Chapter 80 Neurological Problems of the Newborn
General Principles of Investigation and Management
Hypoxic-Ischemic Brain Injury in the Term Newborn
Hemorrhagic and Hypoxic-Ischemic Brain Injury in the Premature Newborn
Intraventricular Hemorrhage in the Term Newborn
Infections of the Central Nervous System
Mechanical Trauma to Extracranial, Central, and Peripheral Nervous System Structures
Traumatic Injury to the Peripheral Nervous System
Neonatal Seizures
Seizures in newborns are rarely idiopathic and represent the most common feature of significant neurological disease in the neonate. Prompt recognition is essential because serious underlying diseases are often the cause of seizures. These diseases require treatment because they may interfere with supportive care such as ventilation and feeding. Experimental studies have shown a decrease in brain glucose concentration during prolonged seizures, an increase in brain lactate concentration, and excessive release of excitatory amino acids, which may interfere with deoxyribonucleic acid (DNA) synthesis and subsequently with glial proliferation, differentiation, and myelination as well as increase in cerebral blood flow velocity (Jensen, 2009; Rennie and Boylan, 2007). Although the implications of these experiments for the human newborn are not entirely clear, their relevance is suggested by in vivo studies with magnetic resonance spectroscopy that demonstrate an association between abnormally low ratios of phosphocreatine to inorganic phosphate during seizures and long-term neurological sequelae.
Diagnosis
Seizure manifestations in newborns differ from those in older children in that newborns generally do not have well-organized, tonic-clonic seizures due to the immaturity of synaptic connections (Volpe, 2008). Table 80.1 summarizes the common types of neonatal seizures. The basis for classification should be a combination of clinical and electroencephalographic (EEG) abnormalities (Clancy, 2006). These seizure types are not specific for cause, but some are seen more often with certain underlying conditions. Tonic seizures, which may represent nonepileptic decerebrate posturing, occur in up to 50% of premature newborns with severe intraventricular hemorrhage (IVH). Focal clonic seizures in the term newborn are most commonly associated with focal cerebral infarction or traumatic injury, such as cerebral contusion.
Table 80.1 Types of Neonatal Seizures
| Neonatal Seizure Types | Clinical Manifestations | Age Distribution |
|---|---|---|
| Subtle | Eye deviation, blinking, fixed stare Repetitive mouth and tongue movements Apnea Pedaling, tonic posturing of limbs | Premature and term |
| Tonic: focal or generalized | Tonic extension of limbs Tonic flexion of upper limbs, extension of legs | Primarily premature |
| Clonic: multifocal or focal | Multifocal, clonic, synchronous, or asynchronous limb movements Nonordered progression Localized clonic limb movements Consciousness often preserved | Primarily term |
| Myoclonic: focal, multifocal, or generalized | Single or several synchronous flexion jerks of upper more than lower limbs | Rare |
Determination of the Underlying Cause
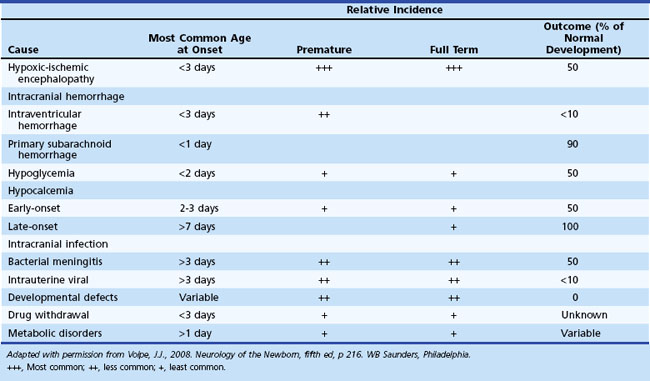
Diagnosis of the underlying cause allows specific treatment and a more precise prediction of outcome (Glass and Wu, 2009; Tekgul et al., 2006). Table 80.2 summarizes the major causes of neonatal seizures, their usual times of onset, and prognosis. Sometimes, several factors cause seizures (e.g., the combination of intracranial hemorrhage, metabolic derangement, and hypoxic-ischemic injury). Benign genetic epilepsies rarely have their onset in the neonatal period; the only example is benign familial neonatal epilepsy, an autosomal dominant trait for which two gene loci, KCNQ2 and KCNQ3, have been identified, which encode for voltage-gated potassium channels (Bellini et al., 2010).
Electroencephalography
EEG, particularly continuous EEG monitoring when available, is a valuable aid in the diagnosis of neonatal seizures, especially in newborns paralyzed to assist ventilation and in those with suspected subtle seizures. Amplitude-integrated EEG is a more recent technique that may assist in detecting subtle and subclinical electrographic seizures (van Rooij et al., 2010). Concern exists that use of amplitude-integrated EEG criteria alone may lead to an overdiagnosis of neonatal seizures, so confirmation by standard EEG is advisable prior to treatment with anticonvulsants (Koh et al., 2010). EEG correlates of neonatal seizures are focal or multifocal spikes or sharp waves and focal monorhythmic discharges. Sharp transients are normal in premature newborns and should not be confused with seizure activity. Similarly, the trace alternant pattern of quiet sleep in normal term infants, in which normal low-amplitude reactivity is preserved between bursts, must be distinguished from the abnormal burst-suppression pattern, in which long periods of voltage suppression or absence of activity are recorded between bursts of high-voltage spikes and slow waves (Lamblin et al., 2010).
Management
Neonatal seizures require urgent treatment. Once adequate ventilation and perfusion are established, the blood glucose concentration is measured. If the glucose concentration is low, 10% dextrose should be administered in a dose of 2 mL/kg. In the absence of hypoglycemia, immediate treatment should be started with anticonvulsant medication, as outlined in Table 80.3. Studies for other underlying causes should proceed concurrently and specific treatment initiated whenever possible.
Table 80.3 Treatment of Neonatal Seizures
| I. Ensure adequate ventilation and perfusion. II. Begin therapy for specific metabolic disturbances (if present). | ||
| Acute Therapy | Maintenance Therapy | |
| Hypoglycemia: glucose (10% solution) | 2 mL/kg IV (0.2 g/kg) | Up to 8 mg/kg/min IV |
| Hypocalcemia: calcium gluconate (5% solution) | 4 mL/kg IV (monitor cardiac rhythm) | 500 mg/kg/24 h PO |
| Hypomagnesemia: magnesium sulfate (50% solution) | 0.2 mL/kg IM | 0.2 mL/kg/24 h IM |
| Pyridoxine deficiency: pyridoxine | 50-100 mg IV | 100 mg PO daily for 2 wk |
| III. Begin anticonvulsant therapy. | ||
| Acute Therapy | Maintenance Therapy (Begin 12 h After Loading Dose) | |
| Phenobarbital | 20 mg/kg IV; if necessary, additional 5-25 mg/kg IV in 5 mg/kg aliquots | 4-6 mg/kg/24 h IV/IM/PO |
| Phenytoin* | 2 doses of 10 mg/kg IV, diluted in normal saline (monitor cardiac rate and rhythm) | 5-10 mg/kg/24 h IV |
| Lorazepam or midazolam continuous infusion | 0.05-0.10 mg/kg IV 1.5 µg/kg/min (ICU) | |
IM, Intramuscularly; IV, intravenously; PO, orally.
* Fosphenytoin is an alternate form of phenytoin that may be used.
Phenobarbital alone controls seizures in most newborns after administering adequate dosages (up to a maximum of 40 mg/kg loading dose). Give phenytoin, 20 mg/kg, if seizures continue. Evaluation of fosphenytoin in the newborn, which is converted to phenytoin, is presently unknown, but initial data suggest that the rate of conversion is identical to that shown for older infants. Unfortunately, seizures are often incompletely controlled by phenobarbital and phenytoin (Jensen, 2009). When these drugs fail, other anticonvulsants (benzodiazepines: midazolam, lorazepam, levetiracetam, and topiramate) may be effective. These are not recommended as first-line drugs (Bassan et al., 2008; Jensen, 2009; Rennie and Boylan, 2007).
Pyridoxine deficiency, a rare cause of neonatal seizures, should be considered whenever no other cause is determined. Most infants have an unusual paroxysmal pattern on EEG, with generalized bursts of synchronous high-voltage activity of 1- to 4-Hz intermixed spikes and sharp waves. The diagnosis of pyridoxine deficiency is not excludable based on lack of response to a single large dose of intravenous (IV) pyridoxine with concurrent EEG recording; rather, large doses (50-100 mg daily) should be given orally for several days (Mills et al., 2010).
Specific metabolic disorders require specific therapeutic considerations with rapid intervention to maximize the chance of a good outcome (Pearl, 2009). Glut-1 deficiency syndrome is another rare cause of seizures and major developmental delay associated with hypoglycorrhachia, which may have implications for treatment in that the ketogenic diet may be most effective for control of seizures (Moers et al., 2002).
Duration of Treatment and Outcome
The optimal duration of maintenance therapy for neonatal seizures is unknown. The duration of maintenance treatment for neonatal seizures depends on the risk for recurrence, underlying cause (see Table 80.3), results of the neurological examination, and EEG findings (Glass and Wirrell, 2009). Phenytoin should be discontinued when stopping IV therapy because adequate serum levels are difficult to maintain with oral phenytoin in the newborn. If seizures have stopped and the neurological examination and EEG are normal, phenobarbital should be discontinued before discharge from the hospital. If continuing phenobarbital after discharge, discontinuation should be considered as early as 1 month later based on the neurological status and EEG. Discontinuing zphenobarbital should be considered in an infant whose examination is not normal if EEG does not show epileptiform activity. The potential deleterious effects of phenobarbital on brain development are a concern. Infants should be treated with phenobarbital for the briefest possible time.
Hypoxic-Ischemic Brain Injury in the Term Newborn
Hypoxic-ischemic encephalopathy results from reduced oxygen delivery to the brain and from the excessive accumulation of metabolites such as lactate, free radicals, and excitotoxic amino acids. It is a major cause of morbidity and mortality in both premature and term infants. Hypoxic-ischemic cerebral injury in the premature newborn is discussed later with IVH (see Hemorrhagic and Hypoxic-Ischemic Brain Injury in the Premature Newborn). In this section, we discuss only hypoxic-ischemic injury in the term newborn. Chapter 61 provides a rational approach to the diagnosis and management of neonatal hypoxic-ischemic encephalopathy. Because most hypoxic-ischemic brain injury in term infants occurs antepartum and intrapartum, prevention depends principally on optimal obstetrical management. Advances in intrapartum monitoring such as fetal heart rate monitoring, assessment of fetal movements, and the use of the biophysical profile and scalp blood gases may result in earlier diagnosis and decrease in incidence of severe hypoxic-ischemic brain injury.
Diagnosis
Because asphyxia is mainly an intrauterine event, careful documentation of maternal risk factors and abnormalities of labor and delivery are important. An accurate history also may provide precise information about the type of insult as well as its severity, duration, and timing, which in turn determines in large part the specific pattern of brain injury. Acute, total, or near-total asphyxia may cause disproportionate injury to thalami, basal ganglia, and brainstem nuclei, whereas prolonged partial asphyxia causes injury principally to the cerebral cortex and subcortical white matter (Miller et al., 2005; Roland et al., 1998).
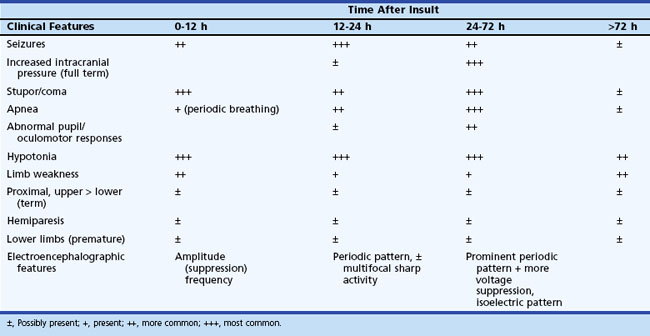
The initial clinical features of severe hypoxic-ischemic encephalopathy include depressed level of consciousness, periodic breathing (due to bilateral hemisphere dysfunction), hypotonia, and seizures. When the hypoxic-ischemic insult is of the acute/near-total type, brainstem dysfunction may be a prominent feature. Between 24 and 72 hours of age, the level of consciousness, seizures, apnea, and other brainstem abnormalities become more prominent, which also correspond to the timing of maximum intracranial pressure. After 72 hours, infants who survive show continued (although diminishing) stupor, abnormal tone, and brainstem dysfunction, with disturbances of sucking and swallowing. Specific patterns of weakness related to the distribution of neuronal injury may become evident (Table 80.4). Table 80.5 summarizes the temporal profile of clinical features of severe hypoxic-ischemic encephalopathy in the term newborn.
Table 80.4 Neuropathological Patterns of Neonatal Hypoxic-Ischemic Brain Injury and Clinical Correlation
| Pattern of Injury | Neuropathological Injury | Clinical Features in Neonatal Period |
|---|---|---|
| Cortical/subcortical watershed | Cortex, subcortical white matter Brainstem nuclei, thalamus, basal ganglia | Term: coma, seizures, hypotonia Term > premature: oculomotor abnormalities, abnormal sucking, swallowing |
| Thalamic/basal ganglia | Cerebral cortex, subcortical white matter in parasagittal regions | Term: proximal limb weakness, upper > lower |
| White matter injury of prematurity | Periventricular and diffuse white matter | Premature: unknown (probably lower limb weakness) |
| Focal/multifocal | Unilateral or bilateral cerebral cortex and subcortical white matter | Premature and term: variable hemiparesis/quadriparesis, stereotyped, nonhabituating reflex responses |
Electroencephalography and Cortical Evoked Responses
The usefulness of the visual, auditory, and somatosensory evoked responses in the diagnosis and prognosis of hypoxic-ischemic encephalopathy is more limited (see Chapter 32A), although there may be a role for visual evoked responses in the diagnosis of periventricular leukomalacia (PVL) and for auditory evoked responses in the diagnosis of brainstem injury.
Metabolic Biomarkers
Hypoglycemia, hypocalcemia, hyponatremia (inappropriate antidiuretic hormone secretion), and lactic acidosis may contribute to the neurological syndrome of hypoxic-ischemic encephalopathy. Uncorrected metabolic derangements may worsen the cerebral injury (Hanrahan et al., 1998).
Neuroimaging
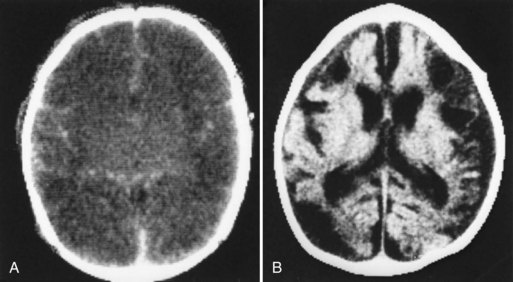
Neuroimaging has major value for locating and quantifying cerebral injury. MRI in the term newborn and US and MRI in the premature newborn are especially valuable. Although MRI has superseded CT in many instances, CT still has a role in the assessment of acute hypoxic-ischemic brain injury, especially in term newborns unable to tolerate the prolonged scanning time required with MRI (Chau et al., 2009). CT performed between 3 and 5 days of age may show decreased attenuation. More precise anatomical delineation of mild brain injury or selective involvement of thalamus and basal ganglia or cerebellum may be assessed more accurately by MRI (Rutherford et al., 2010) (Fig. 80.1). More advanced MRI techniques may prove especially useful (e.g., diffusion tensor imaging, functional MRI, diffusion tractography) (Counsell et al., 2010; Miller and Ferreiro, 2009).
Several techniques may also provide additional insight into the functional disturbances of newborn hypoxic-ischemic cerebral injury. For example, positron emission tomography, single-photon emission computed tomography, and near-infrared spectroscopy show disturbances of cerebral perfusion, and magnetic resonance spectroscopy shows decreased brain levels of high-energy phosphates in asphyxiated infants (Barkovich et al., 2006).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree