Fig. 1
Increasing scientific interest in Acanthamoeba and Balamuthia mandrillaris, causative agents of granulomatous amoebic encephalitis as determined by published articles over the last five decades. A PubMed search using the key words “Acanthamoeba” and “Balamuthia” was carried out
2 The Infectious Agents
Both Acanthamoeba and B. mandrillaris are free-living protist pathogens (Fig. 2). Acanthamoeba was first observed in yeast (Cryptococcus) cultures by Castellani (1930) and Volkonsky (1931) who created the genus Acanthamoeba for these amoebae. The name acanth (Greek: meaning spikes) was added to these amoebae due to the presence of spine-like structures on the surface of these organisms. Later, the pathogenic potential of these organisms was demonstrated for the first time by revealing their ability to produce cytopathic effects on monkey kidney cells in vitro and to kill laboratory animals, i.e., monkeys in vivo (Culbertson et al. 1958, 1959; Jahnes et al. 1957). The first clearly identified GAE case due to Acanthamoeba was observed in 1972 (Jager and Stamm 1972). Soon after, Acanthamoeba was identified as a causative agent of blinding keratitis (Nagington et al. 1974) and then was clearly associated with Legionairre’s disease by serving as a reservoir for Legionella pneumophila (Rowbotham 1980). B. mandrillaris has been discovered more recently, in 1986, from the brain of a baboon, who was a victim of meningoencephalitis, and was described as a new genus, i.e., Balamuthia (Visvesvara et al. 1990), and then associated with GAE in humans and animals (Anzil et al. 1991; Taratuto et al. 1991; Visvesvara et al. 1993). Since then, more than 150 cases of GAE due to B. mandrillaris have been identified (Visvesvara et al. 2007; Schuster et al. 2009). Based on 16S rRNA gene sequences, it is determined that B. mandrillaris is closely related to Acanthamoeba (Booton et al. 2003a, b; Matin et al. 2008; Amaral Zettler et al. 2000). The life cycle of both these pathogens undergoes two stages, a vegetative trophozoite stage and a resistant cyst stage (Figs. 2 and 3) (Matin et al. 2008; Siddiqui et al. 2012; Weisman 1976). The trophozoites are normally in the range of 15–45 μm in diameter, but their size varies significantly between different species. Cell division is asexual and occurs by binary fission. Under harsh conditions (lack of food, extremes in osmolarity, pH and temperatures), the amoebae switch into resistant cyst stage. In simple terms, the trophozoite becomes metabolically inactive (minimal metabolic activity), excess food, water and particulate matter is expelled and the trophozoite encloses itself within a resistant shell to survive harsh periods. The cellular levels of RNA, proteins, triacylglycerides and glycogen declines substantially during the encystment process resulting in decreased cellular volume and dry weight. The trophozoites emerge from the cysts under favourable conditions leaving behind the outer shell and actively reproduce, thus completing the cycle. The ability of these protists to rapidly switch phenotypes into a dormant cyst form is an important property in their defence to resist harsh environmental and physiological conditions as well as drug resistance resulting in disease recurrence.
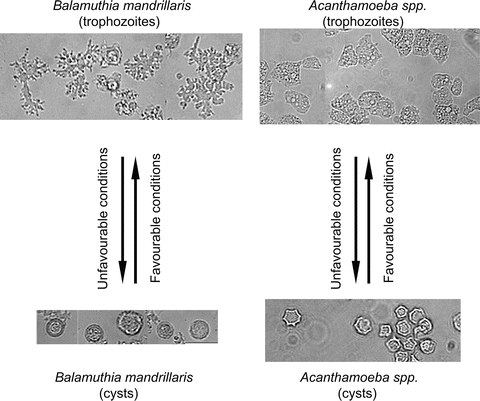
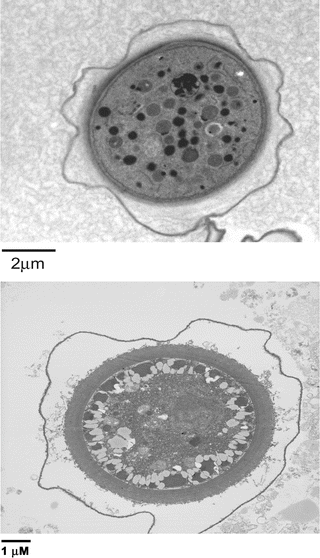

Fig. 2
The life cycle of Acanthamoeba and Balamuthia mandrillaris. The infective form of the amoebae is known as trophozoites. Under unfavorable conditions, trophozoites differentiate into cysts characterized by double wall as indicated by arrows. ×250

Fig. 3
Representative transmission electron micrographs showing a complete multi-walled cyst of Acanthamoeba castellanii belonging to the T4 genotype (a) and Balamuthia mandrillaris (b)
3 Pathophysiology
The clinical picture of GAE may resemble viral, bacterial or tuberculosis meningitis. For example, the clinical symptoms involve headache, fever, behavioural changes, lethargy, stiff neck, aphasia, ataxia, nausea, cranial nerve palsies, confused state, seizures, coma, finally leading to death (Table 1). Patients exhibit haemorrhagic necrotizing lesions or brain abscess (detected by neuroimaging scans) with severe meningeal irritation and encephalitis (Martinez 1991; Martinez and Visvesvara 1997). Patients with respiratory infections, skin ulcerations or brain abscesses should be suspected for infections due to Acanthamoeba and B. mandrillaris. Post-mortem examination often shows severe oedema and haemorrhagic necrosis. It is not known whether this necrotic phase is caused by actively feeding trophozoites or inflammatory processes. The characteristic lesions in the parenchyma of GAE are most numerous in the basal ganglia, midbrain, brainstem and cerebral hemispheres. The granuloma is generally composed of macrophages, CD4+ and CD8+ T cells, B-cells and plasma cells. Granulomatous responses may be absent or minimal in patients with severe impairment of the cellular immune response (Khan 2009; Martinez and Visvesvara 1997; Visvesvara et al. 2007). The affected organs other than the CNS may include subcutaneous tissue, skin, liver, lungs, kidneys, pancreas, prostate, lymph nodes and bone marrow.
Table 1
Features of granulomatous amoebic encephalitis due to Acanthamoeba and Balamuthia mandrillaris
Pathogen | Acanthamoeba | Balamuthia mandrillaris |
|---|---|---|
Cases reported worldwide | ~200 | ~200 |
High-risk groups | Generally immunocompromised individuals | Immunocompetent individuals with soil exposure |
Pathogenesis | Haematogenous spread from primary site of infection, followed by amoebae traversal of the blood-brain barrier and central nervous system invasion | Haematogenous spread from primary site of infection, followed by amoebae traversal of the blood-brain barrier and central nervous system invasion |
Clinical features | Fever, headache, stiff neck, nausea, seizures, disorientation, visual loss, photophobia, coma | Fever, headache, stiff neck, nausea, seizures, disorientation, visual loss, photophobia, coma |
Clinical diagnosis using computed tomography or magnetic resonance imaging | The brain image analyses show multiple space occupying lesions indicating brain abscess | The brain image analyses show cerebral edema, hydrocephalus, and multiple space occupying lesions |
Laboratory diagnosis | PCR on CSF or biopsy, immunofluorescence assays | PCR on CSF or biopsy, immunofluorescence assays |
Treatment | Ketoconazole, fluconazole, itraconazole, pentamidine isethionate, flucytosine, trimethoprim/sulfa-methoxazole and rifampin Experimental: miltefosine | Ketoconazole, fluconazole, albendazole, itraconazole, sulfadiazine, pentamidine isethionate, flucytosine, clarithromycin and azithromycin Experimental: phenothiazines-thioridazine, trifluoperazine |
Case fatality rate | >90 % | >90 % |
4 Predisposing Factors
The predisposing factors in contracting GAE are not clearly understood. Although the majority of GAE cases due to Acanthamoeba are limited to individuals with a weakened immune system, B. mandrillaris infections have been shown to occur also in immunocompetent people (Table 1). Thus, GAE can develop in individuals with no history of syphilis, diabetes mellitus, malignancies, fungal and mycobacterial infections and in patients negative for HIV-1 and HIV-2. However, patients suffering from these as well as other diseases including lymphoproliferative disorders, haematologic disorders, pneumonitis, renal failure, liver cirrhosis, rhinitis, pharyngitis, gammaglobulinemia, systemic lupus erythematosus, glucose-6-phosphate deficiency, tuberculosis, chronically alcoholic, radiotherapy, malnourished, chronically ill, or debilitated, are particularly at risk. Patients undergoing organ/tissue transplantation with immunosuppressive therapy, steroids and excessive antibiotics are also at risk of contracting GAE (Fig. 4). Although the reported number of GAE cases is a few hundred, the burden of GAE worldwide will never be known; this is due to lack of awareness of the disease and/or unavailability of diagnostic methods, and poor public health systems especially in the less developed countries.
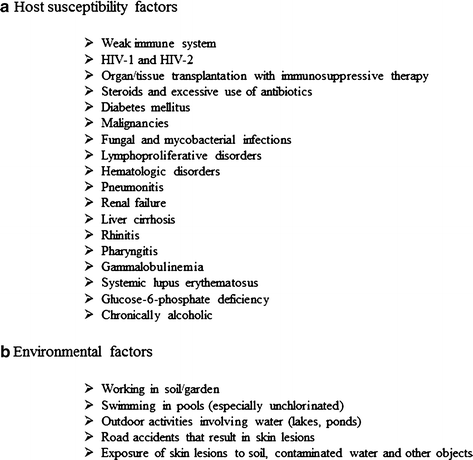

Fig. 4
Risk factors associated with granulomatous amoebic encephalitis
Unlike Acanthamoeba, that is generally limited to immunocompromised patients, GAE due to B. mandrillaris can occur in healthy people of any age, though there is a predominance of cases in the young (under 15 years of age) and the elderly (over 60 years of age), probably due to their weaker immune systems (Maciver 2007). The rarity of the disease suggests the presence of predisposing factors. Notably, GAE due to B. mandrillaris has been predominantly reported in individuals of Hispanic origin (Siddiqui and Khan 2008; Diaz 2011). In support, it is shown that Hispanics are less able to make antibodies against certain Acanthamoeba species (Chappell et al. 2001) and this may be the case also for B. mandrillaris. The genetic predisposition of Hispanics to B. mandrillaris may play a role in contracting GAE, but it needs further investigation as GAE due to B. mandrillaris is reported to occur in a range of mammals including mandrill baboons, monkeys, gibbons, gorillas, sheep, dogs and horses with similar disease presentation to humans (Visvesvara et al. 2007). Other predisposing factors may include working with organic-rich soil (e.g., during agricultural activities), which may explain large number of cases in Hispanics, who are the major workforce in Southern California (Schuster and Visvesvara 2004a, b). Temperature may also be an important factor as the disease seems to be more common in warmer regions, such as Southern California and South America (Deetz et al. 2003; Siddiqui and Khan 2008; Schuster and Visvesvara 2004a, b).
Overall, it is widely accepted that GAE due to free-living protists, Acanthamoeba and in particular B. mandrillaris can occur in healthy individuals, but immunocompromised or debilitated patients due to HIV infection, diabetes, immunosuppressive therapy, malignancies, malnutrition, and alcoholism are particularly at risk (Schuster and Visvesvara 2004a, b; Visvesvara et al. 2007; Siddiqui and Khan 2008). The risk factors for patients suffering from the above diseases include exposure to contaminated water such as swimming pools, on beaches, or working with soil (garden/compost/agriculture). Future studies will determine the precise host and environmental factors contributing to this fatal infection, which may help design preventive strategies and identify the susceptible populations.
5 Pathogenesis
Although GAE is a rare infection, it almost always proves fatal as mentioned above. The mechanisms associated with pathogenesis of GAE remain unclear; however the pathophysiological complications involving the CNS most likely include the invasion of parasites across the blood-brain barrier (BBB), induction of proinflammatory responses, and neuronal damage leading to brain dysfunction. The routes of entry of the amoeba into the body include the lower respiratory tract leading to amoebae invasion of the intravascular space followed by haematogenous spread (Martinez 1991). Skin ulcerations may provide direct amoebae entry into the bloodstream (Fig. 5).
Amoebae entry into the CNS occurs most likely across the BBB (Martinez 1991; Martinez and Visvesvara 1997). The olfactory neuroepithelium route (i.e., invasion of the olfactory part of the nasal epithelium, and migration along nerve fibers, followed by invasion of the olfactory bulb) provides another route of entry into the CNS and has been studied in experimental models (Martinez 1991; Martinez and Visvesvara 1997; Kiderlen and Laube 2004). The cutaneous and/or nasopharyngeal infections can last for months but the involvement of the CNS can result in death within days (Martinez and Visvesvara 1997; Schuster and Visvesvara 2004a, b). Crossing the BBB is a multifactorial process involving pathogen determinants (adhesins, proteases, ecto-ATPases, phospholipases) that allow amoebae to evade the immune system to target the neuronal tissue as well as the host immune responses (interleukin-beta, interleukin-alpha, tumor necrosis factor-alpha, interferon-gamma, host cell apoptosis) that result in the host cell/tissue injury (Table 2). Both Acanthamoeba and B. mandrillaris are extracellular pathogens and are thus exposed to the host immune system. The immune response plays an active role in protection against these pathogens, as well as contributing to BBB perturbations and disease development. For example, characteristic granulomatous lesions in the CNS are a result of the host immune response and are composed of macrophages, T and B cells as described above, and amoebae. The localization of immune cells in the brain suggests the involvement of pro-inflammatory cytokines in protection as well as in the pathophysiology of complications. The overall outcome is increased permeability and/or apoptosis of the brain microvascular endothelial cells, which promote BBB disruption leading to amoebae invasion of the CNS. Both the parasite and host factors are thought to be important in BBB dysfunction but the precise molecular mechanisms are unclear and should be the subject of future studies. The understanding of cross-talk between amoebae-host interactions as well as between BBB and the CNS in disease will provide insights into the disease neuropathogenesis and may help develop novel therapeutic interventions.
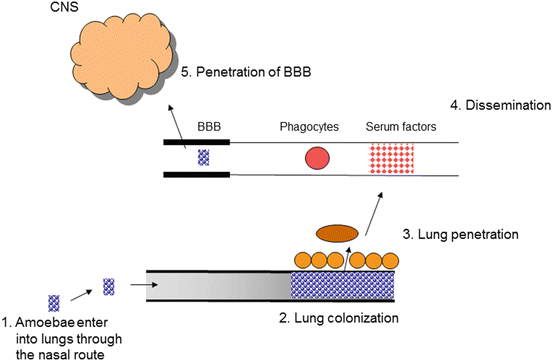

Fig. 5
The model of granulomatous amoebic encephalitis. Amoebae enter lungs via the nasal route. Next, amoebae traverse the lungs into the bloodstream (or directly through skin lesions), followed by haematogenous spread. Finally, amoebae cross the blood-brain barrier and enter into the central nervous system (CNS) to produce disease. The olfactory neuroepithelium may provide an alternative route of entry into the CNS
Table 2
Virulence factors and their potential role in the pathogenesis of granulomatous amoebic encephalitis
Pathogen determinant | Role | Reference |
|---|---|---|
Acanthamoeba | ||
Mannose-binding protein | Cytoadhesion | |
Serine proteases | Paracellular transmigration of the blood-brain barrier; Degradation of extracellular matrix membranes; Immune evasion (degradation of antibodies/complement proteins/cytokines) | |
Ecto-ATPases | ?Host cell cytotoxicity | |
Phospholipases | Unclear | |
Balamuthia mandrillaris | ||
Galactose-binding protein | Cytoadhesion | |
Metalloproteases | Paracellular transmigration of the blood-brain barrier; Degradation of extracellular matrix membranes; ?Immune evasion (degradation of antibodies/complement proteins) | |
Ecto-ATPases | ?Host cell cytotoxicity | Matin and Khan (2008) |
Phospholipases | ?Endothelial injury | |
6 Clinical Diagnosis of the GAE
GAE is an infection with a case fatality rate of more than 90 %. Those cases who survived were diagnosed early, followed by aggressive treatment, which led to successful outcome for these patients. However, the symptoms of GAE can be similar to those caused by other CNS pathogens including virus, bacteria and fungi. This makes GAE diagnosis problematic and requires awareness and solid expertise. Neurological signs are grouped into four categories; (1) impaired consciousness: ranging from confusion to unconsciousness, (2) loss of reflex activity, (3) abnormal speech, e.g. aphasia, and (4) abnormal motor patterns: imbalance or unsteady gait, seizure activities. The advanced stage of the disease is irreversible and includes loss of consciousness, seizures and coma, finally leading to death. Post-mortem examination often shows severe oedema and haemorrhagic necrosis (Martinez 1991; Martinez and Visvesvara 1997; Kiderlen and Laube 2004). Brain image analyses using computed tomography (CT) or magnetic resonance imaging (MRI) may show multifocal areas of altered signal intensities or lesions simulating brain abscesses or tumours. The cerebrospinal fluid findings, although not confirmatory of GAE, are of value in diagnosing CNS involvement. Pleocytosis with lymphocytic predominance is an important feature with elevated polymorphonuclear leukocytes, increased protein concentrations, decreased glucose concentration and slight cloudiness (Martinez and Visvesvara 1997; Schuster and Visvesvara 2004a, b).
The absence of viral and bacterial pathogens should raise the suspicion of GAE. Due to the low density of parasites, the detection of host immune response parameters should be attempted primarily. The demonstration of high levels of Acanthamoeba– or B. mandrillaris-specific antibodies in the patient’s serum may provide a useful and straightforward method to further suspect GAE infection. This is performed using indirect immunofluorescence (IIF) assays. The serial dilutions of the patient’s serum are incubated with fixed amoebae-coated slides, followed by incubation with fluorescein isothiocyanate (FITC)-labelled anti-human antibody and visualized under fluorescence microscopy. It is important to remember that the levels of anti-amoebae antibodies in normal populations may be in the range of 1:20–1:60. However, patients with severely impaired immune system may not develop a high titre, thus other clinical findings should be taken into account for accurate diagnosis (Visvesvara et al. 2007). The confirmatory evidence comes from direct microscopic observation of amoebae in the cerebrospinal fluid (after centrifugation at low speed) or in the brain biopsy but requires familiarity of morphological characters. Giemsa-Wright, acridine orange or calcofluor white staining may facilitate morphology-based positive identification of these amoebae. The lack of familiarity with amoebae morphological characteristics may require immunohistochemical studies using antisera made against Acanthamoeba and B. mandrillaris as aids in the clinical diagnosis of GAE. In addition, it is helpful to inoculate a portion of the cerebrospinal fluid and/or brain biopsy for amoebae culturing. For Acanthamoeba, the clinical specimens can be inoculated onto non-nutrient agar plate seeded with Gram-negative bacteria. Acanthamoeba feeds on bacteria as food source, and depending on the number of amoebae in the specimen, trophozoites can be observed within a few hours (up to 12 h). However in the absence of amoebae, plates should be monitored for up to 7 days. This method is particularly useful if problems are encountered in differentiating Acanthamoeba from monocytes, polymorphonuclear leukocytes and macrophages. Balamuthia mandrillaris do not feed on Gram-negative bacteria and should be inoculated onto mammalian cell cultures, but this procedure may require up to several weeks. Alternatively, PCR-based methods have been developed for the clinical diagnosis of amoebae infections and provide rapid diagnosis from clinical specimens (Booton et al. 2003a, b; Jayasekera et al. 2004).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








