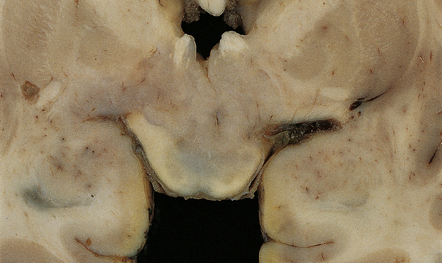
37 Neuroblast-like cells are present in embryonal neoplasms such as primitive neuroectodermal tumors (PNETs), and are admixed with ganglion cells in ganglioneuroblastomas, which are classified with embryonal neoplasms (see Chapter 38). Ganglion cells and/or neurocytes, but not neuroblasts, form a significant component of neoplasms grouped as neuronal and mixed neuronal-glial tumors in the WHO classification (2007): Ganglion cell neoplasms are generally circumscribed; only rare examples have a diffuse glial element (Fig. 37.1). They are usually firm and gray, and frequently contain flecks of calcification and small cysts. They may appear as a mural nodule in a large cyst. Gangliocytomas consist of disorganized ganglion cells set against a neuropil-like background or a lacy, fibrillary background containing a few reactive astrocytes (Fig. 37.2). Groups of ganglion cells may appear in nests enclosed by thin fibrovascular septa. In some cases, extensive desmoplasia may surround elongated cells that have a neuronal immunophenotype. Neoplastic ganglion cells possess abnormal, thickened processes and some have double nuclei. Small round cells with a relatively high nuclear:cytoplasmic ratio, but without nuclear hyperchromasia, represent a further neuronal phenotype encountered in these neoplasms (Fig. 37.2). 37.2 Gangliocytoma. Gangliogliomas also contain neoplastic glial cells, which may greatly outnumber the ganglion cells. Neoplastic glia in gangliogliomas may have an obvious astrocytic morphology, but some ganglion cell neoplasms contain cells of an indeterminate nature (Figs 37.3, 37.4). While the glial component of some gangliogliomas appears as a fibrillary astrocytoma, other gangliogliomas manifest as a pilocytic astrocytoma or pleomorphic xanthoastrocytoma with a ganglion cell component. Eosinophilic granular bodies and neovascularization, of the type seen in pilocytic astrocytomas, may be evident. Degenerative cytologic changes take the form of nuclear pleomorphism and hyperchromasia, in the absence of mitoses. Some gangliogliomas are focally desmoplastic, or may spread into the subarachnoid space, provoking desmoplasia. 37.3 Ganglioglioma. 37.4 Ganglioglioma. Ganglion cells are immunoreactive for NEU-N, synaptophysin, and neurofilament proteins (Fig. 37.4). Electron microscopy reveals dense-core synaptic vesicles. Small round undifferentiated cells with a high nuclear:cytoplasmic ratio and nuclear hyperchromasia are combined with ganglion cells in the ganglioneuroblastoma, which is regarded as a variant of PNET (Fig. 37.5). Mitoses and apoptotic bodies are usually identifiable among the small cells. Recognition of the embryonal element is important because its presence confers a much poorer prognosis and has implications for therapy. The combination of neoplastic neuronal and glial cells that are both undifferentiated and differentiated (i.e. ganglioneuroblastoma/glioblastoma, or malignant glioma/PNET) is recognized as an entity, but extremely rare. Like the ganglioneuroblastoma, these neoplasms should probably be regarded as variants of PNET. 37.5 Ganglioneuroblastoma. In areas of prominent desmoplasia, neoplastic glial cells are spindle-shaped with elongated hyperchromatic nuclei (Fig. 37.6). In some areas, the fascicular architecture and desmoplasia give way to foci of plump astrocytic cells or even limited numbers of poorly differentiated neuroepithelial cells. Ganglion cells with large nuclei are found within DIGGs (Fig. 37.6), but not DIAs, and can be numerous, mimicking a ganglioglioma. A small number of mitoses with a normal configuration may be present among the poorly differentiated cells. 37.6 Desmoplastic infantile ganglioglioma.
Neuroepithelial neoplasms displaying neuronal features
 ganglion cell neoplasms (i.e. gangliocytoma, ganglioglioma, anaplastic ganglioglioma)
ganglion cell neoplasms (i.e. gangliocytoma, ganglioglioma, anaplastic ganglioglioma)
 desmoplastic infantile ganglioglioma (DIGG) and its variant desmoplastic infantile astrocytoma
desmoplastic infantile ganglioglioma (DIGG) and its variant desmoplastic infantile astrocytoma
 neurocytomas (i.e. central and extraventricular neurocytoma, and cerebellar liponeurocytoma)
neurocytomas (i.e. central and extraventricular neurocytoma, and cerebellar liponeurocytoma)
 rosette-forming glioneuronal tumor of the fourth ventricle
rosette-forming glioneuronal tumor of the fourth ventricle
 paraganglioma (of the filum terminale)
paraganglioma (of the filum terminale)
 dysembryoplastic neuroepithelial tumor (DNET)
dysembryoplastic neuroepithelial tumor (DNET)
 dysplastic gangliocytoma of the cerebellum (Lhermitte–Duclos disease).
dysplastic gangliocytoma of the cerebellum (Lhermitte–Duclos disease).
GANGLIOCYTOMA AND GANGLIOGLIOMA
MACROSCOPIC APPEARANCES
MICROSCOPIC APPEARANCES
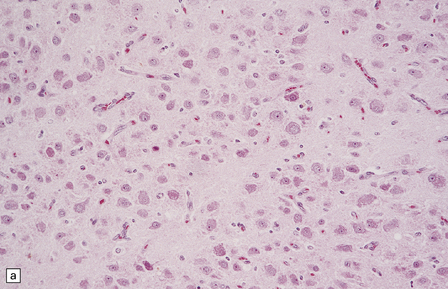
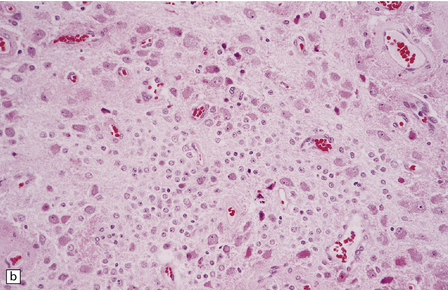
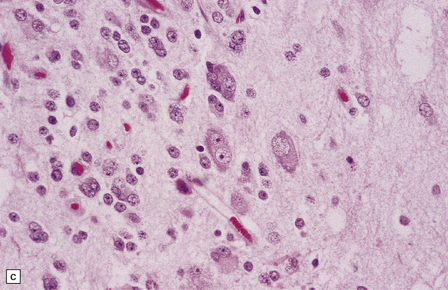
(a) and (b) This neoplasm is composed of large and small cells with a neuronal morphophenotype. Many cells have a prominent nucleolus, and some contain basophilic Nissl substance in the periphery of the cytoplasm. (c) Some ganglion cells have double nuclei.
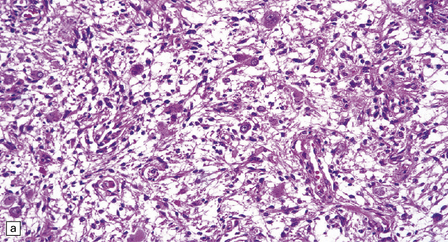
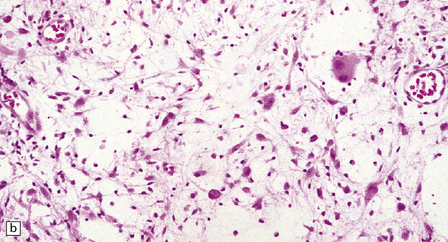
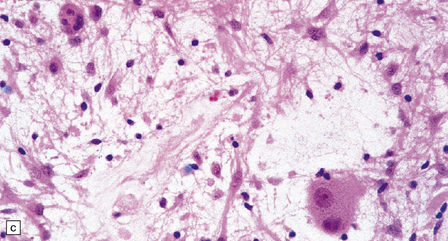
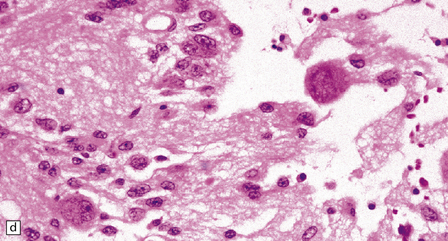
(a) Ganglion cells are seen among disorganized neoplastic cells with an astrocytic morphology. (b) A microcystic pattern is found in another part of the same neoplasm. (c) Astrocytic cells with fibrillary processes surround ganglion cells. While the glial component of a ganglioglioma may clearly have an astrocytic phenotype, its features may not be sufficiently typical to allow classification as fibrillary or pilocytic. (d) The histogenesis of some cells is unclear; in standard histologic preparations, some cells are not readily classified as neuronal or glial.
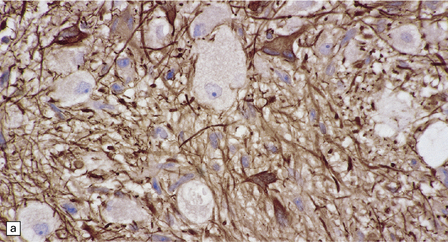
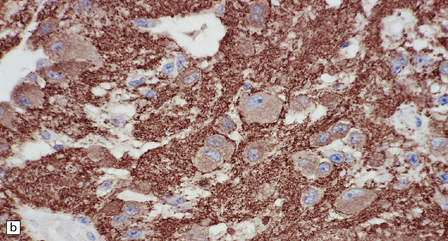
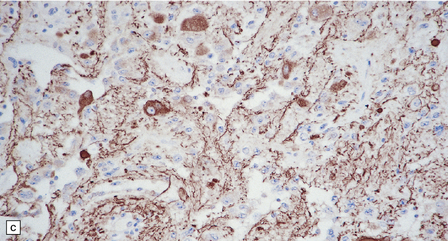
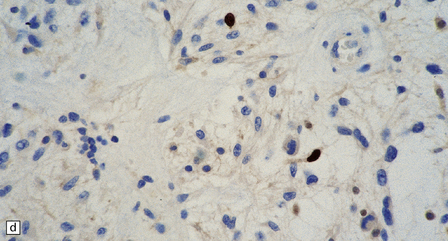
(a) Ganglion cells are surrounded by the processes of astrocytic cells that are immunoreactive for GFAP. (b) The cytoplasm of ganglion cells and a meshwork of neuritic processes are immunoreactive for synaptophysin. (c) The cell bodies of ganglion cells and some fine processes are also immunoreactive for neurofilament proteins. (d) The low growth fraction of a ganglioglioma is illustrated by the paucity of Ki-67-labeled nuclei.
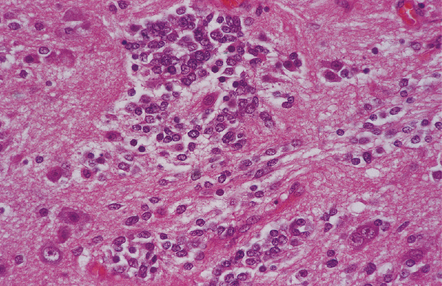
A closely packed group of small undifferentiated cells with hyperchromatic nuclei is seen among ganglion cells.
DESMOPLASTIC INFANTILE GANGLIOGLIOMA (DIGG)
MICROSCOPIC APPEARANCES
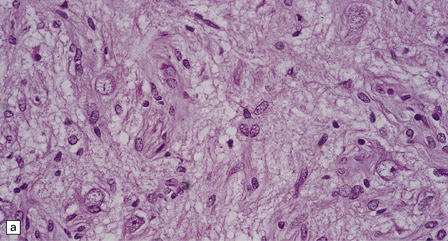
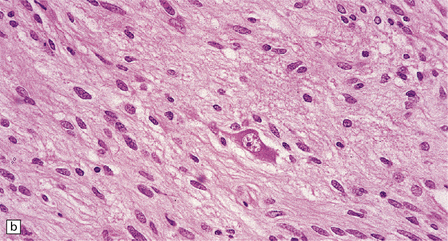
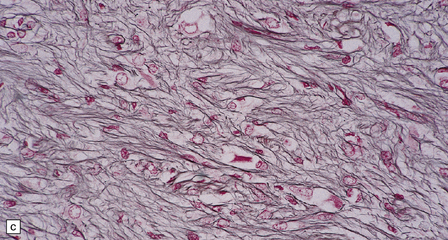
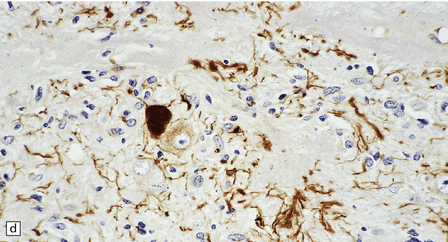
Ganglion cells are scattered among cells with fibrillary cytoplasm and oval nuclei that have either (a) a random or (b) a fascicular arrangement. (c) A dense pericellular reticulin pattern is characteristic. (d) Ganglion cells and some fine processes show immunoreactivity for neurofilament protein.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neuroepithelial neoplasms displaying neuronal features
Only gold members can continue reading. Log In or Register to continue