CHAPTER 353 Nonatherosclerotic Carotid Lesions
Carotid Body Tumors
In 1743, von Haller1 reported the anatomy of the carotid body. He described the carotid body as a nodule about the size of a kernel of wheat at the bifurcation of the common carotid artery (CCA). Anatomic dissection revealed that the structure was contained in a dense meshwork at the base of the carotid bifurcation.2 Believing that the structure was nerve tissue, he called it the “intercarotid ganglion.” In 1862, Luschka, as discussed by Balfour and Wildner,2 characterized the carotid body microscopically after examining hundreds of specimens. He described it as 5 to 7 mm long, 2.5 to 4 mm wide, and 1.5 mm thick and as having the primary histologic characteristics of a gland. Years later, Kohn defined the carotid body as chromaffin cells embedded in the sympathetic nerve fibers about the carotid artery and coined the term paraganglion.3
Lahey and Warren4 reported that Marchand attempted the first surgical resection of a carotid body tumor in 1880. The report detailed an aggressive tumor resection with division of the carotid artery; jugular vein; and vagus, hypoglossal, and sympathetic nerves. The patient died 3 days after surgery. Lahey and Warren4 also noted that in 1886 Maydl reported excising a carotid body tumor with division of the common, internal, and external carotid arteries. The patient developed aphasia and hemiplegia. These early attempts included ligation and resection of the carotid artery bifurcation but no attempts at reconstruction. It was not until 1889 that Albert first resected a carotid body tumor (the size of an apple) by dissecting the tumor, preserving the internal carotid artery (ICA), and ligating the external carotid artery (ECA). The tumor recurred as its original site within a year and required re-excision, as reported by Keen and Funke5 and Paltauf.6 In 1917, Lund7 described the first successful resection of bilateral carotid tumors.
By the 1960s and 1970s, several hundred cases of carotid body tumors had been reported, but the complication rates associated with surgical treatment were still high. The larger series reported mortality rates of 5% to 15% with surgical excision, cerebrovascular complications in 8% to 20% of patients, and postoperative cranial nerve injuries in 32% to 44% of patients.8 These reports, however, preceded the widespread use of intraoperative electroencephalography (EEG) and cerebral blood flow monitoring techniques to determine the usefulness of shunting during carotid cross-clamping. Today, early diagnosis with gadolinium-enhanced magnetic resonance imaging (MRI) of the neck, improved surgical and endovascular techniques, and radiotherapy adjuncts have considerably reduced the morbidity rates associated with treating these tumors.
Anatomy
The carotid body is a light-brown structure within the adventitial layer of the dorsal aspect of the CCA bifurcation. The vascular supply is from the carotid bifurcation, and the primary innervation is the carotid sensory branch of the glossopharyngeal nerve. This branch is also known as the carotid sinus nerve or the nerve of Hering.9 The carotid body is connected to the CCA bifurcation by a fibrovascular band called the ligament of Mayer. Embryologically, the carotid body may have constituents of both the third branchial arch mesoderm and neural elements of the neural crest ectoderm.10
Carotid bodies are part of a group of neuroepithelium-derived aggregates known as paraganglia. Tumors of these cells have also been known as paragangliomas, glomus tumors, and chemodectomas. Tumors of similar histology arise in several locations, including the ciliary body of the orbit, aortic-pulmonary paraganglia, carotid body (glomus caroticum), middle ear (glomus tympanicum), ganglion nodosum of the vagus nerve (glomus vagale), jugular body (glomus jugulare), and adrenal medulla (pheochromocytoma). Although most glomus tumors appear to be composed of nonchromaffin cells that are physiologically silent, some contain secretory granules that may secrete catecholamines similar to pheochromocytomas.10
Histology
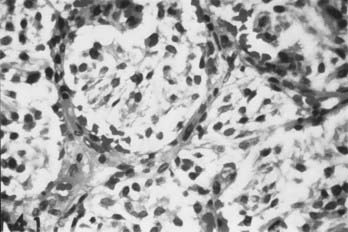
Carotid body tumors consist of epithelioid cells grouped into cords or clusters, also known as Zellballen. Typically, the cells are polyhedral with granular cytoplasm (Fig. 353-1). The stroma is composed of a fine mesh of reticulin fibers.11 Intertwined is a rich network of capillaries, giving the general appearance of a glomerulus. Several reports have detailed the chromaffin-positive nature of the secretory granules, indicating that the carotid body and tumors of it may be capable of secreting catecholamines such as norepinephrine and dopamine.12,15
The angiogenic nature of carotid body tumors is a central issue in the pathophysiology of these lesions, and ultimately it dictates many aspects of their management. Vascular endothelial growth factor (VEGF) and platelet-derived endothelial cell growth factor (PD-ECGF) are expressed in carotid body tumors and may contribute to the extreme vascularity of these tumors.16
The influence of estrogens is another environmental factor that may accelerate the development of carotid body tumors. In high-altitude populations, carotid body tumors show a marked female preponderance, as high as 12 : 1,17 and even at sea level, the predominance of carotid body tumors in women is still apparent, at a ratio of 2 : 1.18 This suggests an interaction between hypoxic and estrogenic factors in the development of carotid body tumors. This interaction might occur at the level of VEGF or PD-ECGF synthesis, both of which are regulated by hypoxia as well as estrogen level.19–22
Genetics
A genetic cause of carotid body tumors is highly suggested from examples of familial occurrence. In the sporadic form, the rate of bilateral tumor occurrence is 5%. An autosomal dominant expression of bilateral tumors, however, occurs in 32% of patients with a familial occurrence.23,24 Comorbid cervical paragangliomas (glomus vagale, glomus jugulare) are relatively common in patients with carotid body tumors. Gardner and coworkers25 reviewed 11 patients with carotid body tumors, 3 of whom had other head and neck paragangliomas.
Seminal genetic studies have indicated an increased expression of the oncogenes c-myc, bcl-2, and c-jun in most carotid body tumor specimens studied.26 Wang and colleagues27 suggested that the expression of the oncoprotein bcl-2 may deregulate apoptosis, thus suppressing programmed cell death, a critical step in the tumorigenesis of carotid body tumors.
Clinical Presentation
The most common manifestation of carotid body tumors is a palpable neck mass in the high cervical region. Less common initial presentations include cranial nerve deficits such as laryngeal dysfunction (hoarseness), difficulty swallowing, and unilateral tongue atrophy or weakness. These symptoms reflect the tumor’s proximity to the vagus and hypoglossal nerves. There have also been reports of patients who became symptomatic with Horner’s syndrome28 or carotid sinus syndrome.29
There is no reported sex predominance in carotid body tumors. Typically, diagnosis is between 30 and 60 years of age. The youngest reported patient was 7 years old.30,32
Radiographic and Medical Evaluation
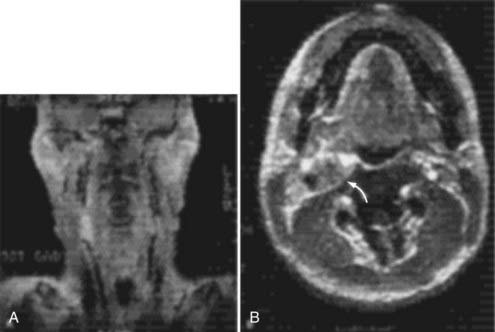
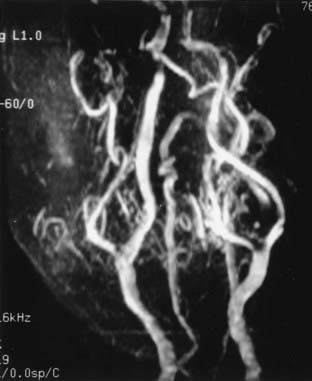
Cervical MRI with and without gadolinium can help differentiate carotid body tumors from other lesions that arise in the same region: carcinomas, enlarged lymph nodes, branchial cleft cysts, and leiomyomas.29,33 MRI demonstrates a brightly enhancing, well-circumscribed mass at the cervical carotid bifurcation (Fig. 353-2). Magnetic resonance angiography can be useful by displaying the hypervascular nature of the tumor and the characteristic splaying of the ECA and ICA (Fig. 353-3).
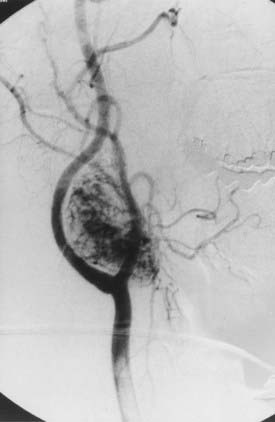
Angiography can help confirm the diagnosis. These tumors are extraordinarily vascular (Fig. 353-4). The ascending pharyngeal artery often provides most of the vascular supply, and the superior thyroid artery almost always provides a minority contribution. The blood supply of the tumor can also be derived from the ICA, vertebral artery, and thyrocervical trunk. If angiography is planned, a concomitant session to embolize the tumor can be coordinated. Studies have shown that preoperative embolization significantly reduces blood loss during resection of a carotid body tumor.34,35
An evaluation of the endocrine system may be warranted, particularly in patients who have elevated blood pressure or tachycardia. Clinicians must be aware of the potential comorbidity of a pheochromocytoma and that some carotid body tumors secrete catecholamines. In such patients, α-adrenergic blockade must be induced pharmacologically 2 weeks before surgery. Once this blockade is established, β-adrenergic blockade is recommended to control heart rate and cardiac arrhythmias.29 Careful preoperative assessment of blood pressure may indicate the need for urine metanephrine analysis.
Characteristics of Tumor Growth
In an effort to assess the preoperative risks of tumor resection, Shamblin and associates36 revised a classification scheme for carotid body tumors. Group I tumors are restricted to the space between the ICA and ECA. Generally, these tumors dissect easily from the surrounding structures. Neither the carotid vessels nor adjacent nerves travel through the tumor. Group II tumors partially involve the ECA and ICA but do not encase the arteries completely. Tumor involving the adventitia of the artery can still be dissected free. Finally, group III tumors totally encase the ICA and invade the adventitia into the muscularis. Dissecting group III tumors from the artery requires reconstruction or sacrifice of the ICA. In this challenging group, the superior laryngeal and hypoglossal nerves may traverse the tumor.
Metastasis
Most carotid body tumors are benign. They are problematic only in their local invasion of vascular and nervous structures and, occasionally, the oropharynx. Staats and coworkers31 reviewed 500 patients and found a 6.4% incidence of malignancy with local or distant metastases. Distant metastases have been reported in lymph nodes, bone, lung, liver, pancreas, thyroid, kidney, brain, and breast.37–39
Surgical Treatment
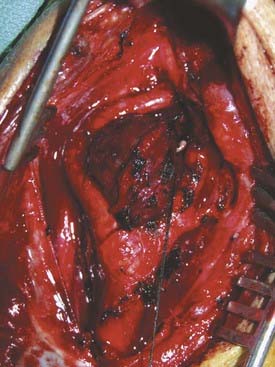
The initial incision primarily reflects the size and extent of the tumor. For example, a small group I tumor may be accessed through a transverse incision along a cervical skin crease, giving the best cosmetic result. Larger tumors with group II or III extension require a larger exposure to control the proximal and distal carotid artery for possible shunting. Here, an oblique incision along the medial border of the sternocleidomastoid muscle is indicated. As in a standard exposure for carotid endarterectomy, the common facial vein is ligated and cut to mobilize the jugular vein laterally. The hypoglossal and vagus nerves are identified. Frequently, the hypoglossal nerve may be displaced superiorly and posteriorly by the tumor8; consequently, care must be exerted during dissection of the superior aspect of the tumor to avoid damaging this nerve (Fig. 353-5). In the more challenging group III tumors, nerves may actually traverse the tumor, making dissection difficult and tedious. In rare cases, the vagus nerve can be involved with the tumor. Tumor invasion into the carotid arterial wall requires temporary occlusion with, or without shunting, to dissect the tumor and, if necessary, top patch the arterial wall defect with a graft.8
Patetsios and colleagues40 reported that in their 30 years of experience with carotid body paragangliomas (29 patients with 34 tumors), complications included two arterial thromboses (7%), five permanent cranial nerve deficits (17%), and one death (3%). There were no strokes. Vascular reconstruction was necessary in eight cases (28%).
Preoperatively, the patient’s blood pressure should be monitored closely, particularly immediately after surgery. Labile blood pressure, presumed to be secondary to baroceptor failure from loss of carotid sinus function, has been reported after resection of a carotid body tumor.41,42 Netterville and coworkers42 reviewed 30 patients with 46 carotid body tumors and found baroreceptor failure in 10 patients. Interestingly, first bite pain occurred in 10 of 25 patients assessed postoperatively.
The role of radiotherapy in managing carotid body tumors remains unclear. Radiotherapy is not used as the sole primary treatment of carotid body tumors, but it is the preferred therapy in recurrent or residual tumor with intracranial extension.8,35,43 A retrospective study by Krych and coworkers44 reported that in 33 patients with paragangliomas who were treated with external-beam radiotherapy or stereotactic radiosurgery, the 10-year tumor control rate was 92%. Radiosurgery may be useful for a metastasis or intracranial extension, however, no significant series of this relatively rare pathology has been reported to date. It has been shown that radiation therapy does not eradicate the tumor cells and probably exerts its major effects by inducing a vasculitis within tumor vessels.45
Fibromuscular Dysplasia
Fibromuscular dysplasia (FMD) can be a unifocal or multifocal disease that manifests as arteriopathy of medium to larger arteries. In 1938, Leadbetter and Burkland46 reported the first clear description of FMD in the pathologic findings of a patient who had hypertension secondary to FMD of the renal artery. In fact, most cases of FMD in the literature involve the renal artery. In 1964, Palubinskas and Ripley47 provided the first angiographic description of FMD of the extracranial ICA in an addendum to their report of FMD in extrarenal arteries. Several reports of cephalocervical FMD followed in the 1960s and 1970s, particularly of the extracranial ICA and vertebral artery.48–53 Subsequent reported cases of intracranial artery involvement with FMD have expanded our understanding of the disease process.54,55 Two major institutional reviews of cerebral angiography, one reviewing 6100 angiograms, the other 13,955, found 0.53% and 0.6% incidences of FMD, respectively.56,57
Histologic studies indicate that the arterial dysplasias present as three primary pathologies: intimal fibroplasia, medial fibroplasias, and subadventitial hyperplasia.58 In medial fibroplasias, also known as fibromuscular hyperplasia, the expression of the disease ranges from fibrodysplasia limited to the outer media to complete involvement of the entire media. Collagen and ground substance accumulate in the inner media in the milder form of the disease, separating in the disorganized smooth muscle cells. In the severe form, the media is profoundly disorganized, with fibroblasts and collagen replacing the smooth muscle.59
Etiology
The exact cause of FMD is becoming better defined as we begin to understand the multifactorial pathology, with a spectrum of phenotypic expressions. Its prevalence in women is much higher than in men, with an approximate 9 : 1 ratio. Stanley and colleagues59 suggested that progestin and estrogen play a role in the development of the disease because 94% of the patients they reviewed were women. Laboratory studies have demonstrated that sex hormones may contribute to the formation of FMD directly by local alterations of the vessel wall (endothelial proliferation, intimal thickening, and focal nodular thickening of the intima, media, and adventitia) or indirectly through an immune response.60,63 However, in a case-control study of 33 patients, endogenous or exogenous sex hormones did not appear to play a role in the etiology of FMD, whereas cigarette smoking and genetics did appear to be linked to the development of FMD.64 Mettinger’s familial analysis of patients with FMD suggests that is an inheritable dominant trait with limited penetrance in men.65,66
Mechanical and anatomic features have also been evaluated in the development of FMD. Stanley and colleagues59 implied that mechanical stresses on the vessel walls play a role in the development of FMD. Likewise, the disease appears to involve primarily arteries with a paucity of vasa vasorum, indicating that arterial wall ischemia may contribute to disease progression. Long-term follow-up of some cases has indicated definite progression of the disease in some patients with carotid FMD.52
Clinical Presentation
Patients with cervical carotid FMD typically present in their 50s to 60s. On physical examination, about two thirds of symptomatic patients have a cervical bruit that is audible on ausculation.56 Symptoms of cerebral ischemia have been reported in 18% to 56% of patients.67 In such cases, the sequential arrangement of fibromuscular rings reduces blood flow while increasing turbulence within the vessel. In addition to ischemic symptoms, FMD has been associated with spontaneous carotid artery dissection,68,69 aneurysm formation,70,71 carotid-cavernous fistula,72,73 and thromboembolism.56 In Mettinger’s review of 284 patients with aortocranial FMD, a surprising 21% had associated intracranial aneurysms.66
Angiographic Findings
In 1977, Osborn and Anderson58 described the angiographic appearance of carotid FMD variants in a summary report. The classic angiographic appearance, present in the more than 80% of patients, is the “string of beads”—multiple, irregularly spaced arterial constrictions with normal or ectatic intervening segments. Angiographically, it is similar to stationary arterial waves or circular spastic contractions, although these show more regularly spaced constrictions with no associated dilated segments. The second variant is that of smooth, concentric tubular stenosis. Angiographically, this variant can be distinguished from Takayasu’s arteritis, which affects the proximal segments of the aortic branches. In contrast, FMD typically spares the origins. The third variant, termed atypical FMD by Houser and Baker,48 usually involves one wall of the affected segment, creating a smooth or corrugated outpouching of the vessel.
Treatment
A report by DeBakey’s group in 1968 described the surgical luminal dilation of the ICA in 12 patients and of the vertebral artery in 1 patient.74 This report is interesting from a historical perspective, but it also documents intraluminal pressure at the proximal and distal ends of the affected segments in 2 patients. Before dilation, they found pressure before 25 and 50 mm Hg; after surgical treatment, there were no discernible gradients. These findings demonstrated that multiple tandem rings, as seen in FMD, significantly reduce arterial pressure and blood flow, although individual stenotic rings do not narrow the carotid artery significantly.
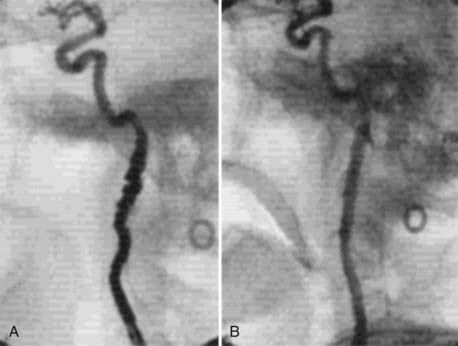
Contemporary treatment has used the endovascular techniques of balloon angioplasty and stenting for medically refractory cervical FMD lesions (Fig. 353-6). In the early 1980s, several reports documented the successful treatment of FMD by percutaneous balloon angioplasty.75,79 By the mid-1980s, the limitations of this technology were revealed in reports of complications of percutaneous transluminal angioplasty of FMD, including dissection80 and aneurysm formation.81 The transfemoral access route has significantly reduced the morbidity of endovascular procedures. Subsequently, endoluminal stenting, performed in coordination with angioplasty for cervical FMD, has become increasingly more prominent as the primary treatment method in medically refractory cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree