CHAPTER 15 Neurourology
Central nervous system disorders are a frequent cause of urologic symptoms and voiding dysfunction. Lower urinary tract symptoms and erectile dysfunction may lead to significant clinical, social, and economic costs for the patient, caregiver, and health care system.1,2 Appropriate recognition and timely management of urologic issues relevant to neurological conditions are important to avoid potentially irreversible adverse outcomes.
Urologic Structures and Innervation
The lower urinary tract has two basic physiologic functions: low-pressure storage of adequate volumes of urine with appropriate sensation and periodic, voluntary expulsion of urine from the bladder in a coordinated and complete fashion. To provide these functions, the bladder, bladder neck, external urethral sphincter, and urethra must have coordinated activity mediated by the autonomic and somatic nervous systems (Fig. 15-1).
The Urinary Bladder
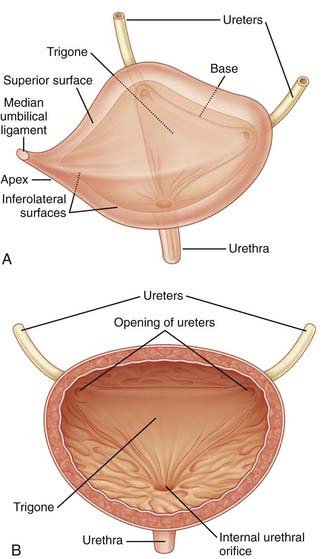
The urinary bladder is a hollow organ composed of a largely impermeable layer of transitional cell epithelium, a layer of thick smooth muscle, and an outer adventitial later of fat and connective tissue (Fig. 15-2). The function of the bladder is related to the intrinsic properties of these tissues, as well as nervous system control. Compliance, defined as the ratio of the change in intravesical pressure to bladder volume, is mostly dependent on the native viscoelastic properties of the bladder wall. The normal bladder is highly compliant and will thus maintain low intravesical pressure during filling of the bladder. Bladder injury, fibrosis of the bladder wall, congenital or acquired neurological denervation, and bladder outlet obstruction can result in detrusor thickening and increased collagen content, conditions that significantly affect compliance.3 Low bladder compliance results in high urinary storage pressure, which if unrecognized and untreated, leads to upper urinary tract deterioration and renal failure.
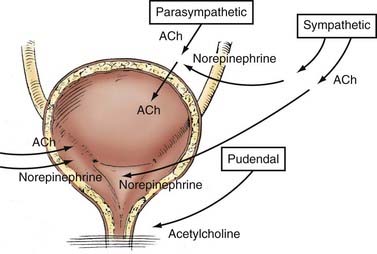
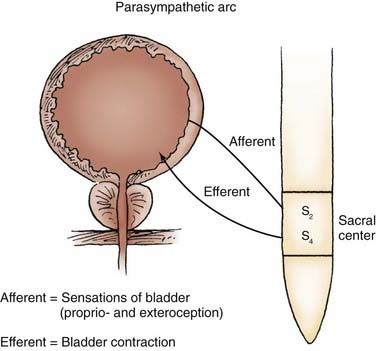
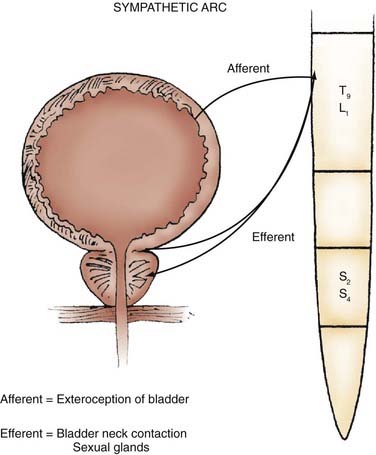
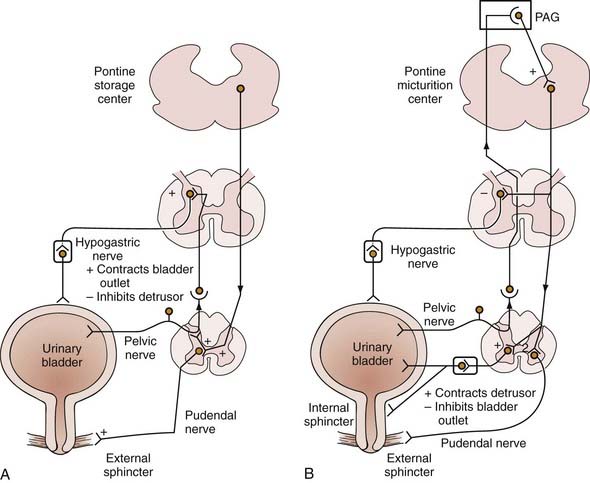
Parasympathetic innervation to the lower urinary tract originates in the S2-4 segments of the spinal cord and provides excitatory motor input to the bladder. Cholinergic preganglionic neurons within the intermediolateral sacral cord send axons to ganglionic cells within the pelvic plexus and the bladder wall. Postganglionic neurons within the bladder wall and pelvic plexus release acetylcholine, which activates cholinergic receptors on the detrusor smooth muscle cells and initiates bladder contraction (Fig. 15-3). Sympathetic pathways originate in the T11-L2 spinal segments, travel in the sympathetic chain ganglia to the prevertebral ganglia in the superior hypogastric and pelvic plexuses, and innervate the bladder via short adrenergic neurons. The bladder contains varying expression of α- and β-adrenergic receptors, and sympathetic stimulation promotes storage of urine via detrusor relaxation and contraction of the bladder neck and outlet (Fig. 15-4). Activation of β-adrenergic receptors within the wall of the bladder provides inhibition and relaxation of the detrusor muscle. Activation of cholinergic receptors stimulates the detrusor to contract, primarily through the M3 and M2 muscarinic receptor subtypes. Additional neurotransmitters, including nitric oxide, adenosine triphosphate, and neuropeptides, also have a proposed modulatory role in bladder relaxation and contraction.4
The bladder outlet is composed of the trigone, ureterovesical junction, the bladder neck, and the proximal urethra. α-Adrenergic receptors predominate in this area, and stimulation of postganglionic α-adrenergic receptors provides excitatory input to the trigone, bladder neck, and proximal urethra that results in increased bladder outlet closure force.4 The bladder neck region and adjacent proximal urethral segment are often referred to as the internal urethral sphincter or smooth muscle sphincter, and although it is not a true anatomic sphincter, it has a role in the maintenance of continence and efficient voiding.
Afferent (sensory) transmission of lower urinary tract stimuli travels via the pelvic, hypogastric, and pudendal nerves to the dorsal root ganglia of the lumbosacral spinal cord. The pelvic nerve afferents monitor the volume of the bladder and the amplitude of bladder contraction via myelinated (Aδ) and unmyelinated (C) fibers within the bladder wall. The unmyelinated fibers are also implicated in the transmission of urgency and pain.5,6 Newer research indicates that cells within the urothelium may also exhibit sensory and signaling properties.7
The Male and Female Urethra
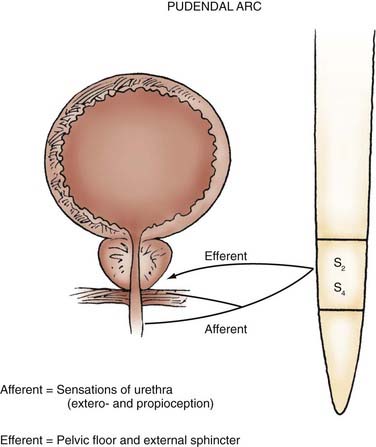
The proximal 2 to 3 cm of the urethra in both males and females maintains its sphincteric properties. Smooth and striated muscle contribute to urethral closure force in the sphincter. In males, the prostatic glandular and fibromuscular stroma tissue surrounding the proximal urethra can affect voiding through extrinsic compression and obstruction. The most proximal segment of the urethra in both sexes consists primarily of smooth muscle, which is not under voluntary control. Innervation of the proximal urethra is similar to that of the bladder neck. There is a high density of α-adrenergic receptors, which when stimulated, produce a rise in intraurethral pressure.8 Conversely, the use of α-adrenergic antagonists can reduce outlet resistance and facilitate release of urine.5 At the level of the pelvic floor in both sexes there is a sheet of striated muscle extrinsic to the urethra, as well as a discrete layer of striated muscle within the wall of the urethra. The extrinsic muscle consists of primarily fast-twitch muscle fibers and is under voluntary control. The intrinsic muscle tissue, also called the intrinsic sphincter or the rhabdosphincter, consists primary of slow-twitch fibers and provides passive urinary continence. Innervation of external sphincter and pelvic floor musculature is primarily somatic from branches of the pudendal nerve. The pudendal nerve pathway begins in the anterior horn cells of the S3 and S4 segments of the spinal cord. Afferent sensory information from the urethra and pelvic muscles also travels via the pudendal nerve (Fig. 15-5).6,9
Neuroanatomy and Physiology of the Lower Urinary Tract
Central Nervous System
Cortex, Basal Ganglia, and Cerebellum
The anteromedial portion of the frontal lobes of the cerebral cortex and cingulate gyrus are involved in the voluntary initiation of micturition and inhibition of reflex voiding activity.10 In general, cortical input is inhibitory on micturition reflexes. Lesions resulting from tumors, aneurysms, or cerebrovascular disease remove the cortical inhibition, which results in increased excitatory input to the brainstem, facilitation of the micturition reflex, and the clinical appearance of urinary frequency and urgency.11 Descending fibers in the corticospinal tracts emanate from the cortical region to innervate sacral parasympathetic neurons and the motor nucleus controlling voluntary sphincter function.12
Pons
Although the process of micturition involves anatomic regions throughout the central and peripheral nervous systems, the organizational center of this reflex can be localized to the pontomesencephalic reticular formation. The pontine micturition center (PMC), or Barrington’s nucleus, functions as a switch to regulate bladder capacity and coordinate bladder and external sphincter activity.13 Multiple anatomic and physiologic studies, building on Barrington’s initial experiments on the cat, have shown that stimulation of the PMC induces the sacral parasympathetic neurons to fire, which results in bladder (detrusor) contraction, suppression of the urethral electromyographic response, and reflex voiding.10,13–15 Descending spinal efferent pathways from the PMC to the sacral cord motor centers are located in the reticulospinal tracts. The pons receives direct ascending input from the bladder wall afferents, which travel through the dorsal root ganglia and the posterior columns of the spinal cord.16
Spinal Cord
The primary excitatory input to the bladder is the parasympathetic preganglionic neurons located in the intermediolateral gray matter of the sacral cord in segments S2-4. Sympathetic preganglionic neurons are located in the anteromediolateral gray matter of the thoracolumbar cord in segments T9-L1. The autonomic pathways of the central nervous system travel in the dorsal aspect of the lateral columns of the spinal cord and have extensive crossover at the thoracolumbar cord level. Sensory and proprioceptive afferents ascend through the posterior columns of the cord.17 Somatic centers innervating the external sphincter and pelvic floor musculature are localized at Onuf’s nucleus within the anterior horn of the sacral cord at the spinal cord level S2-4. Descending input to Onuf’s nuclei is derived from the lateral corticospinal tracts.13
Peripheral Nerves
Sympathetic preganglionic pathways from the T9 to L1 segments of the spinal cord pass through the spinal roots to the sympathetic chain ganglia, the prevertebral ganglia in the superior hypogastric and pelvic plexuses, and the short adrenergic neurons in the bladder and urethra. The sympathetic nerves course vertically along the anteromedial portion of the vertebral bodies in the sympathetic chain and form plexuses at the celiac, superior hypogastric, and inferior hypogastric branches of the aorta. Dissection of the retroperitoneum, particularly around the great vessels, can disrupt the pelvic sympathetic innervation.18
Neurourologic Pathways of Filling and Storage
Suprapontine Control of Voiding
The suprapontine centers are responsible for inhibition of the brainstem and spinal cord voiding reflexes, as well as the initiation of volitional voiding. Volitional voiding starts with activation of the frontal lobe cortex, nuclei within the basal ganglia, and areas of the cerebellum. Injuries to these suprapontine structures, such as those caused by tumor, stroke, or intracranial hemorrhage, remove this inhibition and result in the clinical symptoms of urinary urgency, frequency, and urge incontinence. As long as the pons is spared, normal coordination of bladder and sphincter activity is not affected. Thus, after recovery from such events and related edema and provided that there are no other abnormalities of the lower urinary tract (i.e., prostatic obstruction, urethral stricture, and other conditions), bladder emptying is usually satisfactory, although clinical symptoms of urinary frequency and urgency remain.19 Depending on the location and extent of the injury, voluntary control of micturition may be completely lost and result in regression to spinal reflex voiding.
Sympathetic Pathways and Storage
There is a local sympathetic reflex pathway for urine storage mediated at the level of the spinal cord. The sacrolumbar intersegmental reflex pathway is triggered by vesical afferents via the pelvic nerves. Pelvic afferents detecting bladder stretch stimulate sympathetic efferents at the PMC to inhibit bladder smooth muscle and maintain continence.13 This reflex is inhibited when bladder pressure is raised to a threshold that results in micturition. Voluntary control of the suprapontine centers can suppress this micturition reflex.
Parasympathetic Pathways and Micturition
The parasympathetic pathways to the lower urinary tract originate at the sacral cord levels S2-4. Nerves travel through the sacral spinal nerve roots (primarily S3) to ganglion cells in the pelvic plexus. From this plexus, the parasympathetics innervate the postganglionic cholinergic neurons in the bladder wall and stimulate the bladder smooth muscle cells to contract. Micturition is mediated by the parasympathetic efferents to the bladder, as well as through reflex inhibition of the somatic pathway to the urethral sphincter, which is controlled by the PMC and the spinobulbospinal reflex pathway.13 These parasympathetic pathways are also responsible for the efferent portion of normal erection and reflexogenic erection in patients with spinal cord injury (SCI) in response to a somatic stimulus.20 Afferents in the parasympathetic pathway convey sensory and proprioceptive information to the pons and supraspinal centers through the posterior columns of the cord. Damage to the parasympathetic structures can occur during radical hysterectomy or abdominoperineal resection and result in detrusor acontractility and impotence.
Somatic Pathways and Sphincter Function
Efferent somatic projections to the external sphincter originate in the ventral horn of the sacral cord (Onuf’s nucleus) and travel via the pudendal nerves. Tonic stimulation of the urethral striated sphincter reflexively increases during bladder filling and is inhibited during voiding. This so-called guarding reflex is mediated at the level of the pons. Coordination at the level of the pons and other supraspinal centers results in inhibition of pelvic floor muscle activity during voiding.13
Afferent activity via the pudendal nerves provides pelvic floor proprioceptive information from the striated sphincter. Volitional or involuntary activation of pelvic floor muscle activity suppresses bladder contraction and increases bladder capacity via the urethral sphincter storage reflex.21 These afferent fibers are also responsible for the afferent limb of the erectile reflex (Fig. 15-6).20
Evaluation of Neurourologic Disorders
History
Voiding abnormalities may be the initial symptom of a number of neurological disorders, may result from acute or chronic neurological disease, or can be a sentinel event for progression of neurourologic disease. Symptoms can be categorized as resulting from disorders of bladder filling/storage or voiding. This classification is somewhat artificial because symptoms can often be unreliable in defining specific disease states.22 It is common for patients to have a combination of symptoms or disorders of both bladder filling and voiding. Nevertheless, a history of the onset, duration, and aggravating or ameliorating factors should be noted, especially in relation to the neurological event.
Sudden, urgent micturition implies that the sensory pathways necessary for a desire to void are present, the parasympathetic and pudendal pathways are preserved, and integration of detrusor and sphincter function is appropriate. This is found most commonly with suprapontine lesions; however, incomplete suprasacral lesions may also have a similar pattern. Reflex micturition, or repeated bladder contraction and voiding without a sensation of bladder distention or urgency, occurs with complete suprasacral lesions.23 Voiding by abdominal straining or the Valsalva or Credé (manual suprapubic pressure) maneuvers is consistent with sacral spinal cord lesions, peripheral denervation, and chronic urinary retention secondary to bladder outlet obstruction.24 Paradoxical interruption of the stream without voluntary control is consistent with detrusor sphincter dyssynergia. Patients with poor contractility of the detrusor (detrusor underactivity) as a result of infrasacral lesions void by the Valsalva maneuver, and passive interruption of the urinary stream occurs when the Valsalva or Credé maneuver is stopped.
Additional History
A past history of additional medical, neurological, urologic, obstetric, and gynecologic problems or surgeries can provide additional insight into the neurourologic dysfunction. Frequently, the urologic sequelae of a particular disorder can be complicated by a patient’s prior urologic status. Age-related changes such as atrophic vaginitis, stool impaction or constipation, delirium or changes in mental status, and restricted mobility need to be considered.23 The use of a voiding diary will properly document fluid intake and output over a 48-hour period, provide an idea of functional bladder capacity and patterns of voiding and leakage, and document excessive fluid intake if present.
Physical Examination
In addition to a thorough patient history, the physical examination can provide important information regarding overall evaluation of the patient’s urologic complaints. A routine urologic examination should include an abdominal examination, inspection of the external genitalia, and palpation of the flank. In men, a digital rectal examination should be performed to evaluate prostate size, tenderness, and consistency. In women, a vaginal examination should be performed. The examination should include palpation of the urethra on the anterior vaginal wall to evaluate for discharge or a mass to rule out urethritis, a urethral diverticulum, or tumor. Inspection and palpation of the vaginal mucosa can assess for atrophic vaginitis, which can increase the risk for infection, as well as identify anatomic abnormalities such as cystocele, enterocele, uterine prolapse, and gynecologic cancers. All of the disorders just mentioned can mimic the symptoms of patients with neurourologic complaints.25
Neurourologic Examination
Sensory, motor, and reflex deficits correlate with a specific level of a neurological lesion and can often, but not invariably predict a pattern of bladder and sphincter function. Sensory examination of the anterior abdominal wall, genitalia, and lower extremities reflects the integrity of the thoracic, sacral, and lumbar nerve roots, respectively. The anterior portions of the scrotum and labia majora derive innervation from the thoracolumbar spinal cord, and the sacral nerve roots innervate the posterior portion of these organs. Sensory testing of the saddle area of the perineum can evaluate the afferent limb of the pudendal nerve.23 Basic motor and muscular tone should also be evaluated, specifically, the tibialis anterior, gastrocnemius, and toe extensors, which are innervated by the lower lumbar and upper sacral nerves. A rectal examination to evaluate the external anal sphincter is important for evaluation of the pelvic floor musculature. Voluntary contraction of the external anal sphincter confirms innervation of the pelvic floor and integrity of the corticospinal tract. Preserved sphincter tone in the absence of voluntary contraction is consistent with a suprasacral lesion, whereas diminished tone is consistent with a sacral or peripheral nerve abnormality.22
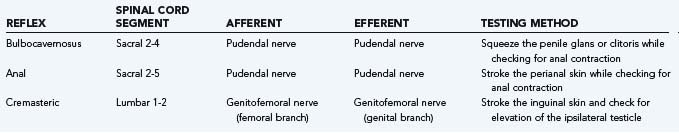
Neuromuscular reflexes are tested to evaluate the integrity of the sensorimotor pathways known as the segmental reflex arcs.26 The sacral reflexes are specialized cutaneous reflexes that permit direct evaluation of sacral spinal cord segments and are outlined in Table 15-1. The bulbocavernosus reflex represents the function of sacral cord segments S2-4. It is elicited by gently squeezing the glans in men or compression of the clitoris in women while checking for contraction of the anal sphincter. Alternatively, pulling gently on an indwelling urethral catheter provokes the afferent pathway. The reflex is mediated by pudendal or pelvic nerve afferents and pudendal nerve efferents. It may be absent in patients with sacral or peripheral nerve injury and in 10% to 15% of normal patients.27 The anal reflex reflects the function of sacral nerves S2-5 and is elicited by stroking the perianal skin, which causes anal contraction. External anal sphincter function can be considered to represent all of the perineal striated musculature.22 The cremasteric reflex (L1-2) can be activated by stroking the inner part of the thigh in a downward direction; it triggers elevation of the ipsilateral testicle as the motor response via activation of the cremasteric muscle. Deep tendon reflexes should also be evaluated (see Table 15-1).
Laboratory Testing
When evaluating a patient with voiding dysfunction secondary to a presumed neurological abnormality, electrolyte, blood urea nitrogen, and creatinine levels should be determined, and urinalysis with culture should be performed. Serum creatinine is a measure of renal function, although it will remain at normal levels until approximately 50% of the total glomerular filtration rate has been lost.28 Abnormal renal function testing results can indicate high-pressure bladder storage, vesicoureteral reflux, intrinsic preexisting renal disorders, or prerenal azotemia.23 Urinalysis and urine culture with sensitivity testing are necessary in all patients with voiding symptoms. Cytologic evaluation of urine should be performed on patients with indwelling catheters, hematuria, and risk factors for urothelial carcinoma such as smoking.
Radiologic Studies
Upper Urinary Tract Imaging
The most devastating complications of neurourologic diseases are related to deterioration of the upper urinary tract, which leads to progressive silent renal failure. Patients with known neurogenic voiding dysfunction or injury that could potentially compromise the lower urinary tract should be routinely screened with upper tract imaging. An upper tract study should be performed at the initial evaluation. Although there is no standard for routine imaging, many experts recommend yearly upper tract imaging, with less frequent evaluation in patients with stable neurological disorders.29,30 Any change in neurological symptoms or voiding habits requires reevaluation of both the lower and upper urinary tracts. Common abnormalities detected by radiologic imaging include hydronephrosis, chronic pyelonephritis or renal scarring, vesicoureteral reflux, and renal calculi.
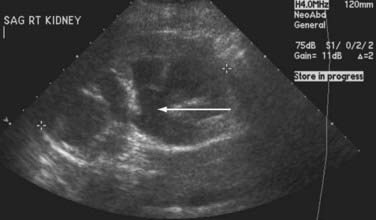
The primary screening modality most frequently used is ultrasound. Although it is limited in evaluation of the ureters and provides no functional information about the kidney, ultrasound is not invasive and does not subject the patient to radiation. It may reveal mass lesions, stones, or hydronephrosis (Fig. 15-7). Excretory urography or intravenous pyelography can provide more information about renal function and urinary tract anatomy. Similarly, contrast-based imaging with higher resolution computed tomography can also be used for the evaluation of renal function and renal, ureteral, and bladder abnormalities. Nuclear isotope renal scanning provides information about scarring or chronic pyelonephritis and can measure differential renal function and excretory function as it relates to obstruction of the upper urinary tract.
Lower Urinary Tract Imaging
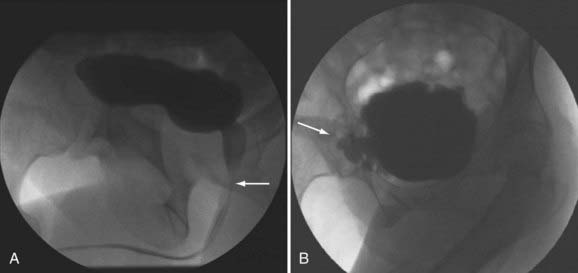
Imaging of the lower urinary tract is commonly performed by direct visualization with cystoscopy or by radiologic methods such as cystography. A voiding cystourethrogram gives information regarding the presence of vesicoureteral reflux and the morphologic characteristics of the bladder, bladder neck, proximal urethra, and striated sphincter during urine storage, bladder filling, and voiding (Fig. 15-8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree