34 Neuromuscular Junction Disorders
Disorders affecting the neuromuscular junction (NMJ) are among the most interesting and rewarding seen in the electromyography (EMG) laboratory. These disorders are generally pure motor syndromes that usually preferentially affect proximal, bulbar, or extraocular muscles. They are confused occasionally with myopathies. With knowledge of normal NMJ physiology (see Chapter 6), most of the abnormalities affecting the NMJ can be differentiated using a combination of nerve conduction studies, repetitive stimulation, exercise testing, and needle EMG.
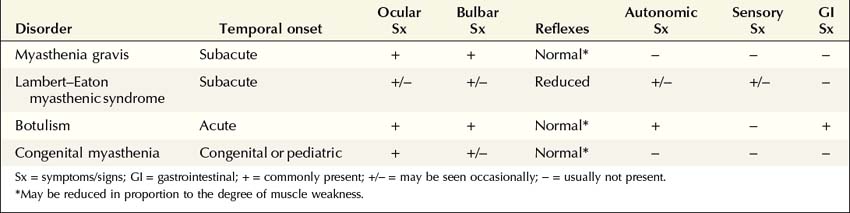
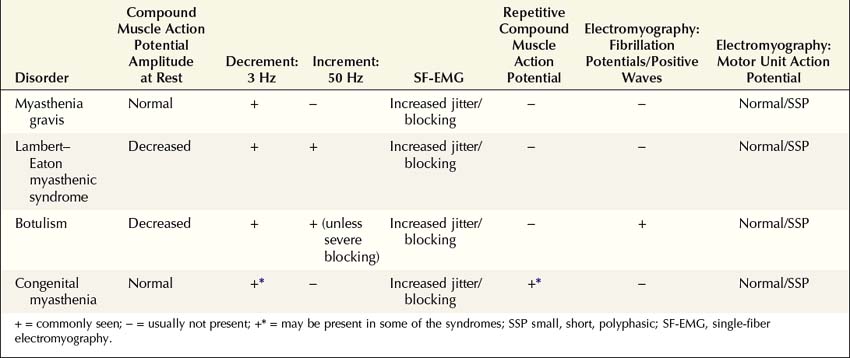
NMJ disorders can be classified into immune-mediated, toxic or metabolic, and congenital syndromes (Box 34–1). They usually are distinguished by their clinical and electrophysiologic findings (Tables 34–1 and 34–2). All are uncommon, but among them, myasthenia gravis (MG) and Lambert–Eaton myasthenic syndrome (LEMS) are the disorders most often encountered in the EMG laboratory. Both are immune-mediated disorders. In MG the autoimmune attack is postsynaptic; in LEMS the presynaptic membrane is the target of attack. Every electromyographer must understand the electrophysiology of these disorders so that appropriate electrodiagnostic tests can be applied and the correct diagnosis not overlooked.
Box 34–1
Disorders of the Neuromuscular Junction
Myasthenia Gravis
Electrophysiologic Evaluation
Like other disorders affecting the NMJ, the electrophysiologic evaluation of MG involves routine nerve conduction studies, repetitive nerve stimulation (RNS), exercise testing, routine EMG, and, in some cases, single-fiber EMG (SF-EMG) (Box 34–2).
Box 34–2
Electrophysiologic Evaluation of Myasthenia Gravis
1. Routine motor and sensory nerve conduction studies. Perform routine motor and sensory nerve conduction studies, preferably a motor and sensory nerve in one upper and one lower extremity. CMAP amplitudes should be normal. If CMAP amplitudes are low or borderline, repeat distal stimulation immediately after 10 seconds of exercise to exclude a presynaptic NMJ transmission disorder (e.g., Lambert–Eaton myasthenic syndrome).
2. Repetitive nerve stimulation (RNS) and exercise testing. Perform slow RNS (3 Hz) on at least one proximal and one distal motor nerve. Always try to study weak muscles. If any significant decrement (>10%) is present, repeat to ensure decrement is reproducible. If there is no significant decrement at baseline, exercise the muscle for 1 minute, and repeat RNS at 1, 2, 3, and 4 minutes looking for a decrement, secondary to post-exercise exhaustion. If at any time a significant decrement is present (at baseline or following post-exercise exhaustion), exercise the muscle for 10 seconds and immediately repeat RNS, looking for post-exercise facilitation (repair of the decrement).
3. Needle electromyography (EMG). Perform routine needle EMG of distal and proximal muscles, especially weak muscles. Patients with moderate to severe myasthenia gravis may display unstable or short, small, polyphasic motor unit action potentials. Recruitment is normal or early. Needle EMG must exclude severe denervating disorders or myotonic disorders, which may display an abnormal decrement on RNS.
4. Single-fiber EMG (SF-EMG). If the above are normal or equivocal in a patient strongly suspected of having myasthenia gravis, perform SF-EMG in the extensor digitorum communis and, if necessary, one other muscle, looking for jitter and blocking. It is always best to study a weak muscle. Normal SF-EMG in a clinically weak muscle excludes an NMJ disorder.
CMAP, compound muscle action potential; NMJ, neuromuscular junction.
Repetitive Nerve Stimulation
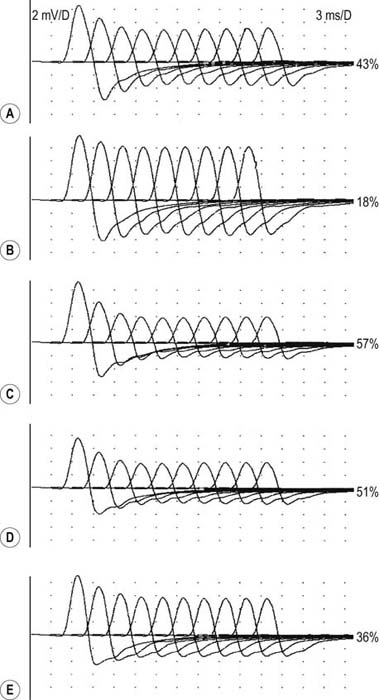
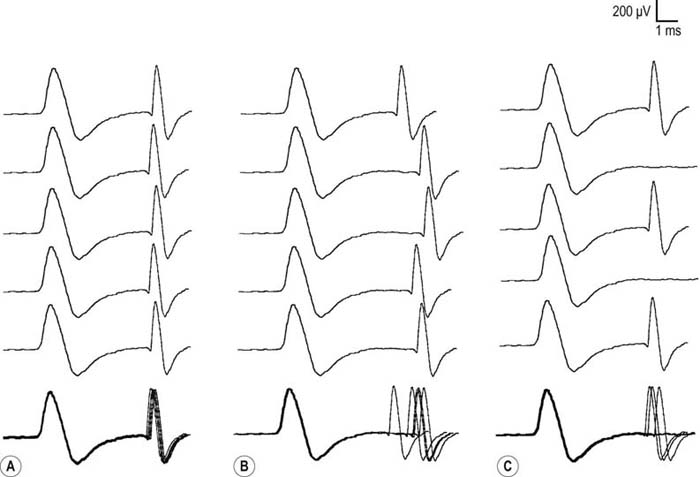
After the routine nerve conduction studies are completed, RNS studies are performed (see Chapter 6). These studies are abnormal in more than 50 to 70% of patients with generalized MG but often are normal in patients with the restricted ocular form of MG. A decremental response on RNS is the electrical correlate of clinical muscle fatigue and weakness. In normal subjects, slow RNS (3 Hz) results in little or no decrement of the CMAP, whereas in MG, a CMAP decrement of 10% or more is characteristically seen (Figure 34–1A). Both distal and proximal nerves should be tested. Although distal nerves are technically easier to study, the diagnostic yield increases with stimulation of proximal nerves (e.g., spinal accessory or facial nerves). This is not unexpected, because the proximal muscles usually are much more involved clinically than the distal ones. Facial RNS is especially important to perform in suspected anti-MuSK MG, where the yield of finding an abnormal decrement is much higher when examining a facial muscle than a limb muscle (probably reflecting the severe facial and bulbar involvement in some patients with anti-MuSK MG).
Exercise Testing
Exercise testing should be routinely used with all RNS studies (see Chapter 6). If there is no significant decrement on RNS studies at baseline (<10% decrement), the patient should perform 1 minute of exercise, followed by RNS at 1-minute intervals for the next 3 to 4 minutes, looking for a CMAP decrement secondary to post-exercise exhaustion. If at any time, either at baseline or following exercise, a significant decrement develops, the patient should perform a brief 10-second maximum isometric contraction, immediately followed by slow RNS, looking for an increment in the CMAP and “repair” of the decrement secondary to post-exercise facilitation (Figure 34–1).
Electromyography
Unstable MUAPs (see Chapter 15) occur when individual muscle fibers are either blocked or come to action potential at varying intervals, which leads to MUAPs that change in configuration from impulse to impulse. If some muscle fibers of a motor unit are blocked and never come to action potential, the motor unit effectively loses muscle fibers, becoming short, small, and polyphasic, similar to MUAPs seen in myopathy. Otherwise, the needle EMG findings in NMJ disorders usually are normal. In general, fibrillation potentials and other abnormal spontaneous activity are not seen in NMJ disorders, with the important exception of botulism (see section on Botulism).
Single-fiber Electromyography
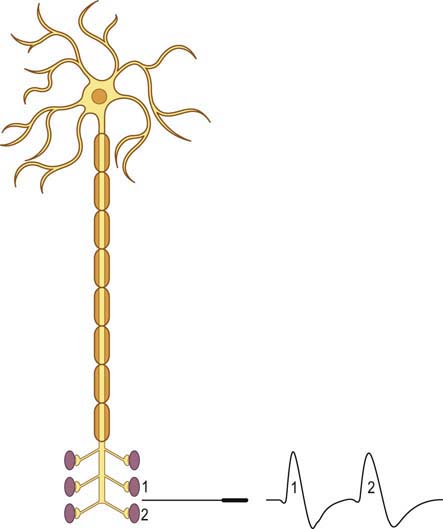
When a motor axon is depolarized, the action potential normally travels distally and excites all the muscle fibers within that motor unit at more or less the same time (Figure 34–2). This variation in the time interval between the firing of adjacent single muscle fibers from the same motor unit is termed jitter and primarily reflects variation in NMJ transmission time. If the NMJ is compromised, the time it takes for the endplate potential to reach threshold is prolonged, which results in greater-than-normal variation between firing of adjacent muscle fibers. If the prolongation is severe enough, the muscle fiber may never reach action potential, resulting in blocking of the muscle fiber.
The goal of SF-EMG is to study two adjacent single muscle fibers, known as a pair, from the same motor unit. This is accomplished by changing the filters on the EMG machine and using a specialized SF-EMG needle. The low-frequency filter (high-pass) is increased to 500 Hz (normally 10 Hz in routine EMG). By using a high-pass filter of 500 Hz, the amplitudes of distant muscle fiber potentials are attenuated while those of the nearby fibers are preserved. The SF-EMG needle is a specially constructed needle with the active electrode (G1) located in a port along the posterior shaft of the needle and with a smaller leading surface area than the conventional concentric needle electrode (Figure 34–3). The reference electrode (G2) is the needle shaft. The result of these two modifications is that single-fiber muscle action potentials are recorded only if they are within 200 to 300 µm of the needle. The needle is placed in the muscle, and the patient is asked to activate the muscle in an even and constant fashion. The needle is moved until a single muscle fiber potential is located. With this single muscle fiber potential triggered on a delay line, the needle is slightly and carefully moved or rotated to look for a second potential that is time locked to the first potential (signifying that it is from the same motor unit).
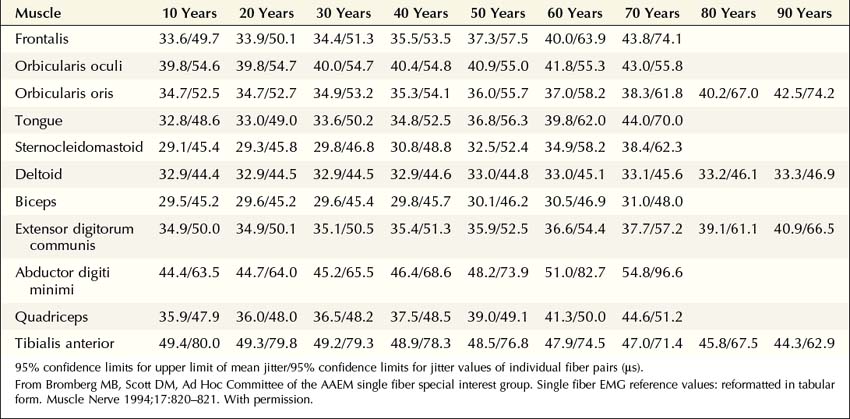
More recently, the regular disposable concentric EMG needle has been used for SF-EMG studies. The standard SF-EMG needle is expensive, and needs to be surgically sanitized between patients. Thus, the cost of the standard SF-EMG needle, along with the theoretical risk of transmitting infection (including prion diseases) despite sanitizing the needle, have prompted this change. In general, the values for jitter are comparable between the traditional SF and the concentric EMG needles. Single-fiber potentials should be accepted for analysis only if the potential is at least 200 µV in amplitude with a rise time of less than 300 µs. If a time-locked second potential is located, an interpotential interval between the two potentials (i.e., the pair) can be measured. By recording multiple consecutive firings of the muscle fiber action potential pairs, the difference between consecutive interpotential intervals can be calculated. This variation between consecutive interpotential intervals is the jitter. By recording 50 to 100 subsequent potentials, the mean consecutive difference (MCD), a measure of jitter, can be calculated between the triggered potential and the time-locked second single muscle fiber potential. Most modern EMG machines have programs that automatically perform the MCD calculation. This procedure is then repeated until 20 separate single-fiber pairs are collected, to calculate a mean MCD. This value is compared with the normal mean MCD for the muscle studied and the patient’s age (Table 34–3). There is also an upper limit of normal jitter for an individual pair, based on the muscle studied and the patient’s age. To call the latter abnormal, more than 10% of the pairs must exceed the limit (e.g., for 20 pairs, at least two must be abnormal). To make a diagnosis of an NMJ disorder, either the mean jitter must be abnormal or the upper limit of normal jitter must be abnormal in more than 10% of individual pairs. However, in most NMJ disorders, both will be abnormal. Increased jitter is consistent with an NMJ disorder (Figure 34–4). In addition to increased jitter, blocking may be seen on SF-EMG. Two time-locked, single-fiber muscle potentials from the same motor unit normally fire together. If the triggered potential fires steadily while the second potential fires only intermittently, blocking is occurring. Blocking, which is another marker of NMJ disease, usually occurs only when the jitter is markedly prolonged (e.g., MCD > 80–100 µs).