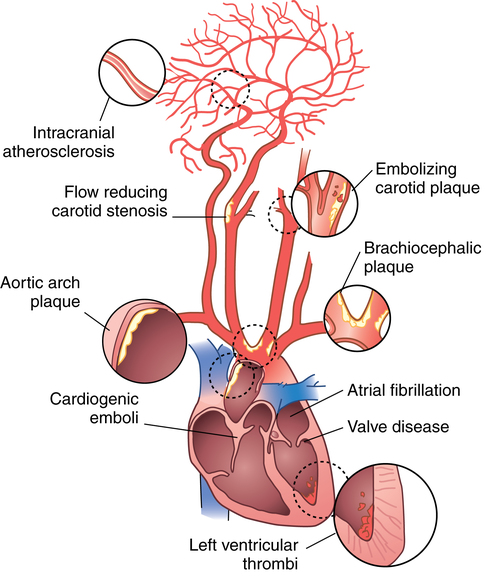
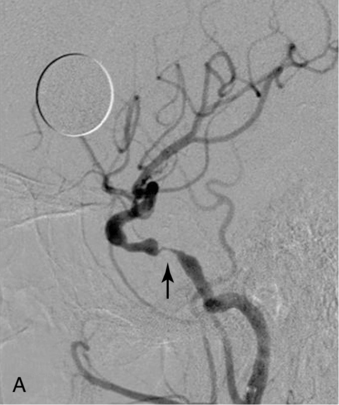
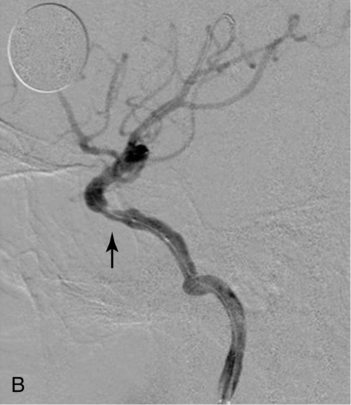
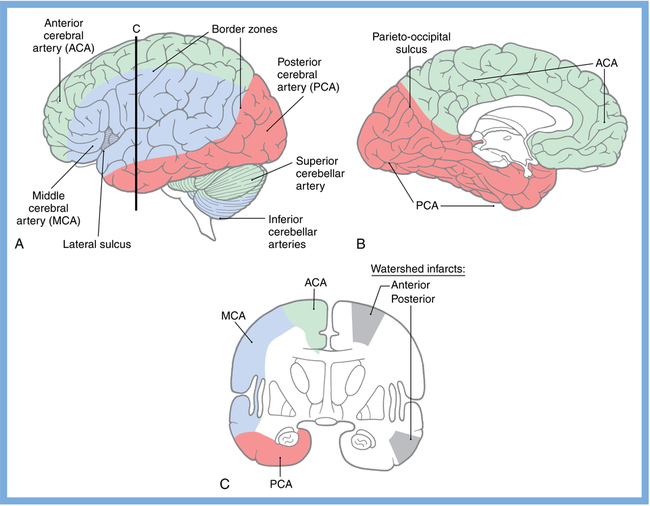
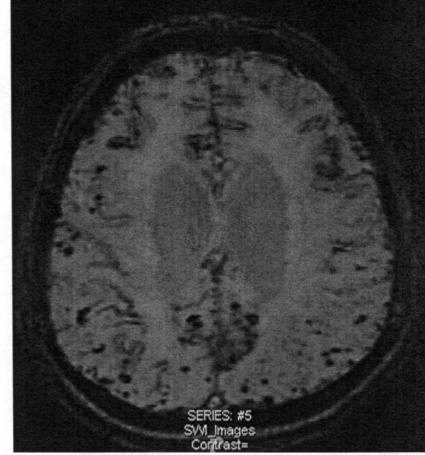
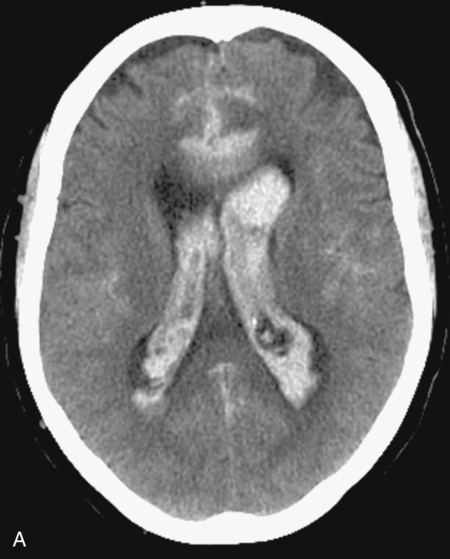
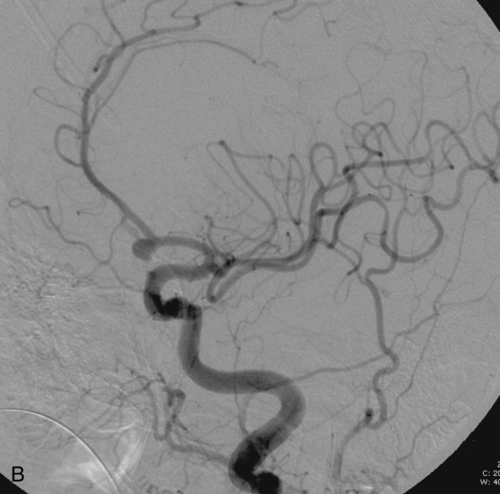
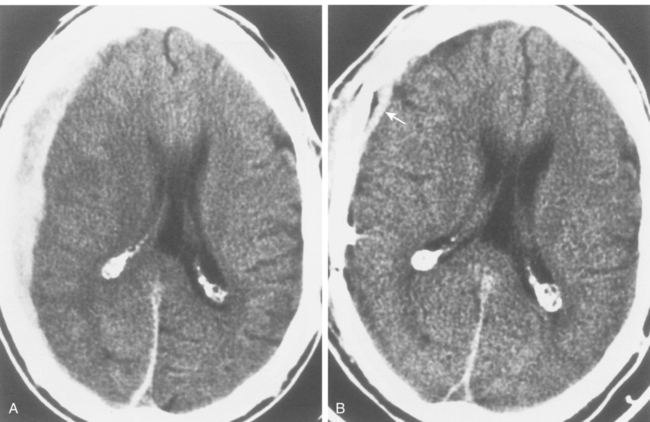
CHAPTER 3 Liana S. Rosenthal and Argye E. Hillis Although it has long been recognized that distinct impairments of language are associated with damage to different locations of the brain, predominantly in the left cortex (Broca, 1865; Dax, 1865; Wernicke, 1881), there has been much less interest in the etiology (underlying disease or other cause) of the brain damage. For example, there has been quite a bit of controversy over the cause of aphasia in Broca’s originally described patient, or “Tan,” who had a progressive illness that has never been clearly identified (Broca, 1865; Selnes & Hillis, 2000). Often studies of aphasia recovery and rehabilitation combine various etiologies of aphasia as though the cause is unimportant, but this assumption may be unwarranted. For the purpose of gaining a broader understanding of what to expect in patients with specific illnesses, we have divided the causes of language disorders based on the expected disease course (Box 3-1). This classification includes diseases that are acute at onset then slowly improve, those that wax and wane, those that are episodic, and those in which function slowly deteriorates over time. The classic example of diseases that have acute onset and usually slowly improve with medical treatment and therapies is stroke. In diseases whose symptoms wax and wane over time, patients may at first return to baseline between exacerbations of the disease, but eventually many patients slowly accumulate deficits and ultimately demonstrate a slow, downhill progression. Multiple sclerosis is the most well known of this disease type. There are also numerous neurodegenerative diseases, including primary progressive aphasia, for which there is currently no disease-modifying therapy. Patients with these illnesses therefore slowly worsen over time, usually developing more symptoms despite our best efforts. Finally, there are three neurological diseases that are episodic, in which the patient should always return to baseline: seizures, migraines, and transient ischemic attacks (TIAs). The most well known example of a disease that presents with language impairment is stroke. While most strokes in the United States are acute ischemic strokes (AISs), a sizeable minority of patients present with primary intracerebral hemorrhage (Brazis, Masdeu, & Biller, 2007). Subarachnoid hemorrhage accounts for an even smaller percentage of strokes. AIS and intracerebral hemorrhage (ICH) share some common risk factors, including hypertension, smoking, diabetes, and obesity. If the AIS or ICH affects the language networks in the brain, patients and observers will describe a sudden onset in difficulty speaking or understanding as well as additional neurological symptoms. Subarachnoid hemorrhage usually presents very differently but can result in delayed AIS from vasospasm. Direct sequelae of the subarchnoid hemorrhage, including diffuse cognitive deficits, as well as possible interventions will be discussed in detail later. For patients with AIS, the first few days after the stroke are the critical period during which some brain tissue may be saved. If the patient is admitted within several hours of onset of symptoms, the initial focus will be on saving brain tissue and restoring immediate brain function, by restoring blood flow to ischemic tissue via thrombolytic agents, clot retrieval, surgical intervention, or other methods. The medical team caring for the stroke patient will then likely institute blood pressure, glucose, and temperature management to reduce the risk of brain swelling or worsening of the patient’s neurological deficits and will take measures to reduce complications of stroke, such as deep venous thrombosis, aspiration pneumonia, and contractures. They will also search for the etiology of the AIS to prevent recurrence. Of patients with AIS, the 30-day case fatality is about 25% (although this rate is lower in dedicated stroke units), with the greatest predictor of mortality being stroke severity (Hankey, 2003). Death is usually secondary to the stroke itself and its resulting sequelae. Especially in the first 3 to 5 days post stroke, significant brain swelling may occur that can result in herniation and subsequent death. Overall, however, patients with small to moderate-size AIS generally do well, with a rapid improvement in deficits within the first week to months after the ischemic event and then a slowing in the rate of improvement over the next year. Although it is often taught that patients show minimal improvement after 1 year from the precipitating event, there is no basis for this teaching. Many patients continue to show functional recovery for the remaining years of their life, if they continue to work toward learning new ways to function better. The mechanisms of recovery are likely to change over time. In the first few days, restoration of tissue function likely accounts for early recovery of language. Reorganization of structure-function relationships, in which undamaged parts of the brain assume functions of the damaged parts, is likely an important part of subacute recovery days to weeks after AIS onset, a process that can be facilitated by intense speech-language therapy (and perhaps augmented by certain medications that affect neurotransmitter release and reuptake) (Hillis, 2005). Finally, reorganization of cognitive processes underlying language and compensation can take place for months or years after stroke and can be facilitated by intense practice at home, guided by a speech-language pathologist, family member, or other coach. The language function in AIS can fluctuate in the first few days and weeks after stroke, and these fluctuations can reflect changes in cerebral blood flow (Croquelois, Wintermark, Reichhart, et al., 2003; Hillis, 2007; Ochfeld, Newhart, Molitoris, et al., 2009) and/or changes in neurotransmitter release and reuptake reflected in, or caused by, fluctuation in motivation, mood, response to rehabilitation, positive and negative responses to medications, and so on. The etiology of AIS is important in understanding the course, the probability if recurrence, and possibility of recovery. For example, AIS due to coagulopathy caused by cancer is likely to recur unless the cancer can be cured. AIS caused by carotid stenosis might never recur if the patient is a candidate for endarterectomy to reverse the carotid stenosis. AIS due to atrial fibrillation will be much less likely to recur if atrial fibrillation can be reversed with cardioversion to a normal cardiac rhythm, and somewhat less likely to recur if the patient is anticoagulated with blood thinner chronically. The course of lacunar strokes (strokes smaller than 1 cm or 1.5 cm) is nearly always stable; the course of strokes caused by narrowing of the middle cerebral artery may be stuttering or progressive over several days (Figure 3-1). Deficits observed in AIS can be explained by the affected vascular territories. The classic aphasia classifications are vascular syndromes, or collections of language symptoms that commonly co-occur because the functions affected are localized to a particular vascular territory. Therefore, the relationship between dysfunction in a particular area (low blood flow or infarct in a vascular territory) and a particular aphasia syndrome is much stronger in the acute stage of stroke than in chronic stroke, after some patients have shown significant recovery but still have the lesion (Croquelois et al., 2003; Ochfeld et al., 2009). For example, blockage in the superior division of the left middle cerebral artery (MCA) typically results in ischemia to the left posterior inferior frontal cortex and a Broca’s aphasia. These patients typically have nonfluent, apractic, agrammatic speech output, poor repetition, and relatively spared comprehension at least of simple syntactic structures at onset. As the motor strip is often also involved, these patients typically also have right face and arm weakness greater than right leg weakness. The left face, arm, and leg as well as visual fields will be spared. If the lesion is relatively restricted to this area, many recover relatively quickly and may have no deficits 6 months later (Ochfeld et al., 2009). In contrast, ischemia in the posterior superior temporal cortex secondary to a blockage in the inferior division of the left MCA generally leads to fluent but meaningless speech output, poor repetition, and poor comprehension, classified as Wernicke’s aphasia (Croquelois et al., 2003; Ochfeld et al., 2009). These patients may also have a subtle, usually clinically underappreciated, right-sided hemispatial neglect as well as superior quadrantanopia but no arm or leg weakness. Because emboli from the heart tend to travel down the inferior division, rather than up the superior division of the MCA, cardioembolic strokes are more likely to cause Wernicke’s aphasia than Broca’s aphasia (Urbinelli, Bolard, Lemesle, et al., 2001), again underscoring that these are vascular syndromes (Figure 3-2). “Watershed” strokes are a special case of AIS with a somewhat different mechanism from simple occlusion of an artery leading to loss of blood flow to the territory of one artery. They often occur when there is severe narrowing of one or more cerebral vessels, combined with sudden drop in blood pressure. Imagine having two sprinklers that provide water to your yard. You have positioned them such that they just cover the yard but do not overlap. If suddenly the water pressure drops, there will be a strip of yard between the two sprinklers where the water from neither sprinkler will reach. Likewise, if there is a sudden drop in blood pressure, especially if there is narrowing of the carotid artery that supplies the MCA and anterior cerebral artery (ACA), there will be a strip of brain that will not receive adequate blood from either the MCA or the ACA. Ischemia in the watershed areas between the left MCA and left ACA territories generally results in transcortical motor aphasia with relatively preserved comprehension and repetition but nonfluent speech with difficulty initiating and organizing responses (Hillis, 2007). In addition, patients with a transcortical motor aphasia may also have right leg greater than arm weakness and a relative sparing of facial musculature, because the leg area of the motor strip, medial to the arm and face is in this “watershed” area. Ischemia in the watershed area between the left MCA and left posterior cerebral artery (PCA) often results in a transcortical sensory aphasia in which the patient has poor comprehension and meaningless speech but relatively preserved repetition. These patients may also have a hemianopsia and a hemihypesthesia, where patients have difficulty on the affected side with two-point discrimination and stereoagnosia because parts of the sensory strip lie in this territory. There are, of course, numerous other aphasia syndromes and aphasic deficits that do not fit within vascular syndromes, which are beyond the scope of the chapter (Figure 3-3). While not strictly resulting in aphasia, right hemisphere strokes will also lead to communication disorders. These deficits include reduced understanding of the humor, intent, connotation, and affective prosody of speech, in part because of difficulty integrating language and its context. Patients with right hemisphere lesions have difficulty understanding the nonliteral meaning of words and sentences. For example, when observing someone carrying a load of books, a patient with a right hemisphere lesion might reply “yes” when asked “could you open the door for me?” Most others would understand that the book-carrier actually wants the door opened for them (Mitchell & Crow, 2005). Patients with right hemisphere lesions can also have great difficulty understanding the emotion conveyed by a person’s tone of voice or can have trouble conveying emotion through prosody (Ross & Monnot, 2008). For example, the phrase “She stole my money” has slightly different meanings if the emphasis is placed on the she versus an emphasis on the my or the money. Patient with right cortical lesions also sometimes have difficulty with metaphors. Subjects with right cortical lesions asked to select which of two drawings conveyed the meaning of the phrase “he had a heavy heart” more often chose the photograph with the literal meaning of a person stumbling with a large heart tied to his back as opposed to the photograph of a person crying (Winner & Gardner, 1977). Some of these patients also perform poorly on tasks of discourse comprehension, such as understanding the main theme of a paragraph or a conversation (Hough, 1990). The relationships between site of lesion and type of communication deficit have not been clearly identified after right hemisphere ischemic stroke but are under investigation. ICH accounts for approximately 10% to 15% of all strokes in the United States (Brazis et al., 2007) and has a significantly higher mortality compared to AIS, with only 38% of affected patients surviving the first year (Qureshi, Tuhrim, Broderick, et al., 2001). A low score on the Glasgow Coma Scale (GCS), a large volume of the hematoma, and the presence of intraventricular blood on the initial CT scan are three factors that have consistently been associated with a high mortality rate (Qureshi et al., 2001). The increased mortality associated with intraventricular blood may be secondary to a direct mass effect of the blood on periventricular structures or may relate to the development of obstructive hydrocephalus. In an effort to decrease the morbidity and mortality associated with intraventricular blood, these patients often have catheters placed in their ventricles to facilitate external drainage of cerebrospinal fluid. The goal is to relieve the pressure buildup and hydrocephalus that develop because of the blood clotting in the ventricles. These catheters are not ideal because they have a high infection risk and they often clot with the very blood they are supposed to be draining. There is, therefore, continued interest in administering thrombolytic agents into the ventricles of patients with intraventricular hemorrhages, and small studies have shown an improvement in mortality with this approach (Qureshi et al., 2001). Similar to AIS, the deficits related to ICH are directly related to the localization of the damaged tissue. ICHs, however, do not respect the vascular territories so patients do not present with the classic aphasia syndromes described earlier. Instead, ICHs have five typical locations where they occur: the cerebral lobes (“lobar hemorrhage”), basal ganglia, thalamus, brainstem, and cerebellum (Figure 3-4). Patients with hemorrhage in the deep brain structures of the left hemisphere including the basal ganglia, internal capsule, and the adjacent white matter lesions have been noted to suffer from articulatory impairment in addition to comprehension and naming impairments. Left hemisphere thalamic lesions led to impairments in naming and repetition. Right hemisphere thalamic lesions led to a deficit in the elaboration of narratives and integrating elements within a context. For example, these patients had difficulty describing the activity of the subjects in a picture (Radanovic & Scaff, 2003). Patients with large ICHs typically present with a decreased level of consciousness. In addition, patients often have headaches, nausea, and emesis due to increased intracranial pressure and may have meningismus secondary to blood in the ventricles. The most common risk factor for ICH is idiopathic hypertension, especially ICH in the basal ganglia, thalamus, or brainstem. However, ICH may also be secondary to amyloid angiopathy, arteriovenous malformations, intracranial aneurysms, and other vascular malformations. Arteriovenous malformations are abnormal communications between arteries and veins that tend to bleed; their treatment with radiosurgery, surgery, or embolization is controversial, as rebleeding is common with and without treatment (Stapf, Mohr, Choi, et al., 2006). In addition, dural venous sinus thrombosis can lead to an ICH as can an intracranial primary neoplasm or metastasis of a systemic neoplasm. Melanoma, renal cell carcinoma, and choreocarcinoma are cancers that commonly bleed. Other common cancers that metastasize to the brain and sometime hemorrhage are breast and lung cancer. Cocaine and alcohol use have also been associated with an increased ICH risk. Finally, the ICH may be secondary in part to a coagulopathy, most often caused by anticoagulant use (Qureshi et al., 2001). Venous hemorrhage can be caused by thrombosis of the cerebral veins (also called cerebral sinuses). It is common for large AISs to show hemorrhagic conversion, but this does not change the prognosis for the AIS unless it is large and causes acute increased intracranial pressure (e.g., in some cases after thrombolysis); hemorrhagic conversion of ischemic stroke is not considered ICH. Although patients with ICH initially seem much more ill and have a lower level of consciousness than do patients with AIS, if they survive the acute period, they generally do well. Once the blood is reabsorbed, there may be little permanent damage. An exception to this brighter prognosis is the increasingly recognized etiology of cerebral amyloid angiopathy, the most common cause of lobar hemorrhage, particularly in people over 60. It is typically diagnosed by the presence of multiple microhemorrhages in the cortex (see Figure 3-5) and is associated with high risk of recurrent lobar hemorrhage and progressive dementia and, to a lesser extent, with infarct and SAH. SAH accounts for most of the remaining 2% to 5% of all strokes. The average case-fatality rate is about 51%. While SAH accounts for 5% of deaths from stroke, it accounts for 27% of all stroke-related years of potential life lost before the age of 65 (Suarez, Tarr, & Selman, 2006). This high morbidity rate reflects the fact that SAH often occurs at a younger age than other strokes. About 85% of all nontraumatic SAHs are the result or a ruptured intracranial aneurysm (Van Gijn & Rinkel, 2001). Head trauma is the most common cause of SAH, but SAH is rarely the isolated injury in these cases (Figure 3-6). Headache and decreased level of consciousness are the most common presentations of aneurysmal SAH. Localizable deficits are related to the location of the ruptured aneurysm and subsequent bleed. Common initial signs include a third nerve palsy, a sixth nerve palsy, bilateral lower extremity weakness, abulia, visuospatial neglect, or the combination of hemiparesis and aphasia. Subsequent localizable deficits are often secondary to vasospasm, which is symptomatic in 46% of patients after SAH (Suarez et al., 2006). Delayed cerebral vasospasm, causing infarcts and increased intracranial pressure, is now the leading cause of death and disability among patients with aneurysmal SAH (Brazis et al., 2007). Other common sequelae include hydrocephalus in 20% of patients and rebleeding in 7% of patients (Suarez et al., 2006). Initial management of SAH generally includes a conventional angiogram for evaluation of a possible aneurysm. If an aneurysm is found, early management of the aneurysm with either microvascular surgical clipping or endovascular coiling improves mortality and allows for better management of neurological complications (Whitfield & Kirkpatrick, 2001). Both methods of securing a ruptured aneurysm have been shown to be effective, and the decision regarding which method to use is based on characteristics of both the patient and the aneurysm. Most survivors of SAH are able to live with their families and gain independence in activities of daily living. In one study, two-thirds of the patients who had been working prior to the SAH had returned to that position 1 year later; on average, they were away from work for 20 weeks (Hackett & Anderson, 2000). Despite these positive outcomes, many survivors report difficulties with neuropsychological functioning. One year after SAH, there was a significant reduction in patient-reported health-related quality of life and difficulties with memory, mood, speech, and self-care. Overall, one-third to one-half of the patients report reductions in their ability to perform their social role (Hackett & Anderson, 2000). The grade, or severity, of SAH is the best predictor of impairment of cognition and memory (Ogden, Mee, & Henning, 1993). Many benefit from cognitive rehabilitation to return to work. Similar to strokes, subdural hematomas (SDHs) (Figure 3-7) may also present with aphasia or other focal neurological deficits (Dell, Batson, Kasdon, & Peterson, 1983; Kaminski, Hlavin, Likavec, & Schmidley, 1992; Mori & Maeda, 2001; Moster, Johnston, & Reinmuth, 1983). The focal presentations are often secondary to the blood pushing on the brain and therefore inhibiting cortical function. Alternative presentations include headache, seizure, and psychiatric abnormalities (Ernestus, Beldzinski, Lanfermann, & Klug, 1997). SDHs may be acute or chronic and are most often a result of trauma. Other etiologies include neurosurgical treatment for other reasons, anticoagulant therapy, coagulopathy, and alcoholism (Mori & Maeda, 2001; Ernestus et al., 1997). Management usually involves correcting any coagulopathy, considering prophylactic antiepileptic medication, and also considering surgical evacuation. For acute SDHs, surgical evacuation is recommended for all SDHs with a thickness greater than 10 mm or those that result in midline shift greater than 5 mm. For smaller SDHs or those with less of a midline shift, surgical evacuation should be performed in patients with a rapidly decreasing GCS score, an enlarged pupil, and/or an intracranial pressure greater than 20 mm Hg (Bullock, Chesnut, Ghajar, et al., 2006). Surgical evacuation in chronic subdural hematomas often follows similar guidelines. For smaller, chronic SDHs, that do not appear to be leading to devastating neurological sequalae, or in patients with too high of a surgical risk, it is reasonable to manage the SDH expectantly. Patients often undergo numerous computed tomography (CT) scans and monitoring of their neurological symptoms and, overtime, the SDH may spontaneously resolve (Parlato, Guarracino, & Moraci, 2000).
Neuropathologies underlying acquired language disorders
Acute-onset diseases that remain stable or improve
Acute ischemic stroke
Intracerebral hemorrhage

Subarachnoid hemorrhage
Subdural hematoma
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neuropathologies underlying acquired language disorders