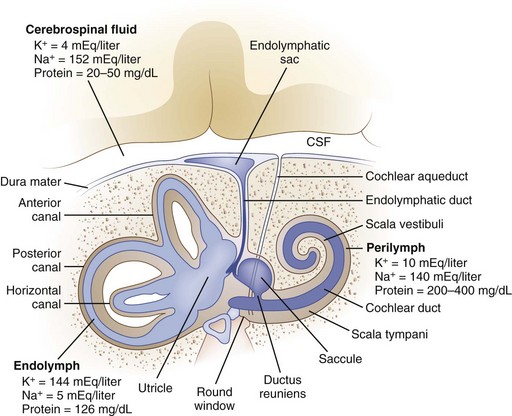
Fig. 37.1 Anatomy of the inner ear. CSF, Cerebrospinal fluid.
(From Baloh, R.W., 1998. Dizziness, Hearing Loss, and Tinnitus. F.A. Davis Company, Philadelphia, Figure 6, p. 16.)
The cochlea senses sound waves after they travel through the external auditory canal and are amplified by the tympanic membrane and ossicles of the middle ear (Baloh and Kerber, 2011). The stapes, the last of three ossicles in the middle ear, contacts the oval window, which directs the forces associated with sound waves along the basilar membrane of the cochlea. These forces stimulate the hair cells, which in turn generate neural signals in the auditory nerve. The auditory nerve enters the lateral brainstem at the pontomedullary junction and synapses in the cochlear nucleus. The trapezoid body is the major decussation of the auditory pathway, but many fibers do not cross to the contralateral side. Signals then travel to the superior olivary complex. Some projections travel from the superior olivary complex to the inferior colliculus through the lateral lemnisci, and others terminate in one of the nuclei of the lateral lemniscus. Next, fibers travel to the ipsilateral medial geniculate body, and then auditory radiations pass through the posterior limb of the internal capsule to reach the auditory cortex of the temporal lobe.
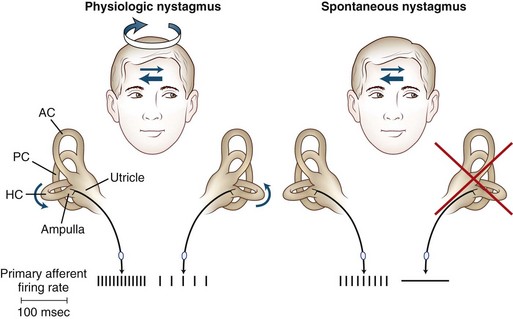
The peripheral vestibular system is composed of three semicircular canals, the utricle and saccule, and the vestibular component of the eighth cranial nerve (Baloh and Kerber, 2011). Each semicircular canal has a sensory epithelium called the crista; the sensory epithelium of the utricle and saccule is called the macule. The semicircular canals sense angular movements, and the utricle and saccule sense linear movements. Two of the semicircular canals (anterior and posterior) are oriented in the vertical plane nearly orthogonal to each other; the third canal is oriented in the horizontal plane (horizontal canal). The crista of each canal is primarily activated by movement occurring in the plane of that canal. When the hair cells of these organs are stimulated, the signal is transferred to the vestibular nuclei via the vestibular portion of cranial nerve VIII. Signals originating from the horizontal semicircular canal then pass via the medial longitudinal fasciculus along the floor of the fourth ventricle to the abducens nuclei in the middle brainstem and the ocular motor complex in the rostral brainstem. The anterior (also referred to as the superior) and posterior canal impulses pass from the vestibular nuclei to the ocular motor nucleus and trochlear nucleus triggering eye movements roughly in the plane of each canal. A key feature is that once vestibular signals leave the vestibular nuclei they divide into vertical, horizontal, and torsional components. As a result, a lesion of central vestibular pathways can cause a pure vertical, pure torsional, or pure horizontal nystagmus.
The primary vestibular afferent nerve fibers maintain a constant baseline firing rate of action potentials. When the baseline rate from each ear is symmetrical (or an asymmetry has been centrally compensated), the eyes remain stationary. With an uncompensated asymmetry in the firing rate, either resulting from increased or decreased activity on one side, slow ocular deviation results. By turning the head to the right, the baseline firing rate of the horizontal canal is physiologically altered, causing an increased firing rate on the right side and a decreased firing rate on the left side (Fig. 37.2). The result is a slow deviation of the eyes to the left. In an alert subject, this slow deviation is regularly interrupted by quick movements in the opposite direction (nystagmus) so the eyes do not become pinned to one side. In a comatose patient, only the slow component is seen because the brain cannot generate the corrective fast components.
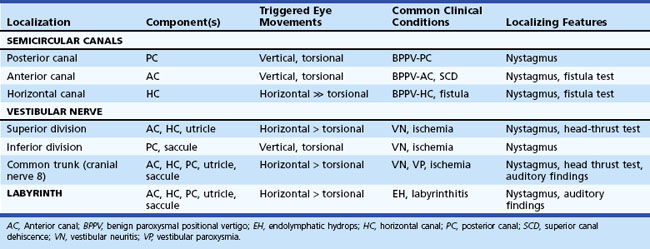
The plane in which the eyes deviate as a result of vestibular stimulation depends on the combination of canals that are stimulated (Table 37.1). If only the posterior semicircular canal on one side is stimulated (as occurs with BPPV), a vertical-torsional deviation of the eyes can be observed, which is followed by a fast corrective response generated by the conscious brain in the opposite direction. However, if the horizontal canal is the source of stimulation (as occurs with the horizontal canal variant of BPPV), a horizontal deviation with a slight torsional component (because this canal is slightly off the horizontal plane) results. If the vestibular nerve is lesioned (vestibular neuritis) or stimulated (vestibular paroxysmia), a horizontal greater than torsional nystagmus is seen that is the vector sum of all three canals—the two vertical canals on one side cancel each other out.
Table 37.1 Physiological Properties and Clinical Features of the Components of the Peripheral Vestibular System
History of Present Illness
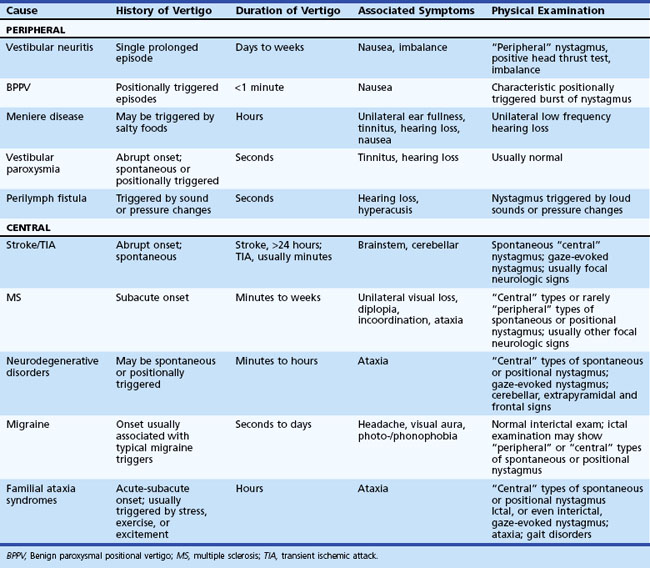
The history and physical examination provide the most important information when evaluating patients complaining of dizziness (Colledge et al., 1996; Lawson et al., 1999). Often, patients have difficulty describing the exact symptom experienced, so the onus is on the clinician to elicit pertinent information. The first step is to define the symptom. No clinician should ever be satisfied to record the complaint simply as “dizziness.” For patients unable to provide a more detailed description of the symptom, the physician can ask the patient to place their symptom into one of the following categories: movement of the environment (vertigo), lightheadedness, or strictly imbalance without an abnormal head sensation. Because patient descriptions about dizziness can be unreliable and inconsistent (Newman-Toker et al., 2007), other details about the symptom become equally important. The physician should also ask the following questions: Is the symptom constant or episodic, are there accompanying symptoms, how did it begin (gradual, sudden, etc.), and were there aggravating or alleviating factors? If episodic, what was the duration and frequency of attacks, and were there triggers? Table 37.2 displays the key distinguishing features of common causes of dizziness. One key point is that any type of dizziness may worsen with position changes, but some disorders such as BPPV only occur after position change.
Physical Examination
General Neurological Examination
The general neurological examination is very important in patients complaining of dizziness, because dizziness can be the earliest symptom of a neurodegenerative disorder (de Lau et al., 2006) and can also be an important symptom of stroke, tumor, demyelination, or other pathologies of the nervous system.
Neuro-otological Examination
Ocular Motor
The first step in assessing ocular motor function is to search for spontaneous involuntary movements of the eyes. The examiner asks the patient to look straight ahead while observing for nystagmus or saccadic intrusions. Nystagmus is characterized by a slow- and fast-phase component and is classified as either spontaneous, gaze-evoked, or positional. The direction of nystagmus is conventionally described by the direction of the fast phase, which is the direction it appears to be “beating” toward. Recording whether the nystagmus is vertical, horizontal, torsional, or a mixture of these provides important localizing information. Spontaneous nystagmus can have either a peripheral or central pattern. Although central lesions can mimic a “peripheral” pattern of nystagmus (Lee and Cho, 2004; Newman-Toker et al., 2008), some very unusual and unlikely circumstances are required for peripheral lesions to cause “central” patterns of nystagmus. A peripheral pattern of spontaneous nystagmus is unidirectional, that is, the eyes beat only to one side (![]() Video 37.1). Peripheral spontaneous nystagmus never changes direction. It is usually a horizontal greater than torsional pattern because of the physiology of the asymmetry in firing rates within the peripheral vestibular system whereby the vertical canals cancel each other out. The prominent horizontal component results from the unopposed horizontal canal. Other characteristics of peripheral spontaneous nystagmus are suppression with visual fixation, increase in velocity with gaze in the direction of the fast phase, and decrease with gaze in the direction opposite of the fast phase. Some patients are able to suppress this nystagmus so well at the bedside, or have partially recovered from the initiating event, that spontaneous nystagmus may only appear by removing visual fixation. Several simple bedside techniques can be used to remove the patient’s ability to fixate. Frenzel glasses are designed to remove visual fixation by using +30 diopter lenses. An ophthalmoscope can be used to block fixation. While the fundus of one eye is being viewed, the patient is asked to cover the other eye. Probably the simplest technique involves holding a blank sheet of paper close to the patient’s face (so as to block visual fixation) and observing for spontaneous nystagmus from the side.
Video 37.1). Peripheral spontaneous nystagmus never changes direction. It is usually a horizontal greater than torsional pattern because of the physiology of the asymmetry in firing rates within the peripheral vestibular system whereby the vertical canals cancel each other out. The prominent horizontal component results from the unopposed horizontal canal. Other characteristics of peripheral spontaneous nystagmus are suppression with visual fixation, increase in velocity with gaze in the direction of the fast phase, and decrease with gaze in the direction opposite of the fast phase. Some patients are able to suppress this nystagmus so well at the bedside, or have partially recovered from the initiating event, that spontaneous nystagmus may only appear by removing visual fixation. Several simple bedside techniques can be used to remove the patient’s ability to fixate. Frenzel glasses are designed to remove visual fixation by using +30 diopter lenses. An ophthalmoscope can be used to block fixation. While the fundus of one eye is being viewed, the patient is asked to cover the other eye. Probably the simplest technique involves holding a blank sheet of paper close to the patient’s face (so as to block visual fixation) and observing for spontaneous nystagmus from the side.
Ocular flutter. Spontaneous back-and-forth saccades without an intersaccadic delay are seen.
Gaze Testing
Gaze-evoked downbeating nystagmus. Downbeating nystagmus occurs with gaze to either side.
Smooth Pursuit
Smooth pursuit refers to the voluntary movement of the eyes used to track a target moving at a low velocity. It functions to keep the moving object on the fovea to maximize vision. Though characteristically a very smooth movement at low frequency and velocity testing, smooth pursuit inevitably breaks down when tested at high frequencies and velocities. Though smooth pursuit often becomes impaired with advanced age, a recent study found no significant decline in smooth pursuit in a group of healthy elderly individuals (>75 years) tested yearly for at least 9 years (Kerber et al., 2006). Patients with impaired smooth pursuit require frequent small saccades to keep up with the target, thus the term saccadic pursuit is used to describe this finding (see Video 37.3). Abnormalities of smooth pursuit occur as the result of disorders throughout the CNS and with tranquilizing medicines, alcohol, inadequate concentration or vision, and fatigue. Patients with diffuse cortical disease, basal ganglia disease, or diffuse cerebellar disease consistently have bilaterally impaired smooth pursuit. Patients with early or mild cerebellar degenerative disorders may have markedly impaired smooth pursuit with mild or minimal truncal ataxia as the only findings.
Vestibular Nerve Examination
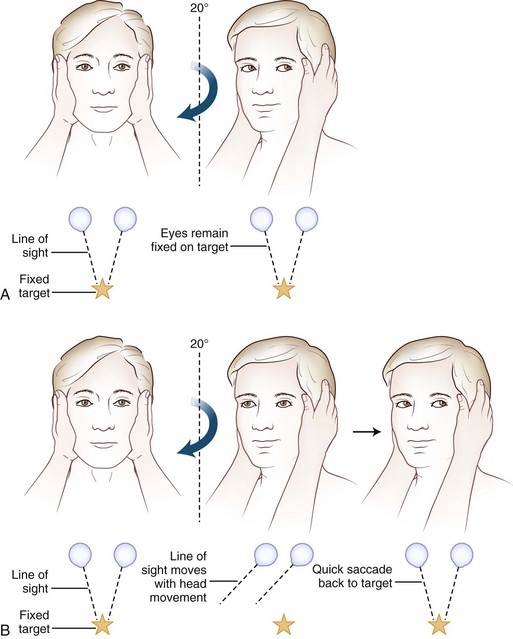
Often omitted as part of the cranial nerve examination in general neurology texts, important localizing information can be obtained about the functioning of the vestibular nerve at the bedside. A unilateral or bilateral vestibulopathy can be identified using the head-thrust test (Halmagyi et al., 2008) (Fig. 37.3 and ![]() Video 37.6). To perform the head-thrust test, the physician stands directly in front of the patient, who is seated on the exam table. The patient’s head is held in the examiner’s hands, and the patient is instructed to focus on the examiner’s nose. The head is then quickly moved about 5 to 10 degrees to one side. In patients with normal vestibular function, the VOR results in movement of the eyes in the direction opposite the head movement. Therefore the patient’s eyes remain on the examiner’s nose after the sudden movement. The test is repeated in the opposite direction. If the examiner observes a corrective saccade bringing the patient’s eyes back to the examiner’s nose after the head thrust, impairment of the VOR in the direction of the head movement is identified. Rotating the head slowly back and forth (the doll’s eye test) also induces compensatory eye movements, but both the visual and vestibular systems are activated by this low-velocity test, so a patient with complete vestibular function loss and normal visual pursuit will have normal-appearing compensatory eye movements on the doll’s eye test. This slow rotation of the head, however, is helpful in a comatose patient who is not able to generate voluntary visual tracking eye movements. Slowly rotating the head can also be a helpful test in patients with impairment of the smooth-pursuit system, because smooth movements of the eyes during slow rotation of the head indicates an intact VOR, whereas continued saccadic movements during slow rotation indicates an accompanying deficit of the VOR (Migliaccio et al., 2004).
Video 37.6). To perform the head-thrust test, the physician stands directly in front of the patient, who is seated on the exam table. The patient’s head is held in the examiner’s hands, and the patient is instructed to focus on the examiner’s nose. The head is then quickly moved about 5 to 10 degrees to one side. In patients with normal vestibular function, the VOR results in movement of the eyes in the direction opposite the head movement. Therefore the patient’s eyes remain on the examiner’s nose after the sudden movement. The test is repeated in the opposite direction. If the examiner observes a corrective saccade bringing the patient’s eyes back to the examiner’s nose after the head thrust, impairment of the VOR in the direction of the head movement is identified. Rotating the head slowly back and forth (the doll’s eye test) also induces compensatory eye movements, but both the visual and vestibular systems are activated by this low-velocity test, so a patient with complete vestibular function loss and normal visual pursuit will have normal-appearing compensatory eye movements on the doll’s eye test. This slow rotation of the head, however, is helpful in a comatose patient who is not able to generate voluntary visual tracking eye movements. Slowly rotating the head can also be a helpful test in patients with impairment of the smooth-pursuit system, because smooth movements of the eyes during slow rotation of the head indicates an intact VOR, whereas continued saccadic movements during slow rotation indicates an accompanying deficit of the VOR (Migliaccio et al., 2004).
Positional Testing
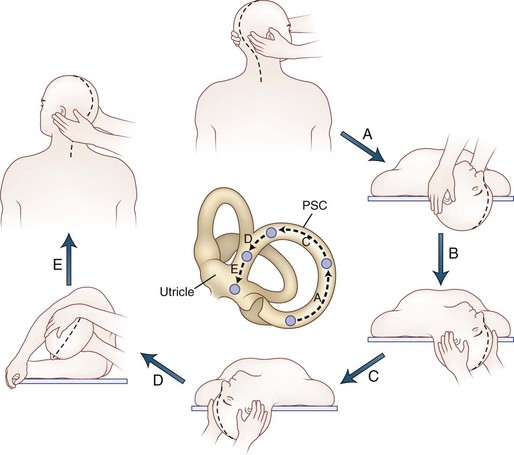
Positional testing can help identify peripheral or central causes of vertigo. The most common positional vertigo, BPPV, is caused by free-floating calcium carbonate debris, usually in the posterior semicircular canal, occasionally in the horizontal canal, or rarely in the anterior canal. The characteristic burst of upbeat torsional nystagmus is triggered in patients with BPPV by a rapid change from an erect sitting position to supine head-hanging left or head-hanging right (the Dix-Hallpike test) (![]() Video 37.7). When present, the nystagmus is usually only triggered in one of these positions. A burst of nystagmus in the opposite direction (downbeat torsional) occurs when the patient resumes the sitting position. A repositioning maneuver can be used to liberate the clot of debris from the posterior canal. We use the modified Epley maneuver (Fig. 37.4 and
Video 37.7). When present, the nystagmus is usually only triggered in one of these positions. A burst of nystagmus in the opposite direction (downbeat torsional) occurs when the patient resumes the sitting position. A repositioning maneuver can be used to liberate the clot of debris from the posterior canal. We use the modified Epley maneuver (Fig. 37.4 and ![]() Video 37.8), which is more than 80% effective in treating patients with posterior canal BPPV, compared to 10% effectiveness of a sham procedure (Fife et al., 2008). The key feature of this maneuver is the roll across in the plane of the posterior canal so that the clot rotates around the posterior canal and out into the utricle. Once the clot enters the utricle, it may reattach to the membrane, dissolve, or may even remain free-floating in the utricle, but the debris no longer disrupts semicircular canal function. Recurrences are common, however.
Video 37.8), which is more than 80% effective in treating patients with posterior canal BPPV, compared to 10% effectiveness of a sham procedure (Fife et al., 2008). The key feature of this maneuver is the roll across in the plane of the posterior canal so that the clot rotates around the posterior canal and out into the utricle. Once the clot enters the utricle, it may reattach to the membrane, dissolve, or may even remain free-floating in the utricle, but the debris no longer disrupts semicircular canal function. Recurrences are common, however.
If the debris is in the horizontal canal, direction-changing horizontal nystagmus is seen. Patients are tested for the horizontal canal variant of BPPV by turning the head to each side while lying in the supine position. The nystagmus can be either paroxysmal geotropic (beating toward the ground) or persistent apogeotropic nystagmus (beating away from the ground). In the case of geotropic nystagmus, the debris is in the posterior segment (or “long arm”) of the horizontal canal, whereas the debris is in the anterior segment (or “short arm”) when apogeotropic nystagmus is triggered. When geotropic nystagmus is triggered, the side with the stronger nystagmus is the involved side. However, when apogeotropic nystagmus is observed, the involved side is generally opposite the side of the stronger nystagmus. With the geotropic variant, the debris can be removed from the canal by rolling the patient (barbecue fashion) toward the normal side. Other repositioning maneuvers for horizontal canal BPPV include the Gufoni maneuver and the “forced prolonged position” (Fife et al., 2008; Vannucchi et al., 1997). In cases of the apogeotropic variant, performing the barbeque maneuver toward the affected side can convert the nystagmus to geotropic because it moves the particles from the short arm of the canal to the long arm. Once the nystagmus is converted to geotropic, the typical treatments for the geotropic variant are used.
Positional testing can also trigger central types of nystagmus (usually persistent downbeating), which may be the most prominent examination finding in patients with disorders like Chiari malformation or cerebellar ataxia (Kattah and Gujrati, 2005; Kerber et al., 2005a). Central positional nystagmus can mimic the nystagmus of horizontal canal BPPV. Positional nystagmus may also be prominent in patients with migraine-associated dizziness (von Brevern et al., 2005).
Auditory Examination
Finger rubs at different intensities and distances from the ear are a rapid, reliable, and valid screening test for hearing loss in the frequency range of speech (Torres-Russotto et al., 2009). If a patient can hear a faint finger rub stimulus at a distance of 70 cm (approximately one arm’s length) from one ear, then a hearing loss on that side—defined by a gold-standard audiogram threshold of greater than 25 dB at 1000, 2000, and 4000 Hz—is highly unlikely. On the other hand, if a patient cannot hear a strong finger rub stimulus at 70 cm, a hearing loss on that side is highly likely. The whisper test can also be used to assess hearing at the bedside (Bagai et al., 2006). For this test, the examiner stands behind the patient to prevent lip reading and occludes and masks the non–test ear, using a finger to rub and close the external auditory canal. The examiner then whispers a set of three to six random numbers and letters. Overall, the patient is considered to have passed the screening test if they repeat at least 50% of the letters and numbers correctly. The Weber and Rinne tests are commonly used bedside tuning fork tests. To perform these, a tuning fork (256 Hz or 512 Hz) is gently struck on a hard rubber pad, the elbow, or the knee about two-thirds of the way along the tine. To conduct the Weber test, the base of the vibrating fork is placed on the vertex (top or crown of the head), bridge of the nose, upper incisors, or forehead. The patient is asked if the sound is heard and whether it is heard in the middle of the head or in both ears equally, toward the left, or toward the right. In a patient with normal hearing, the tone is heard centrally. In asymmetrical or a unilateral hearing impairment, the tone lateralizes to one side. Lateralization indicates an element of conductive impairment in the ear in which the sound localizes, a sensorineural impairment in the contralateral ear, or both. The Rinne test compares the patient’s hearing by air conduction with that by bone conduction. The fork is first held against the mastoid process until the sound fades. It is then placed 1 inch from the ear. Normal subjects can hear the fork about twice as long by air as by bone conduction. If bone is greater than air conduction, a conductive hearing loss is suggested.
Specific Disorders Causing Vertigo
Peripheral Vestibular Disorders
Peripheral vestibular disorders are important for neurologists to understand because they are common, readily identified at the bedside, and often missed by frontline physicians (see Table 37.2).
Vestibular Neuritis
A common presentation to the ED or outpatient clinic is the rapid onset of severe vertigo, nausea, vomiting, and imbalance. The symptoms gradually resolve over several days, but some symptoms can persist for months. The etiology of this disorder is probably viral, because the course is generally benign and self-limited, it occurs in young healthy individuals, and occasionally occurs in epidemics. Histopathological studies provide evidence of a peripheral vestibular localization and support the etiology of a viral cause. A viral etiology is also the likely cause of most cases of Bell palsy and sudden sensorineural hearing loss. The key to the diagnosis of vestibular neuritis is recognizing the peripheral vestibular pattern of nystagmus and identifying a positive head-thrust test in the setting of a rapid onset of vertigo without other neurological symptoms. Magnetic resonance imaging studies (MRIs) are usually normal in patients with vestibular neuritis (Strupp et al., 1998b). The course of vestibular neuritis is self-limited, and the mainstay of treatment is symptomatic. A recent study of patients with vestibular neuritis showed improvement of peripheral vestibular function, as measured by caloric testing at 1 year, after receiving methylprednisolone within 3 days of onset, compared to placebo (Strupp et al., 2004). A formal vestibular rehabilitation program can help patients compensate for the vestibular lesion (Strupp et al., 1998a).
Benign Paroxysmal Positional Vertigo
Benign paroxysmal positional vertigo may be the most common cause of vertigo in the general population. Patients typically experience brief episodes of vertigo when getting in and out of bed, turning in bed, bending down and straightening up, or extending the head back to look up. As noted earlier, the condition is caused when calcium carbonate debris dislodged from the otoconial membrane inadvertently enters a semicircular canal. The debris can be free-floating within the affected canal (canalithiasis) or stuck against the cupula (cupulolithiasis). Repositioning maneuvers are highly effective in removing the debris from the canal, though recurrence is common (see Fig. 37.4) (Fife et al., 2008). Once the debris is out of the canal, patients are instructed to avoid extreme head positions to prevent the debris from reentering the canal. Patients can also be taught to perform a repositioning maneuver should they have a recurrence of the positional vertigo.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree