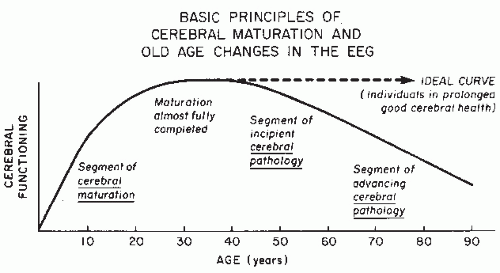
|
Premature (24-27 Wk) |
Premature (28-31 Wk) |
Premature (32-35 Wk) |
Full-Term Newborn (36-41 Wk) |
Continuity |
Discontinuous, long flat stretches |
Discontinuous |
Continuous in waking state and REM sleep, discontinuous in non-REM sleep |
Continuous except for tracé alternant in non-REM (quiet) sleep |
Interhemispheric synchrony |
Short bursts in synchrony |
Mostly asynchronous activity leads |
Partly synchronous, especially in occipital |
Minor asynchronies still present |
Differentiation of waking and sleeping |
Undifferentiated |
Undifferentiated |
Waking distinguished from sleep early in the period, then differentiation of non-REM and REM sleep |
Good |
Posterior basic (alpha) rhythm |
None |
None |
None |
None |
Slow activity (awake) |
Very slow bursts, high voltage (state of vigilance undifferentiated) |
Very slow activity predominant |
Slow (delta) with occipital maximum |
Slow (delta), mostly of moderate voltage |
Temporal theta |
Present and increasing |
Prominent |
Decreasing and disappearing |
Disappearing or absent |
Occipital theta |
Prominent |
Decreasing |
Decreasing |
Absent |
Fast activity (awake) |
Very little beta activity |
Frequent ripples or brushes around 16 per second |
Frequent ripples or brushes (16-20 per second) |
Decreasing ripples, sparse fast activity |
Low voltage |
Long flat stretches |
Flat stretches, mainly asynchronous |
Low-voltage record suspect of serious cerebral pathology |
Very low voltage records are due to severe cerebral pathology; prognosis ominous |
Hyperventilation |
Not feasible |
Not feasible |
Not feasible |
Not feasible |
Intermittent photic stimulation |
Unknown |
Unknown |
Unknown |
Driving response below 4 flashes/second may occur, not easily elicited |
Drowsiness |
Undifferentiated |
Undifferentiated |
Undifferentiated |
Undifferentiated |
Tracé alternant |
None |
None |
Present in non-REM (quiet) sleep |
Present in non-REM (quiet) sleep |
Spindles |
None |
None (but ripples present) |
None (but ripples present) |
None (but scanty ripples) |
Vertex waves and K complexes |
None |
None |
None |
None |
Positive occipital sharp transients of sleep |
None |
None |
None |
None |
Slow and fast activity in sleep |
Slow activity of high voltage, little slow activity (stage of vigilance undifferentiated) |
Much slow activity, more irregular; little fast activity |
Irregular slow activity of occipital predominance |
Much delta and theta activity, continuous in REM sleep |
REM sleep |
Undifferentiated |
Undifferentiated |
Continuous slow activity; oculographically, REM present |
Continuous slow activity, REM in EOG (more REM or “active” than non-REM sleep) |
Rhythmical frontal theta activity (6-7 per second) |
None |
None |
None |
None |
14 and 6 per second positive spikes |
None |
None |
None |
None |
Psychomotor variant (marginal abnormality) |
None |
None |
None |
None |
Sharp waves, spikes |
Some intermixed sharp activity in bursts (normal) |
Some intermixed sharp activity (normal) |
Often prominent sharp waves or spikes (normal) |
Some minor sharp transients (normal) (abnormal spikes more consistent and prominent) |
Infancy (2-12 mo) |
Early childhood (12-36 mo) |
Preschool age (3-5 yr) |
Older children (6-12 yr) |
Adolescents (13-20 yr) |
Continuous |
Continuous |
Continuous |
Continuous |
Continuous |
No significant asynchrony |
No significant asynchrony |
No significant asynchrony |
No significant asynchrony |
No significant asynchrony |
Good |
Good |
Good |
Good |
Good |
Starting at age 3-4 mo at 4 per second, reaching about 6 per second at 12 mo |
Rising from 5-6 per second to 8 per second (seldom 9 per second) |
Rising from 6-8 per second to 7-9 per second |
Reaching 10 per second at age 10 yr |
Averaging 10 per second |
Considerable |
Considerable |
Marked admixture of posterior slow activity (to alpha rhythm) |
Varying degree of posterior slow activity mixed with alpha |
Posterior slow activity diminishing |
None |
None |
None |
None |
None |
None |
None |
None |
None |
None |
Very moderate |
Mostly moderate |
Mostly moderate |
Mostly moderate |
Moderate, except for low voltage fast records |
Uncommon, usually abnormal |
Uncommon, usually abnormal |
Uncommon, usually abnormal |
Seldom as variant of normalcy |
Occasionally and (at end of teenage period more often) as variant of normalcy |
Not feasible |
Mostly not feasible |
Often marked delta response |
Often marked delta response |
Delta responses become less impressive |
Improving driving to low flash rates after age 6 mo |
Often good driving response to low flash rates |
Often good driving response to low flash rates |
Often good driving response, chiefly at medium flash rates (8-16 per second) |
Often good driving response, chiefly at medium flash rates |
Around age 6 mo, appearance of rhythmical theta |
Marked “hypnagogic” rhythmical theta (4-6 per second) |
Rhythmical theta gradually vanishing, other types of slow activity predominant |
Gradual alpha dropout with increasing slow activity |
Gradual alpha dropout with low-voltage stretches (mainly slow) |
Disappears in first (seldom second) month |
None |
None |
None |
None |
Appear after second month; 12-15 per second, sharp, shifting |
In second year, sharp and shifting, then symmetrical with vertex maximum |
Typical vertex maximum |
Typical vertex maximum |
Typical vertex maximum |
Appear mainly at 5 mo, fairly large, blunt |
Large, becoming more pointed |
Large with an increasingly impressive sharp component |
Large with a prominent sharp component |
Not quite as large, sharp component not quite as prominent |
None |
Poorly defined |
Poorly defined |
Still poorly defined but gradually evolving |
Often very well developed |
Much diffuse 0.75-3 per second activity with posterior maximum; moderate fast activity |
Marked posterior maximum of slow activity; often a good deal of fast activity |
Predominant slowing but less prominent posterior maximum |
Much diffuse slowing, slightly decreasing voltage |
Much diffuse slowing with further attenuation of voltage |
REM portion decreasing; mostly slow activity |
Mostly slow, starting to become more desynchronized |
Slow activity with some desynchronization |
Less slowing and increasing desynchronization |
Mature desynchronization |
None |
Seldom in third year of life |
May occur, not very common |
A bit more common |
A bit more common, declining at end of period |
None |
Rare |
May occur, not very common |
Fairly common |
Fairly common |
None |
None |
Probably none |
Uncommon |
More common (although relatively rare) |
Essential as abnormal phenomena |
Spikes in seizure- free children, mainly occipital (mild abnormalities) |
Spikes in seizure-free children, mainly occipital, also Rolandic (slight abnormalities) |
Spikes in seizure-free children, mainly Rolandic (central-mid-temporal), slight to moderate abnormalities; physiological occipital spikes in congenitally blind children |
Benign Rolandic spikes usually disappear before beginning of this period |