Fig. 1
Onchocerciasis-affected countries of the world (drawn by Njamnshi EN, BSc Geography). Countries in Africa: 1. Angola, 2. Benin, 3. Burkina Faso, 4. Burundi, 5. Cameroon, 6. Central African Republic, 7. Chad, 8. Congo, 9. Côte d’Ivoire, 10. Democratic Republic of Congo, 11. Equatorial Guinea, 12. Ethiopia, 13. Gabon, 14. Ghana, 15. Guinea, 16. Guinea-Bissau, 17. Kenya, 18. Liberia, 19. Malawi, 20. Mali, 21. Mozambique, 22. Niger, 23. Nigeria, 24. Rwanda, 25. Senegal, 26. Sierra Leone, 27. Sudan, 28. South Sudan, 29. Togo, 30. Uganda, 31. United Republic of Tanzania. Countries in Asia: 32. Saudi Arabia, 33. Yemen. Countries in Latin America: 34. Venezuela, 35. Mexico, 36. Guatemala, 37. Ecuador, 38. Colombia, 39. Brazil
Classically, descriptions of the clinical features of onchocerciasis have been dominated by two groups of symptoms, namely skin and eye symptoms. The skin condition which manifests initially as a severe, stigmatizing and troublesome itch in the acute phase usually progresses to a chronic skin disease (Fig. 2). A clinical classification and grading system is now being used as follows: acute papular onchodermatitis (APOD), chronic papular onchodermatitis (CPOD), lichenified onchodermatitis (LOD), atrophy and depigmentation.
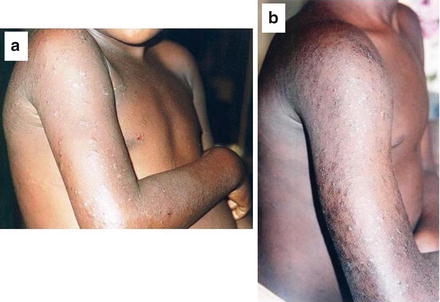

Fig. 2
Onchodermatitis in the acute (a) and chronic (b) forms
Onchocercal skin disease is more frequent in the forest regions, whereas onchocercal ocular disease and blindness are more prevalent in the savannah regions. These clinical differences are thought to be due to a variety of factors including differences in Onchocerca volvulus strains, vector type, host immunity and co-infection with other parasites (Enk 2006; Amazigo et al. 2006). Onchocercal ocular disease involves mainly keratitis, chorioretinitis and optic nerve disease (OND). In a study by Coffeng et al. (2012) in Cameroon, it was found that dermatological and ocular onchocercal diseases significantly occur concurrently. Also the association between onchocercal visual impairment and skin depigmentation could be partly explained, among other factors, by duration of exposure to infection and host characteristics, which may play a role in the pathogenesis of these co-morbidities (Coffeng et al. 2012).
In the last 15 years, there has been growing research interest in the association between onchocerciasis and epilepsy (Pion and Boussinesq 2012; Kaiser et al. 2010, 2011; Konig et al. 2010; Pion et al. 2009). Recently, with the introduction of mass control programmes for onchocerciasis by the World Health Organisation and its partners, especially the use of ivermectin through the Mectizan Donation Programme (Thylefors 2008), cases of encephalopathy related to the concurrence of Loa loa infection have been described in Central Africa (Gonzalez et al. 2012; Hoerauf et al. 2011; Babalola 2011; see also Bisoffi et al. 2014). Loa loa is a filarial nematode that causes recurrent, transitory skin edema and eosinophilia. As described further in this chapter, the development of encephalopathy following treatment of onchocerciasis has been associated with certain levels of concomitant Loa loa infection.
The efficacy of mass treatment has been demonstrated, leading to the hope for possible elimination of onchocerciasis from the tropics (Mackenzie et al. 2012; Thylefors 2008; Sauerbrey 2008; Boatin 2008). However, the many logistic challenges observed in these control programs, coupled with the recent observation of cases of filarial resistance to ivermectin, has led to the trial of new molecules (Nana-Djeunga et al. 2012; Mackenzie et al. 2012; Takesue et al. 2012; Osei-Atweneboana et al. 2011). These issues suggest that elimination may not be achievable in the near future. Thus, there is still need for research on clinical manifestations for better disease classification or staging for management purposes, and on pathogenetic mechanisms that will contribute to new drug development. In this chapter, we have attempted to summarize the major clinical nervous system manifestations of onchocerciasis, highlighting where possible, the current state of knowledge of the disease mechanisms and knowledge gaps.
2 Involvement of the Peripheral Nervous System: Pruritus (Onchocercal Itch)
2.1 Definition and Clinical Assessment of Itch
Itch or pruritus has been described for many years as an unpleasant sensation that evokes the urgent desire to scratch (Ikoma et al. 2011). The clinical measurement of pruritus intensity can be done using self-reporting scales similar to those used in pain medicine. These include the visual analogue scale (VAS), which is the most commonly used tool, the numerical rating scale (NRS) and the verbal rating scale (VRS) (Reich et al. 2011; Phan et al. 2011; Furue et al. 2013).
2.2 Clinical Importance of Onchocercal Itch
Although itch is the most disturbing symptom associated with onchocercal skin disease, it has not received a high priority. Nevertheless, several studies in Africa have shown the importance of this symptom. In a review by Murdoch (2010), troublesome itching was found to affect 32 % of the population aged 5 years and above. In another study in Nigeria, onchocercal itching (40 %) and onchocercal skin manifestations (34.3 %) were identified as the most troublesome signs and symptoms (Mbanefo et al. 2010). In a multicenter study comprising seven sites in five different African countries to evaluate the impact of the community-directed treatment with ivermectin, it was found that severe onchocercal itching varied between 2 and 38 % before ivermectin mass treatment and dropped to 0.2–12 % after 5–6 years of treatment, indicating the importance of this treatment on symptom alleviation (Ozoh et al. 2011). This observation has interesting implications for the patient’s quality of life given that the most worrisome consequence of onchocerciasis may be social exclusion or stigmatization (see Tabah et al. 2014). In the Nigerian study, about 35 % of the participants complained of stigmatization and psychological impact of the disease affecting almost all facets of their lives (Mbanefo et al. 2010).
2.3 The Neuroscience of Itching: Scratching the Brain for an Understanding of Itching
Although some evidence from the study of the neurobiology, neurophysiology and functional neuroimaging of itch has contributed progressively to a better understanding of the peripheral and central mechanisms of itching (Ikoma et al. 2011), the origin, transmission and perception of this socially embarrassing symptom is still a subject of discussion. Other open issues concern nerves and receptors involved in itch induction, relevant neural pathways and brain encoding of itch impulses. The answers to these questions are important not only for the understanding of the mechanisms but also for the development of novel, effective therapeutic agents (Tey and Yosipovitch 2011).
Concerning itch induction, the “intensity theory” hypothesizes that depending on the signal intensity, signal transduction on the same nerves leads to either pain (high intensity) or itch (low intensity) (Ikoma et al. 2011). On the other hand, the “labeled-line coding theory” hypothesizes the complete separation of pain and itch pathways (Ikoma et al. 2011).
The pathogenesis of acute and chronic (>6 weeks duration) pruritus is complex and involves the skin network of resident cells such as sensory neurons and transient inflammatory cells (lymphocytes) (Grundmann and Ständer 2011). Several classes of histamine-sensitive or histamine-insensitive C-fibers are involved in itch transmission in the skin. Ringkamp et al. (2011) have recently investigated, in humans and in an experimental model, the role of small nociceptive, myelinated fibers in non-histaminergic itch sensation. They tested the effect of a differential nerve block on itch produced by intradermal insertion of spicules from the pods of a cowhage plant (Mucuna pruriens) in psychophysical studies and also carried out electrophysiological experiments in the anesthetized monkey to investigate the responsiveness of cutaneous, nociceptive, myelinated afferents to different chemical stimuli (cowhage spicules, histamine, capsaicin). Their findings provided some evidence that activity in nociceptive, myelinated afferents contributes to cowhage-induced sensations, and that non-histaminergic itch is mediated through activity in both unmyelinated and myelinated afferents.
Itch-dedicated nociceptor neurons have been recently discovered (Greaves and Khalifa 2004). Specific receptors have been discovered on cutaneous and spinal neurons to be exclusively involved in the processing of pruritic signals. Some intracutaneous itch mediators have also been identified such as endovanilloids, proteases, cannabinoids, opioids, neurotrophins and cytokines. Relevant receptors are vanilloid receptor channels and proteinase-activated, cannabinoid, opioid, cytokine, and new histamine receptors. New data indicate that specific pruritic ligands carrying both itch and pain information are selectively recognized by different G protein-coupled receptors (GPCRs), and this information may be transduced through different intracellular circuits in the same neuron (Han and Simon 2011). These findings raise questions about the intracellular mechanisms that process and perhaps encode GPCR-mediated signals. In rodents, second-order neurons expressing gastrin-releasing peptide receptor (GRPR) and spinothalamic tract neurons are also involved (Jeffry et al. 2011). Some data suggest that the newly identified itch-specific neuronal pathways in the spinothalamic tract are distinct from pain pathways and relay to CNS regions that process peripheral pruritogenic stimuli (Paus et al. 2006).
Recent studies have suggested that GRPR-positive neurons constitute a long-sought labeled line for itch sensation in the spinal cord (Sun et al. 2009). It has been demonstrated that different dorsal horn neurons respond to histamine and allergic itch stimuli (Nakano et al. 2008). Recent studies also demonstrated that various neuronal receptors in the spinal cord are involved in pruritus (Cevikbas et al. 2011). Glutamate is the principal excitatory transmitter between C fibers and gastrin-releasing peptide (GRP)-positive dorsal horn neurons (Koga et al. 2011). Spinal bombesin-recognized neurons are critical to both the histamine-dependent and histamine-independent pathways for itch, and they mediate more non-histaminergic than histaminergic sensation of itch in mice (Han et al. 2012).
It has even been demonstrated that genetically susceptible persons would develop itch more intensely than others when exposed to visual cues, suggesting that interpersonal social cues can dramatically alter the subjective sensory experience of itch (Papoiu et al. 2011). The perception of the sensation of itch depends on various processes by a network of different brain regions contributing to the encoding of sensory, emotional, attention-dependent, cognitive-evaluative and motivational patterns (Pfab et al. 2012). These networks are yet to be fully understood.
3 Optic Neuropathy: The Blind Spot of River Blindness?
3.1 Epidemiology and Burden
The ocular disease associated with onchocercal infection has been well described, with sclerosing keratitis (Fig. 3), iridocyclitis, optic neuritis or atrophy, and chorioretinitis or chorioretinal atrophy accounting for visual morbidity (McKechnie et al. 1997). In a well illustrated study of the fundus oculi of 244 patients in Cameroon, Bird et al. (1976) working in Cameroon found that optic nerve disease, alone or in the presence of choroido-retinal changes, was responsible for a large proportion (about 88 %) of the blindness due to posterior segment lesions in onchocerciasis. In a forest-saving mosaic zone of south-eastern Nigeria, endemic for onchocerciasis, onchocerciasis-related eye disease was present in about 14 % of the investigated patients’ cohort (Umeh et al. 1996) and constituted 21 % of the total number of eye disorders. In this study, a total of 78 of 235 subjects with visual impairment had onchocerciasis-related eye lesions, 35 of whom were blind in both eyes, and onchocerciasis-induced eye disease was the cause in 28. The prevalence of bilateral blindness from all causes in the study area was 4.1 %, while that from onchocerciasis-related causes was 3.3 %. The commonest onchocerciasis-induced lesions responsible for visual impairment and blindness were choroidoretinitis and OND.
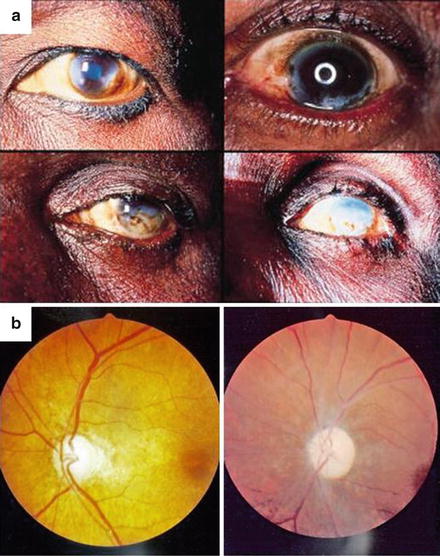

Fig. 3
(a) Ocular onchocerciasis: sclerosing keratitis (reproduced with permission from Etya’ale 2001). (b) Optic atrophy (APOC) and optic atrophy plus chorioretinitis (APOC) in onchocerciasis
A study in Nigeria (Yang et al. 2001) reported that the mean intraocular pressure was 1.58 mmHg lower in the individuals from the meso-endemic communities compared with those from the non-endemic communities despite the prevalence of peripheral anterior synechiae being higher in the meso-endemic communities. Glaucomatous optic nerve damage may therefore not be the primary cause of visual loss in ocular onchocerciasis as this occurs late and is probably preceded by other blinding onchocercal pathology. In the meso-endemic savannah communities of Kaduna State in Nigeria with a relatively high prevalence (9 %) of OND, it has been suggested that onchocercal infection was the single most important cause of OND, accounting for 50 % of all cases (Cousens et al. 1997). About 13 % of cases were associated with signs suggestive of glaucoma. Di-ethyl carbamazine use for the treatment of filariasis might be responsible for up to 30 % of all OND.
A survey of onchocercal eye disease was performed in the hyperendemic area of a rain forest focus of onchocerciasis in Esmeraldas Province in Ecuador (Cooper et al. 1995). A total of 785 skin snip positive individuals from black and Chachi American Indian communities were examined. The blindness rate attributable to onchocerciasis was 0.4 and 8.2 % were visually impaired. Onchocercal ocular lesions were seen in a high proportion of the study cases: punctate keratitis (34 %), microfilariae in the anterior chamber (29 %) and cornea (34 %), iridocyclitis (1.5 %), optic atrophy (5.1 %), and chorioretinopathy (28 %).
During a field trial of ivermectin, 6,831 persons age 5 years and above, living in 34 mesoendemic onchocercal communities in Kaduna State, northern Nigeria, were examined for ocular disease (Abiose et al. 1994). Visual function assessments included tests of visual acuity and visual fields. A total of 185 individuals (2.7 %) were bilaterally blind by acuity criteria with a further 28 blind by field constriction. The overall prevalence of blindness was 3.1 %. Additional 118 individuals were visually impaired by WHO criteria. The cause of blindness in 43 % of eyes in bilaterally blind patients was onchocerciasis. A further 11 % were blind from optic atrophy probably mostly onchocercal in origin. Glaucoma was the next most common cause of blindness in the bilaterally blind (11 %).
3.2 Effect of Mass Treatment of Onchocerciasis on Optic Nerve Disease
Reduction in incidence of OND with annual ivermectin to control onchocerciasis was reported by Abiose et al. (1993). There was evidence that ivermectin reduced the incidence of OND in subjects with microfilarial loads above 10 mf/mg but had little effect in those with lower loads. An updated Cochrane review (Ejere et al. 2012) showed evidence for a protective effect of mass treatment with ivermectin on visual field loss and OND in communities meso-endemic for the savannah strain of O. volvulus. However, whether these findings can be applied to communities with different endemicity and affected by the forest strain is unclear.
3.3 Etiopathogenesis of Optic Nerve Disease
If the anterior segment eye pathology has been associated with the intensity of onchocercal infection and host responses to the inflammatory process from degrading microfilariae, the posterior segment pathology is less well understood (McKechnie et al. 1997) although an immunogenetic mechanism has been proposed based on experimental models. The pathogenesis of OND in onchocerciasis is not well known, though it could involve host inflammatory responses to the degrading parasite antigens, as postulated in anterior ocular disease. A review by Hall and Pearlman (1999) pointed out that onchocerca-mediated keratitis results from an inflammatory response in the anterior portion of the eye. Furthermore, based on experimental models, the pathogenesis could be due to the host inflammatory response to degenerating parasites in the eye, with sensitized T helper cells and cytokines playing an important role (Hall and Pearlman 1999). Glaucoma may not be a major contributor to OND in onchocerciasis as it is not very common and occurs late (Yang et al. 2001). It may be suspected that, as in skin disease (Murdoch et al. 1997), the spectrum of onchocercal ocular disease, including OND, also has an immunogenetic basis possibly with specific HLA-DQ molecules associated with the level of immune response to parasite antigens present locally or systemically.
Thus, to date the basic mechanisms of onchocercal OND are unknown. A few experimental studies suggest an immunogenetic process but this needs to be confirmed. Due to this state of knowledge, we consider this the “blind spot of river blindness”.
4 Epilepsy
4.1 Epidemiology and the Concept of “River Epilepsy”
Following the Lancet report of an improvement of epileptic seizures after ivermectin treatment (Kipp et al. 1992), there has been a growing amount of literature, based primarily on epidemiological data, on the association of onchocerciasis and epilepsy, ranging from classical seizures to more exotic forms of paroxystic manifestations like head nodding syndrome (Williams 2012; Pion and Boussinesq 2012; Kaiser et al. 1996, 1998, 2000, 2007, 2008, 2009, 2010, 2011; Konig et al. 2010; Pion et al. 2009; Winkler et al. 2008; Prischich et al. 2008; Katabarwa et al. 2008; Marin et al. 2006; Dozie et al. 2006; Twum-Danso 2004; Druet-Cabanac et al. 1999, 2004; Kamgno et al. 2003; Boussinesq et al. 2002; Farnarier et al. 2000; Arborio and Dozon 2000; Maegga 1998; Duke 1998; Burnham 1998; Newell et al. 1997; Kabore et al. 1996; Kipp et al. 1994; Kilian 1994; Ovuga et al. 1992). Although this association has been an issue of debate in the past two decades with some of the controversy arising probably from methodological issues in some of the studies, a recent systematic review and meta-analysis and other studies have provided further evidence of the existence of an association between onchocerciasis and epilepsy (Pion and Boussinesq 2012; Kaiser et al. 2010, 2011; Konig et al. 2010; Pion et al. 2009).
A review of eight studies (Pion et al. 2009) included a total of 79,270 individuals screened for epilepsy from west Africa (Benin and Nigeria), central Africa (Cameroon and Central African Republic) and east Africa (Uganda, Tanzania and Burundi). The prevalence of epilepsy ranged from 0 to 8.7 % whereas that of onchocerciasis ranged from 5.2 to 100 %. According to this review, the variation in epilepsy prevalence was consistent with a logistic function of onchocerciasis prevalence, with epilepsy prevalence being increased, on average, by 0.4 % for each 10 % increase in onchocerciasis prevalence (Fig. 4). Data from these studies on the association of the two disease conditions led to suggest the concept of “river epilepsy” as a parallel of “river blindness” (Pion et al. 2009).
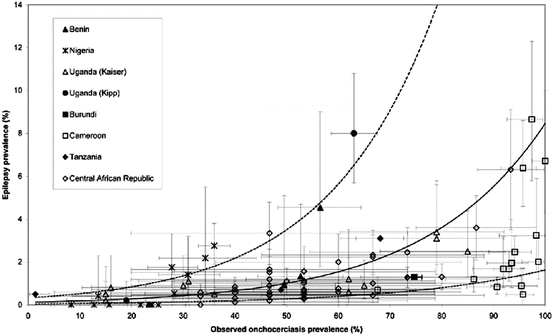

Fig. 4
Epilepsy prevalence versus onchocerciasis prevalence (reproduced with permission from Pion et al. 2009). Error bars represent 95 % exact confidence intervals. Solid line: predicted relationship estimated by random-effect logistic regression; dashed lines: 95 % confidence interval of the model predictions
A trend associating the presence and the mean number of subcutaneous onchocerca nodules has been demonstrated in onchocerciasis-associated epilepsy (Pion and Boussinesq 2012; Kaiser et al. 2011, 2012), suggesting some contribution of the severity of the infection to the pathogenesis of associated epilepsy. It has been suggested that other factors, including parasitic infections such as intestinal worms, may play a role in this association (Njamnshi et al. 2007).
4.2 Seizures and Evolution Following Onchocerciasis Treatment
One clinical and electroencephalographic study of epilepsy in an onchocercal endemic area in west Uganda revealed that 78 % of the seizures were partial while 22 % were generalized (Kaiser et al. 2000). The types of epileptic seizures and syndromes associated with onchocerciasis do not appear to show specific characteristics but it is worth cautioning that excellent clinical descriptions are rare.
4.3 Open Questions on Onchocerciasis-Associated Epilepsy
The types of epileptic seizures and epileptic syndromes associated with onchocerciasis are yet to be well characterized in large studies. Furthermore, only few studies have involved the correlation of clinical and electrophysiological characteristics of “river epilepsy”. What we now need to know is whether there is any causal relationship in the onchocerca-epilepsy association. If this suspected causality is confirmed, the involved pathogenetic mechanisms, the role of onchocerciasis severity in inducing epilepsy and relevant determining factors remain to be clarified. We also do not know the role of parasitic infections other than onchocerciasis and co-morbidities in inducing epilepsy and if these modify the course of epilepsy.
5 Post-treatment Encephalopathy
5.1 Epidemiology and Clinical Manifestations
The first reports of serious reactions, including encephalopathy, following mass treatment of onchocerciasis with ivermectin have been from Cameroon in areas with Loa loa co-endemicity (Gardon et al. 1997; Boussinesq et al. 1998; Kamgno et al. 2000; Bockarie and Deb 2010). These cases of encephalopathy are rare but can be fatal (Kamgno et al. 2008) and have been reported to be similar to those described following treatment with diethyl carbamazine (Gardon et al. 1997).
The clinical picture is characterized by disorders of consciousness progressing to coma, agitation, involuntary movements, urinary incontinence, motor and sensory deficits, hypertonia, sometimes absent myotatic reflexes, paraesthesia, transient grasping reflex, and brisk tendon reflexes (Gardon et al. 1997; Kamgno et al. 2008). The electroencephalogram shows diffuse abnormalities which disappear after 3–6 months (Boussinesq et al. 2003). A pretreatment Loa loa microfilarial count of more than 50,000 mf/mL (Loa loa microfilariae were found in the cerebrospinal fluid), has been shown to predispose to the development of encephalopathy, with an incidence of 7 % (Gardon et al. 1997), decreasing to 0.7 % with a microfilarial count of 30,000 mf/mL (Kamgno et al. 2008). Lower doses of ivermectin have failed to prevent Loa loa encephalopathy in onchocerciasis (Kamgno et al. 2000). Genetic predisposition is thought to play a role in the development of encephalopathy as haplotypes associated with altered drug bioavailability especially in brain tissue were present as homozygotes in two of the patients with encephalopathy (50 %), but absent in controls (Bourguinat et al. 2010).
5.2 Neurobiology of Loa loa Encephalopathy
The exact physiopathological mechanisms of Loa loa encephalopathy associated with mass treatment of onchocerciasis are not well known. However, observations from a few autoptic cases have revealed vascular changes with a thickened basement membrane, perivascular inflammatory reaction and some neuronal degeneration (Kamgno et al. 2008). Suggested possible mechanisms include microfilarial obstruction of cerebral blood vessels leading to ischemia and degeneration, as retinal hemorrhages have been described in these patients (Moussala et al. 2004; Boussinesq et al. 2003), or microfilarial invasion of brain parenchyma as microfilariae escape from blood vessels following treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








