CHAPTER 4 Neuroembryology
Classic neuroembryology dealt primarily with documentation of the timing and location of morphologic changes in embryonic development, both gross and microscopic, but an acceleration of genetic, molecular, and radiologic (i.e., neuroimaging) advances in recent years has fundamentally changed the way that we perceive and understand both normal formation and malformation of the brain. Completion of the first draft of the sequence of the human genome will have profound implications on our understanding of the genetics of brain development.1–4 An accelerating pace of genetic discovery is accompanied by the risk that reviews become obsolete before their publication. We now speak of genetics and genomics, but since the human genome project met its initial goals, we will necessarily move onto proteomics (i.e., study of all proteins expressed by the genome)5 and an ever-growing list of “omics” as we delve deeper into the complex molecular genetic interactions required for creating the brain.
Although we do not dispute the critical role of these advances in elucidating development, we strongly believe that this information is best used within the framework of a solid understanding of the timing of structural changes during brain development. In this chapter we offer a unified vision of neuroembryology, with molecular genetic details presented along with classic structural information. It is beyond the scope of this chapter to review the principles of genetics or the revolutionary techniques of gene cloning, polymerase chain reaction, and positional cloning or to attempt to catalog the genes responsible for neurological abnormalities.6 More information is available in review articles or book chapters on neurogenetics.7,8 We do provide examples of important genes relevant to neurodevelopment and extensive references that can aid in understanding basic concepts.
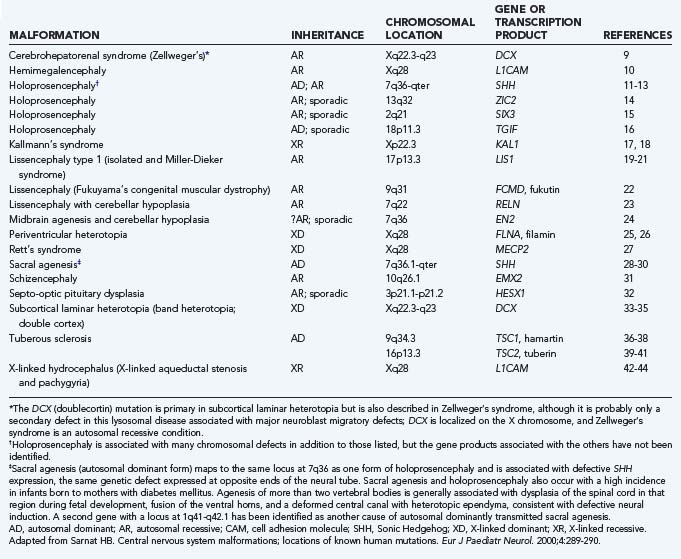
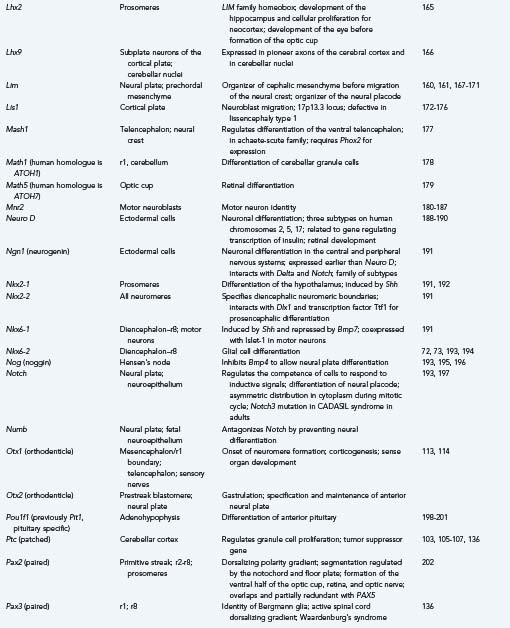
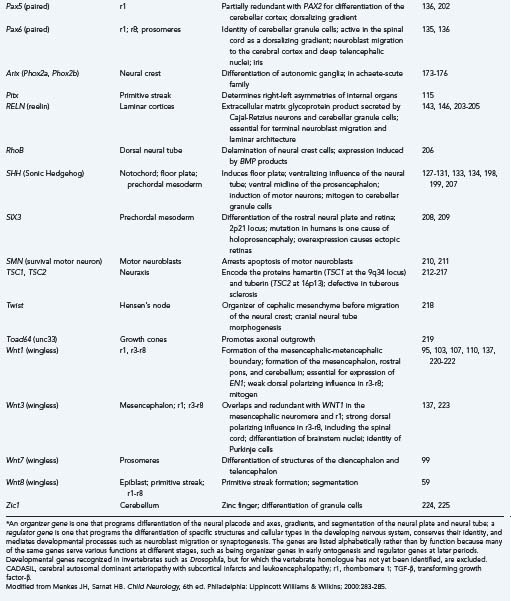
Because much of our knowledge about human brain development has resulted from searching for human homologues to developmental genes discovered in animals from Drosophila to mice, we include examples from these studies along with our discussions of the stages, processes, and abnormalities of human neuroembryology. Table 4-1 lists known gene mutations responsible for several important human central nervous system (CNS) malformations.9–44 When specifically referring to a human gene, we use the convention of denoting the gene symbol in italicized, capital letters.
We once thought of brain insults arising from either environmental or genetic factors, but we now recognize that these causes are interconnected and inseparable. Environmental factors act by influencing gene regulation and expression, and genetic differences determine responses to environmental agents, including toxins and transcription factors. The molecular details of how thousands of genes and the proteins that they encode work together to determine normal or abnormal structure and function are becoming clearer daily but are still overwhelmingly complex. Although the estimated number of genes in the human genome is only about 30,000 (far less than the previously estimated 100,000 and only 2.5 times larger than the fly genome), the human proteome is estimated to contain between 130,000 and 400,000 distinct proteins. Each has many potential ways of interacting with other proteins or genes and of being posttranslationally modified.4
Improvements in neuroradiologic techniques are helping to uncover relationships between genetic abnormalities and structural malformations and to provide another justification for learning more about the basics of embryologic and fetal development. As imaging has improved, so has our appreciation that many developmental disorders represent a spectrum of abnormalities much more complex than previously appreciated. The quality of the image and the speed of acquisition of fetal magnetic resonance imaging (MRI) are constantly improving, and it is already capable of very good anatomic definition of fetal brain malformations.45 The next major step, which will almost certainly occur in the near future, will be the development of MRI techniques for evaluating functional gene expression. In combination with the other molecular genetic advances described, improved imaging will provide an unprecedented opportunity to understand brain development and its disorders.
Classic Neuroembryology
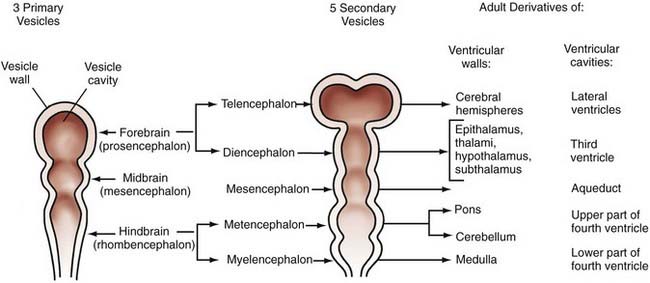
The technical meaning of the term embryology should restrict the study of development to the first 6 postconceptional weeks in humans, the embryonic period proper, but traditional use extends the term to include fetal life until birth, and this is the application that we use in this chapter. Although nonhuman embryologic studies have long been important to our understanding of brain development, meticulous descriptive morphogenic studies of serially sectioned human embryos over the past several decades have provided unique and invaluable information. Based on studies of internal and external morphology that originated from the Carnegie collection of embryos, the 8 weeks of embryonic development have been subdivided into 23 morphologic (Carnegie) stages.46 Stages 8 to 23 are relevant to neuroembryology, with the neural groove and folds first appearing in stage 8, which occurs at about 23 days’ gestation. At this time, the embryo is only about 1 mm long. No accepted morphologic staging system has been developed for the fetal period. These studies remain valuable in using known milestones in structural development to identify the termination period, or the gestational day beyond which the onset of a specific malformation could not have occurred. Knowledge of the exact times when defects such as anencephaly or meningomyelocele may occur is critical for molecular genetic and epidemiologic investigations and can provide important clues to pathophysiologic mechanisms. Further detail can be obtained from O’Rahilly and Muller’s updated classic atlas of developmental stages.46 From careful studies such as these, the adult derivatives of embryonic structures were determined long before we began to understand the signals and processing underlying their formation (Fig. 4-1).
Along with careful work over the past several decades that resulted in this embryonic staging system, similar analysis of the development of the cerebral vasculature led to a staging system usually referred to as Padgett stages.47–49 Knowledge of how the vascular system evolves leads to a clearer understanding of vascular malformations and common vascular anomalies (covered elsewhere in this book) and the patterns of secondary embryonic and fetal brain injuries and malformations. The arterial system essentially achieves an adult pattern by the end of the embryonic period, whereas the venous system develops much later in the fetal period. Although neither the seven stages of arterial evolution developed by Padgett (and still used) nor cerebral venous development will be considered further here, more information can be found in any of several excellent reviews of vascular development.50–53
Developmental Organization: Stages, Genes, and Regulatory Factors
Gastrulation
As the primitive streak extends forward, cells aggregate at one end, a collection designated the primitive node or Hensen’s node. Hensen’s node defines rostral. Cells of the epiblast on either side move toward the primitive streak, stream through it, and emerge beneath it to pass into the narrow cavity between the two sheets of cells, with the epiblast above and the hypoblast below; these migratory cells give rise to the mesoderm and endoderm internally, and some then replace the hypoblast.54
After extending about halfway across the blastoderm (epiblast), the primitive streak with Hensen’s node reverses the direction of its growth and retreats, moving posteriorly as the head fold and neural plate form anterior to Hensen’s node. As the node regresses, a notochordal process develops in the area rostral to it, and somites begin to form on either side of the notochord, with the more caudal somites differentiating first and successive ones differentiating anterior to the somites already formed. The notochord induces epiblast cells to form neuroectoderm (see “Induction”). Several genes essential in creating the fundamental architecture of the embryo and its nervous system are already expressed in the primitive node,55 and many reappear later to influence more advanced stages of ontogenesis.
Induction
Induction was discovered in 1924, when Hans Spemann and Hilde Mangold demonstrated that the dorsal lip of the newt gastrula was capable of inducing the formation of an ectopic second nervous system when transplanted to another site in a host embryo, into another individual of the same species, or to a ventral site of the same embryo.56 This dorsal lip of the amphibian gastrula, also called the Spemann organizer, is homologous with the Hensen node of embryonic birds and mammals.
The first gene isolated from the Spemann organizer was Gsc (goosecoid), which encodes a homeodomain protein (see the later section “Transcription Factors and Homeoboxes”) able to recapitulate transplantation of the dorsal lip tissue when injected into an ectopic site. It also normally induces the prechordal mesoderm and contributes to prosencephalic differentiation.55,57,58 In Hensen’s node in the chick, even before the primitive streak is fully formed, Wnt8c is expressed and is essential for the regulation of axis formation and later for hindbrain patterning in the region of the future rhombomere 4 (r4).59 The regulatory gene Cnot, with major domains in the primitive node, notochord, and prenodal and postnodal neural plate, is also involved in the induction of prechordal mesoderm and in formation of the notochord in particular.60
The specificity of induction lies not in the inductive molecule but rather in the receptor in the induced cell. This distinction is important because foreign molecules similar in structure to the natural inductor molecule may sometimes be erroneously recognized by the receptor as identical; such foreign molecules may act as teratogens if the embryo is exposed to such a toxin. Induction occurs during a very precise temporal window; the period of responsiveness of the induced cell is designated its competence, and the cell is incapable of responding before or after that precise time.61
Induction receptors are not necessarily in or on the plasma membrane of the cell but may be in the cytoplasm or in the nucleus. Retinoic acid is an example of a nuclear inducer. In some cases, the stimulus acts exclusively at the plasma membrane of target cells and does not require actual penetration of the cell.61,62 The receptors that represent the specificity of induction are also genetically programmed. Notch is a particularly important gene in regulating the competence of a cell to respond to inductive cues from within the neural tube and from surrounding embryonic tissues.63 Some mesodermal tissues, such as smooth muscle of the fetal gut, can act as mitogens on the neuroepithelium by increasing the rate of cellular proliferation,64,65 but this phenomenon is not true neural induction because the proliferating cells do not differentiate or mature. Some organizer and regulatory genes of the nervous system, such as Wnt1, also exhibit mitogenic effects,66 and insulin-like growth factor and basic fibroblast growth factor act as mitogens as well.67–69
Early formation of the neural plate is not accomplished exclusively by mitotic proliferation of neuroepithelial cells; surrounding cells are also converted to a neural fate. In amphibians, a gene known as Xash (achaete-scute) is expressed very early in the dorsal part of the embryo from the time of gastrulation and acts as a molecular switch to change the fate of undifferentiated cells to become neuroepithelium rather than surface ectodermal or mesodermal tissues.70 Some cells differentiate as specific types because they are actively inhibited from differentiating into others. All ectodermal cells are preprogrammed to form neuroepithelium, and neuroepithelial cells are preprogrammed to become neurons if not inhibited by genes that direct them along a different lineage, such as epidermal, glial, or ependymal.71–73
Neurulation
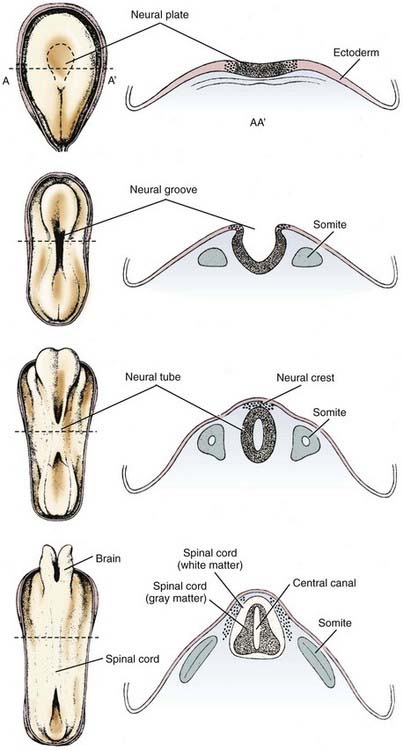
These forces arise in part from growth of the surrounding mesodermal tissues on either side of the neural tube, the future somites (Table 4-2).74 After surgical removal of mesoderm and endoderm from one side of the neuroepithelium in experimental animals, the neural tube still closes, but it is rotated and becomes asymmetric.75 The mesoderm appears to be important for orientation but not for closure of the neural tube. Expansion of the surface epithelium of the embryo is the principal extrinsic force for folding of the neuroepithelium to form the neural tube.76 Cells of the neural placode are mobile and migrate beneath the surface ectoderm, which causes the lateral margins of the placode to become raised toward the dorsal midline. Growth of the whole embryo itself does not appear to be an important factor because neurulation proceeds equally well in anamniotes (e.g., amphibians), which do not grow during this period, and in amniotes (e.g., mammals), which grow rapidly at this time.77
TABLE 4-2 Factors Involved in Closure of Neuroepithelium to Form the Neural Tube
From Menkes JH, Sarnat HB. Child Neurology, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:289.
Among the intrinsic forces of the neuroepithelium, the cells of the floor plate have a wedge shape—narrow at the apex and broad at the base—that facilitates bending.78 Although the width of the floor plate is small, its site in the ventral midline is crucial and sufficient to allow a significant influence. It represents yet another aspect of induction of the floor plate by the notochord, apart from its influence on the differentiation of neural cells.79 The ependymal cells that form the floor plate are the first neural cells to differentiate, and they induce growth of the parenchyma of the ventral zone more than the dorsal regions.80,81 This mechanical effect may also facilitate curving of the neural placode. The direction of proliferation of new cells in the mitotic cycle, determined in part by the orientation of the mitotic spindle, becomes another mechanical force shaping the neural tube.77,78 Adhesion molecules are also probably an important mechanical factor for neurulation. In later stages, the ependymal cell–lined central canal, which is much larger in the fetus than in the newborn, may have a role in exerting a centrifugal force to create the tubular shape, although in early spinal cord development the central canal is a tall, narrow, midline slit and only later in fetal life does it assume a rounded contour as seen in transverse sections.80
Neuroepithelial cells of the neural placode or plate downregulate the polarity of their plasma membrane so that the apical and basilar surfaces are not as distinct before neural tube closure. Cell differentiation in general involves such changes in cell polarity.82 The rostrocaudal orientation of most mitotic spindles of the neuroepithelium and the direction in which they push by the mass of daughter cells that they form also influence the shape of the neural tube (Fig. 4-2).83
The neural tube closes in the dorsal midline first in the cervical region, with the closure then extending rostrally and caudally such that the anterior neuropore of the human embryo closes at 24 days and the posterior neuropore closes at 28 days, with the distances from the cervical region being unequal. This traditional view of a continuous zipper-like closure is an oversimplification. In the mouse embryo, the neural tube closes in the cranial region at four distinct sites, with the closure proceeding bidirectionally or unidirectionally and in general synchrony with somite formation.84,85 An intermittent pattern of anterior neural tube closure involving multiple sites has also been described in human embryos.86 In this closure pattern, the principal rostral neuropore closes bidirectionally87 to form the lamina terminalis, an essential primordium of the forebrain.80
Bending of the neural plate to form the neural tube is termed primary neurulation. Failure of the anterior neuropore to close by 24 days results in anencephaly. Because the lamina terminalis does not form, its derivatives (including the basal ganglia and other forebrain structures) do not develop. The lack of forebrain neuroectoderm results in failure of induction of the overlying mesoderm, and the cranium, meninges, and scalp fail to close in the midline.88 The term secondary neurulation refers only to the most caudal part of the spinal cord (i.e., conus medullaris), which develops from neuroepithelium caudal to the site of posterior neuropore closure. More details on abnormalities that occur because of problems with secondary neurulation are offered in other chapters in this textbook.
Neural crest cells arise from the dorsal midline of the neural tube at or shortly after the time of closure and migrate extensively along prescribed routes through the embryo to differentiate as the peripheral nervous system. This includes the dorsal root and sympathetic ganglia, adrenal medulla and carotid body chromaffin cells, melanocytes, and a few other cell types of ectodermal and mesodermal origin.89,90
Segmentation and Regionalization
Segmentation of the neural tube creates intrinsic compartments that restrict the movement of cells by physical and chemical boundaries between adjacent compartments. These embryonic compartments are known as neuromeres. The spinal cord has the appearance of a highly segmented structure; however, it is not intrinsically segmented in the embryo, fetus, or adult but rather corresponds in its entirety to the most caudal of the eight neuromeres that create the hindbrain. The apparent segmentation of the spinal cord results from clustering of nerve roots imposed by true segmentation of surrounding tissues derived from the mesoderm, tissues that form the neural arches of the vertebrae, somites, and associated structures. Neuromeres of the hindbrain are designated rhombomeres.91–94 The entire cerebellar cortex, vermis, flocculonodular lobe, and lateral hemispheres develop from rhombomere 1 (r1), with a small contribution to the anterior vermis from the mesencephalic neuromere, but the dentate and other deep cerebellar nuclei are formed in rhombomere 2 (r2).95,96 The rostral end of the neural tube forms a mesencephalic neuromere and probably six forebrain neuromeres (i.e., two diencephalic and four telencephalic prosomeres), although these may be subdivided further.97–99 The segmentation of the human embryonic brain into neuromeres is summarized in Table 4-3.
TABLE 4-3 Segmentation of the Neural Tube
| NEUROMERE | DERIVED STRUCTURES IN MATURE CENTRAL NERVOUS SYSTEM |
|---|---|
| Rhombomere 8 (r8) | Entire spinal cord; caudal medulla oblongata; cranial nerves XI, XII |
| Rhombomere 7 (r7) | Medulla oblongata; cranial nerves IX, X; neural crest |
| Rhombomere 6 (r6) | Medulla oblongata; cranial nerves VIII, IX |
| Rhombomere 5 (r5) | Medulla oblongata; cranial nerves VI, VII; no neural crest |
| Rhombomere 4 (r4) | Medulla oblongata; cranial nervess VI, VII; neural crest |
| Rhombomere 3 (r3) | Caudal pons; cranial nerve V; no neural crest |
| Rhombomere 2 (r2) | Caudal pons; cranial nerves IV, V; cerebellar nuclei |
| Rhombomere 1 (r1) | Rostral pons; cerebellar cortex |
| Mesencephalic neuromere | Midbrain; cranial nerve III; neural crest |
| Diencephalic prosomere 2 | Dorsal diencephalons |
| Diencephalic prosomere 1 | Ventral diencephalon |
| Prosencephalic prosomere 2 | Telencephalic nuclei; olfactory bulb |
| Prosencephalic prosomere 1 | Cerebral cortex; hippocampus; corpus callosum |
From Menkes JH, Sarnat HB. Child Neurology, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:280.
The segments of the embryonic neural tube are distinguished by physical barriers formed by processes of early specializing cells that resemble the radial glial cells that appear later in development100,101 and by chemical barriers from secreted molecules that repel migratory cells. Cell adhesion is increased in the boundary zones between rhombomeres, which also contributes to the creation of barriers against cellular migration in the longitudinal axis. Limited mitotic proliferation of the neuroepithelium occurs in the boundary zones between rhombomeres. Although cells still divide in this zone, their nuclei remain near the ventricle during the mitotic cycle and do not move as far centrifugally within the elongated cell cytoplasm during the interkinetic gap phases as they generally do.101 The rhombomeres of the brainstem may also be visualized as a series of transverse ridges and grooves on the dorsal surface, the future floor of the fourth ventricle; these ridges are gross morphologic markers of the hindbrain compartments.94,102
The first evidence of segmentation is a boundary that separates the future mesencephalic neuromere from r1 of the hindbrain. More genes play a role in this initial segmentation of the neural tube than in any boundaries that subsequently form to separate other neuromeres. The mesencephalic-metencephalic region appears to develop early as a single, independent unit or “organizer” for other neuromeres rostral and caudal to that zone.103,104 The organizer genes recognized at the mesencephalic-metencephalic boundary for this earliest segmentation of the neural tube include Pax2, Wnt1, En1, En2, Pax5, Pax8, Otx1, Otx2, Gbx2, Nkx2-2, and Fgf8.
The earliest known gene with regional expression in the mouse is Pax2, and it is expressed even before the neural plate forms. It is the earliest gene recognized in the presumptive region of the midbrain-hindbrain boundary.105,106 In invertebrates, Pax2 is important for the activation of Wg (wingless) genes; this relationship is relevant because the first gene definitely associated with an identified midbrain-hindbrain boundary in vertebrates is Wnt1, a homologue of Wg. Regulation of Wnt1 may be divided into two phases. In the early phase (1 or 2 somites), the mesencephalon broadly expresses the gene throughout; in the later phase (15 to 20 somites), expression is restricted to the dorsal regions, the roof plate of the caudal diencephalon, the mesencephalon, the myelencephalon, and the spinal cord, but it is also expressed in a ring that extends ventrally just rostral to the midbrain-hindbrain boundary and in the ventral midline of the caudal diencephalon and mesencephalon.107–109 Wnt1 is essential in activating and preserving the function of the mouse engrailed genes En1 and En2. En1 is coexpressed with Wnt1 at the 1-somite stage in a domain only slightly caudal to Wnt1, which includes the midbrain and r1, the rostral half of the pons, and the cerebellar cortex but excludes the diencephalon.110 Activation of En2 begins at the 4-somite stage, and its function in mesencephalic and r1 development is similar, with differences in some details, particularly their roles in cerebellar development.111,112 The homeobox gene Otx2 appears early in the initial boundary zone of the midbrain-hindbrain, and as with Wnt1, it appears to be essential for the later expression of En1, En2, and Wnt1.113,114
Patterning of the Neural Tube
Development of the basic characteristics of the body plan is called patterning.91 These patterns are the anatomic expression of the genetic code within the nuclear DNA of every cell, but they may also result from signals from neighboring cells carried by molecules that are secretory translation products of various families of organizer genes, each in a highly precise and predictable temporal and spatial distribution.
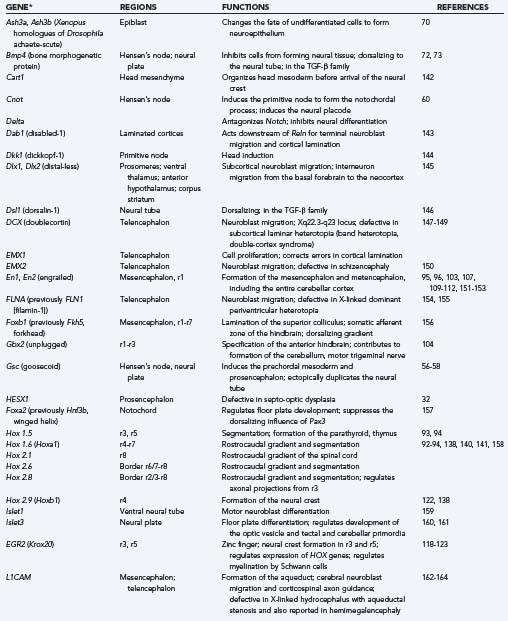
Early development of the CNS in all vertebrates, even before closure of the neural placode or plate to form the neural tube, requires the establishment of a fundamental body plan of bilateral symmetry, with cephalization, or the identity of head and tail ends, and determination of the dorsal and ventral surfaces. These axes of the body itself and the CNS require the expression of genes that impose gradients of differentiation and growth. The genes that determine the polarity and gradients of the anatomic axes are called organizer genes. Many express themselves in the CNS and in other organs and tissues.54,109 The bilateral symmetry of many organs and programmed asymmetries, probably including neural structures such as the different targets of the left and right vagal nerves and left-right asymmetries in the cerebral cortex, is determined in large part by Pitx2, a gene expressed as early as in the primitive node.115 Some genes function to stimulate or inhibit the expression of others, or there is an antagonism or equilibrium between certain families of genes, as exemplified by those that exert dorsoventral or ventrodorsal gradients. The difference between an organizer gene and a regulator gene is its function, and the same gene often subserves both roles at different stages of development. The definitions and programs of these two groups are summarized in Table 4-4.
TABLE 4-4 Programs of Developmental Genes
| Organizer Genes |
| Regulator Genes |
From Menkes JH, Sarnat HB. Child Neurology, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:281.
Transcription Factors and Homeoboxes
Transcription factors are proteins expressed by regulatory genes that bind to the regulatory regions of other genes and control transcription. These transcription factors are essential for functional expression of the gene, and several different protein structural motifs have been discovered to be highly evolutionarily conserved. One such motif is the basic helix-loop-helix structure, which is so fundamental to the evolution of life that it appears for the first time in certain bacteria even before evolution of a cell nucleus to concentrate the DNA.116
The zinc finger is another DNA-binding, gene-specific transcription factor motif. It consists of 28 amino acid repeats with pairs of cysteine and histidine residues and with each sequence folded around a zinc ion.117 Krox20 (this gene name is applied to the mouse; the human form is designated EGR2) is a zinc finger gene expressed in alternating rhombomeres, especially r3 and r5; neural crest tissue does not differentiate from these two rhombomeres, although it does in adjacent segments, including r4.118 Krox20 serves an additional function in the peripheral nervous system, where it regulates myelination by Schwann cells,119 and it also regulates the expression of some other genes, most notably those of the Hox family.118,120–123 Another developmentally important zinc finger gene is PLZF (human promyelocytic zinc finger). Studies of mouse and chick homologues of this gene have revealed that it is expressed in a restricted zone surrounding hindbrain rhombomere boundaries, thus suggesting an important functional role for it and other zinc finger genes in vertebrate hindbrain regionalization.124
Some transcription factor genes include homeoboxes. These restricted DNA sequences of 183 base pairs of nucleotides encode a class of proteins sharing a common or very similar 60–amino acid motif called the homeodomain.91 Homeoboxes or homeotic genes are classified into various families with common molecular structures and similar general expression during ontogenesis. They are especially associated with genes that program segmentation and the rostrocaudal gradients of the neural tube. Some of the families of homeobox genes important in development of the vertebrate nervous system are Gsc, Hox, En, Wnt, Shh, Nkx, Lim, and Otx.
Growth factors may also influence the pattern of the neural tube by behaving biologically as transcription factors: basic fibroblast growth factor behaves as an auxiliary inductor of the longitudinal axis with a rostrocaudal gradient during formation of the neural tube.125
Developmental Gene Families of the Central Nervous System
The genes that program the axes and gradients of the neural tube may be classified as families based on their similar nucleic acid sequences and their similar general functions, although important differences occur within a family in the site or neuromere where each gene is expressed and the anatomic structures that they form. A dorsalizing gene has a dorsal territory of expression and causes the ventral parts of the neural tube to differentiate as dorsal structures if influences from ventralizing genes do not antagonize them sufficiently and vice versa. In development of the somite, the sclerotome (which forms the cartilage and bone of the vertebral body) is normally situated ventral to the myotome (which forms muscle cells) and the dermatome. Ectopic cells of the floor plate or notochord implanted next to the somite of the chick embryo cause ventralization of the somite such that excess cartilage and bone are formed and there is a deficiency of muscle and dermis.126,127 The floor plate, or notochord in this instance, is the ventralizing inductor of the mesodermal somite, and this is caused by expression of Shh (Sonic Hedgehog), which also serves as a strong ventralizing gradient force in the neural tube.128–131 If a section of notochord is ectopically implanted dorsal or lateral to the neural tube, a second floor plate forms opposite the notochord, and motor neurons differentiate on either side of it despite the presence of a normal floor plate and motor neurons in the normal position.132 Shh, which is expressed as early as in the primitive node, induces ventralization of a dorsal region of the neural tube or duplicates the neural tube. Such an influence in the human fetus, the so-called split notochord, could be an explanation for the rare cases of diplomyelia or diastematomyelia.80 Excessive Shh expression, particularly its amino-terminal cleavage product, upregulates floor plate differentiation at the expense of motor neuron formation129 and may induce duplication of the neuraxis. Shh exerts a strong influence on differentiation of the ventral and medial structures of the prosencephalon,133 and defective expression of this gene has been found to be one molecular basis of human holoprosencephaly.134
To establish an equilibrium with genes with ventralizing influence, other genes exercise a dorsalizing influence. The Pax family is an example of genes that cause differentiation of the dorsal structures of the neural tube.135,136 The Wnt family is also dorsalizing in the hindbrain; in situ hybridization shows its transcription products to be expressed diffusely only in the early neuroepithelium and to be restricted to dorsal regions as the neural tube develops.137 The zinc finger gene Zic2 has a dorsalizing gradient in the forebrain. The rostrocaudal axis of the neural tube and segmentation, or the formation of neuromeres, are directed in large part by a family of 38 homeobox genes that are divided into four groups called Hox genes.92,93,138–141 Each of 13 Hox genes is expressed in certain rhombomeres and not in others (Table 4-5). Hox genes are not expressed in the forebrain. In addition to their functions in establishing the compartments or rhombomeres of the brainstem and effecting the differentiation of certain anatomic structures, Hox genes guide the growth cones forming the long descending and ascending pathways between the brain and spinal cord.158
Genes that direct the specific differentiation of structures are called regulator genes, and in many cases they have served as organizer genes in an earlier period. The most important families for development of the brainstem and midbrain in vertebrates are En, Wnt, Hox, Krox, and Pax. Table 4-5 summarizes the sites of expression and functions of selected important organizer and regulator genes. Many regulatory genes change their territories of expression in different stages of development; they can increase their territory to include more rhombomeres or have broader expression early in development and more restricted domains later. Sometimes, expression of a gene in the wrong neuromere, called ectopic expression, interferes with normal development (see Table 4-5).
Function of Retinoic Acid
Retinoic acid, the alcohol of which is vitamin A, is a hydrophobic molecule secreted by the notochord and by ependymal floor plate cells.226 Retinoid-binding proteins and receptors are already strongly expressed in mesenchymal cells of the primitive streak and in the preoptic region of the early developing hindbrain.227 Ependymal cells other than those of the floor plate and neuroepithelial cells have retinoic acid receptors but do not secrete this compound. Retinoic acid diffuses across the plasma membrane without requiring active transport and binds to intracellular receptors; it then enters the nucleus, where it binds to a specific nuclear receptor protein, changes the structural configuration of that protein, and thereby enables it to attach to a specific receptor on a target gene, where the complex formed by retinoic acid and its receptor then functions as a transcription factor for neural induction.228,229
Retinoic acid functions as a polarity molecule for determining the anterior and posterior surfaces of limb buds and, in the nervous system, is important in segmentation polarity. It produces a strong rostrocaudal polarity gradient.54,230,231 Excessive retinoic acid acts on the neural tube of amphibian tadpoles to transform anterior neural tissue to a posterior morphogenesis, which results in extreme microcephaly and suppression of optic cup formation.229 In vertebrates, optic cup formation may fail because of suppression of retinoic acid by genes of the Lim family, such as Islet3 and Lim1.160,165 Retinoic acid upregulates homeobox genes, those of the Hox family and Krox20 in particular, and can cause ectopic expression of these genes in rhombomeres where they are not normally expressed.122,232 An excess of retinoic acid, whether endogenous or exogenous (i.e., excess maternal intake of vitamin A or analogues during early gestation), results in severe malformations of the hindbrain and spinal cord. A single dose of retinoic acid administered intraperitoneally to maternal hamsters on embryonic day 9.5 results in the Chiari II malformation and frequently meningomyelocele in the fetuses.233–235 In cell cultures of cloned CNS stem cells from the mouse, retinoic acid enhances neuronal proliferation and astroglial differentiation.236
Flexures and Sulci of the Brain
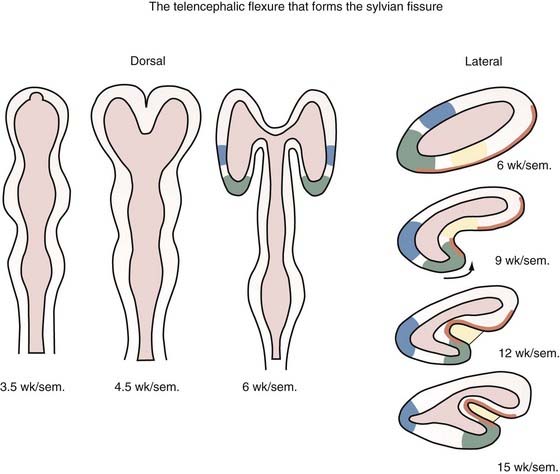
The neural tube begins development as a straight structure, but further growth of surrounding structures exerts an external physical force that causes bending at precise points. The pontine and cervical flexures thus form. At 6 weeks’ gestation the telencephalic hemisphere is oval shaped, but ventral bending then occurs to create the operculum and eventually the sylvian fissure (Fig. 4-3). Because both the dorsal (frontal lobe) and ventral (temporal lobe) lips of the sylvian fissure correspond to the ventral surface of the primitive telencephalon, disorders of this region, such as schizencephaly and perisylvian syndromes of polymicrogyria at the lips of the sylvian fissure, follow a ventrodorsal genetic gradient in the vertical axis, and both lips are involved in these malformations.237
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree