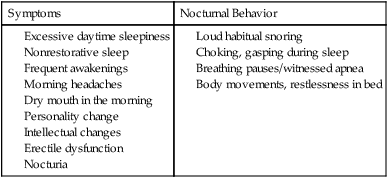
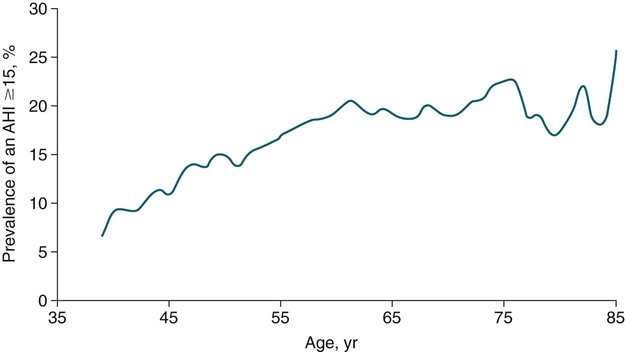
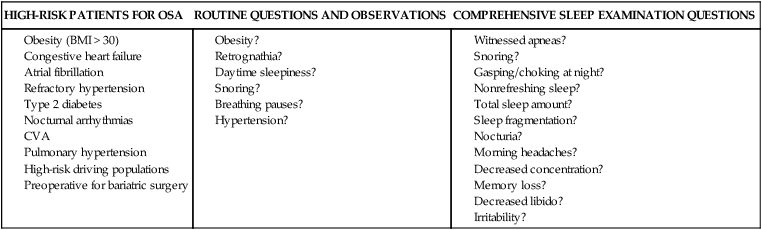
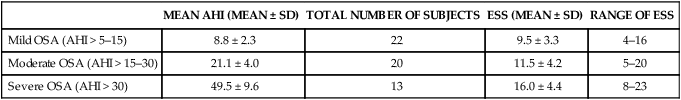
• Risk factors for the presence of OSA include obesity, male gender, older age, and postmenopausal status (not on HRT). Hypothyroidism, acromegaly, cigarette smoking, and chronic alcohol use are also considered risk factors but the evidence is less compelling. • A significant proportion of patients diagnosed with OSA based on an increased AHI do not complain of daytime sleepiness. • A high Mallampati (or modified Mallampati) score for the upper airway is a predictor of the presence of OSA. • Patients with habitual snoring, witnessed apnea or gasping, hypertension, and a large neck circumference are at increased risk for having OSA. • Questionnaires are not sufficiently sensitive or specific to obviate the need for objective testing to determine the presence or absence of OSA. • In patients with milder OSA, the amount of supine and REM sleep may have a large impact on the overall AHI. • The two groups of patients with OSA who have daytime hypercapnia are those with the OHS and the combination of obstructive airways disease (COPD) and OSA. • Approximately 80% of patients with the OHS have OSA. The other 20% have sleep-related worsening of daytime hypercapnia and hypoxemia with relatively few discrete apneas or hypopneas. • Patients with OSA and obstructive airway disease (COPD) may have severe arterial oxygen desaturation during sleep and daytime hypercapnia. • Pediatric patients with OSA often present with a different symptom complex than that of adults. Common manifestations include daytime hyperactivity, labored breathing during sleep, diaphoresis, and paradoxical chest movement. Enuresis can also be a manifestation of pediatric OSA. • The age range of highest prevalence of pediatric OSA patients is typically from 2 to 8 years when hypertrophy of the adenoids and tonsils occurs. • The values of respiratory indices considered to be diagnostic of pediatric OSA vary but are typically an obstructive AI > 1/hr or obstructive AHI > 1–2/hr. The decision to treat is based on symptoms as well as PSG findings. The obstructive sleep apnea (OSA) syndrome was first recognized as a significant health problem only over the last half of the 20th century. In 1956, Burwell and coworkers1 used the term pickwickian syndrome to describe individuals with obesity, hypersomnolence, hypercapnia, cor pulmonale, and erythrocytosis. The term pickwickian was based on the character Fat Boy Joe from Charles Dickens’ The Posthumous Papers of the Pickwick Club (1837), who was markedly obese and tended to fall asleep uncontrollably during the day. The current terminology describing such individuals is the obesity hypoventilation syndrome (OHS). We now know that such patients represent only 10% to 15% of the total number of patients with OSA. Guilleminault and colleagues2 described the OSA syndrome in patients with daytime sleepiness and obstructive apneas on polysomnography (PSG). An apnea index of 5/hr or greater was considered abnormal.3 An apnea was defined as absent airflow at the nose and mouth for 10 seconds or more. Obstructive apneas are secondary to airway closure at a supraglottic location that reverses at apnea termination often associated with a brief awakening (arousal).4 Obstructive apneas are followed by a fall in arterial oxygen saturation (SaO2) of varying severity. It was soon realized the episodes of reduced airflow and tidal volume (hypopneas) that are the result of upper airway narrowing are also clinically significant.5,6 Patients with primarily hypopneas had the same symptoms, arousals, and arterial oxygen desaturation as patients with obstructive apneas. The term obstructive sleep apnea hypopnea syndrome (OSAHS) has been used to be more inclusive. However, many clinicians still use the term obstructive sleep apnea (OSA) to refer to the syndrome and the term OSA is used in this chapter with the understanding that patients may have variable amount of apneas and hypopneas. As discussed in Chapter 8, the definition of hypopnea has varied considerably.7,8 The American Academy of Sleep Medicine (AASM) scoring manual9 recommends scoring apnea on the basis of an oronasal thermal sensor and hypopnea on the basis of nasal pressure monitoring. The recommended hypopnea definition requires a 30% reduction in nasal pressure signal for 10 seconds or longer in association with a 4% or greater arterial oxygen desaturation. The alternative definition of hypopnea requires a 50% or greater reduction in the nasal pressure signal associated with either an arousal or a 3% or greater arterial oxygen desaturation. Patients with OSA have variable proportions of obstructive, mixed, and central apneas as well as hypopneas. As discussed in Chapter 8, the apnea-hypopnea index (AHI) is defined as the number of apneas and hypopneas per hour of sleep. The respiratory disturbance index (RDI) is the number of apneas, hypopneas, and respiratory effort–related arousals (RERAs) per hour of sleep (RDI = AHI + RERA index). RERAs are events associated with increased respiratory effort (as evidenced by esophageal pressure manometry or flattening of the nasal pressure signal) for 10 seconds or longer that are associated with arousal but do not meet criteria for hypopnea9 (see Chapter 8). Whereas the recent AASM scoring manual defines RERAs, the manual does not define the term RDI. The Centers for Medicare and Medicaid Services (CMS) guidelines10 regarding reimbursement criteria for continuous positive airway pressure (CPAP) define the RDI as the number of respiratory events per hour of monitoring time. This definition refers to the respiratory event index obtained from portable monitoring (PM) devices that do not record sleep. To add to the confusion, some publications and sleep centers use the term RDI as the number of apneas and hypopneas per hour of sleep. Therefore, careful attention to the respiratory event definition is required when reading the literature or a sleep study interpretation. A commonly used diagnostic criteria for OSA syndrome has been an AHI ≥ 5/hr associated with symptoms or an AHI ≥ 15/hr with or without associated symptoms.11,12 Some diagnostic criteria have used the RDI ([apneas + hypopneas + RERAs]/hour of sleep) instead of the AHI. The International Classification of Sleep Disorders, 2nd edition (ICSD-2), criteria for a diagnosis of the OSA syndrome in adults13 (Box 15–1) requires either scoreable respiratory events (apneas, hypopneas, or RERAs) of 5/hr or greater with associated symptoms or scoreable respiratory events of 15/hr or greater with or without symptoms. Of note, the ICSD-2 criteria mentions only scoreable respiratory events (apneas, hypopneas, RERAs) per hour of sleep and does not define the parameter “RDI.” Prevalence is defined as the proportion of a population with a condition. The prevalence of OSA depends on the defining RDI or AHI criteria, the definition of hypopnea, the method used to detect airflow, and presence or absence of a requirement that symptoms be present. The Wisconsin-based cohort study of state employees younger than 65 years of age found a prevalence of sleep-disordered breathing (SDB) defined as an AHI of 5/hr or greater (with hypopneas based on a definition of discernible change in airflow and ≥ 4% desaturation) to be 9% in women and 24% in men.14 The sleep apnea syndrome, defined as the presence of both SDB and self-reported sleepiness, was present in 2% of women and 4% of men. Bixler and associates15 found a 17% and 7% prevalence of an AHI greater than 5/hr in and 15/hr in men, respectively. Higher prevalence rates may be present in referral or clinical populations. It is estimated that in Western countries, up to 5% of the population have an undiagnosed OSA syndrome (elevated AHI and symptoms).16 Another publication in 1997 presented data that 93% of women and 82% of men with moderate to severe OSA were undiagnosed.17 There is also evidence that OSA severity can progress with time. Analysis of 8-year follow-up of 282 participants of the Wisconsin cohort study showed a mean increase in the AHI from 2.6 events/hr to 5.1.16 In obese individuals with a body mass index (BMI) over 30, the mean AHI increased from 4.8/hr to 10.1/hr. However, not all studies of untreated patients with OSA have also shown progression in AHI severity. A number of population-based studies have documented several risk factors for the presence of OSA (Table 15–1).16 Of these, the most consistent findings have been the presence of obesity and male gender.16,18 TABLE 15–1 Risk Factors for Obstructive Sleep Apnea OSA = obstructive sleep apnea. Adapted from Malhotra A, White DP: Obstructive sleep apnea. Lancet 2002;360:237–245. An association between AHI and obesity has been documented in many studies.16–21 Peppard and coworkers19 followed the effects of weight change on AHI. A 10% weight gain predicted an approximate 32% increase in the AHI. A 10% weight loss predicted a 26% reduction in the AHI. A 10% increase in weight was associated with a sixfold increase in the risk of developing moderate to severe OSA. Other studies have documented a decrease in the AHI with weight loss.22,23 Whether the type of obesity or areas of excess fat (neck vs. abdomen) are important is currently under investigation. Davies and colleagues21 found that neck circumference correlated with AHI better than general obesity. Prediction models for OSA have used neck circumference as a predictor.24 However, a recent study found neck circumference to correlate best with AHI in women whereas abdominal girth correlated better in men.25 In the Wisconsin cohort study by Young and associates,14,16 men had about twice the incidence of the OSA syndrome compared with women (4% vs. 2%) (AHI ≥ 5/hr + symptoms). Bixler and coworkers26 found the prevalence of OSA defined as an AHI greater than 10/hr + symptoms to be 3.9% in men and 1.2% in women (P = .0006). Analysis of the Sleep Heart Health data also showed the risk of OSA in men was greater (odds ratio 1.5).27 Further discussion of the effect of gender on the pathophysiology of OSA is contained in Chapter 16. The prevalence of OSA appears to be higher in the elderly than in middle-aged populations. The prevalence of a chronic nonfatal disease would increase as cases accumulate even if the incidence (new cases/yr) was constant or declining. Ancoli-Israel and colleagues28 studied 427 community-dwelling elderly age 65 years or older using limited channel ambulatory monitoring. A prevalence of OSA, defined as an AHI greater than 10/hr, was found to be 62%. Here, hypopneas were based on changes in flow (based on two channels of respiratory inductance plethysmography) independent of changes in the SaO2 (no oximetry). This is a prevalence of about three times that in middle-aged populations. There is evidence from the Sleep Heart Health Study that the SDB prevalence increases from age 40 to around 60.27 After that, the prevalence appears to plateau (Fig. 15–1). In older adults, the AHI may be less correlated with excessive sleepiness and increased cardiovascular risk. Enright and coworkers29 studied a large group of subjects over age 65 with questionnaires and echocardiography, ultrasound of the carotids, and an electrocardiogram. Snoring was very common but, interestingly, reported snoring seemed to decrease after age 75. Loud snoring, observed apneas, and daytime sleepiness were not associated with hypertension or the prevalence of cardiovascular disease. Conversely, decreased snoring reports could be secondary to a lower frequency of having a bed partner or hearing deficits in the elderly. It is possible that the presence of OSA in the elderly population has different implications.30 Conventional wisdom is that postmenopausal women have a greater incidence of OSA than premenopausal women. However, the confounders of increased age and often increased BMI in the postmenopausal group complicate the analysis. An analysis of the Wisconsin cohort study of the risk of having an AHI greater than 15 (adjusted for age and body habitus) found an odds ratio of 3.5 for greater risk in postmenopausal compared with premenopausal women.31 Another frequently quoted study found a greater risk but included men in the control group.26 The Sleep Heart Health Study analysis of women older than 50 years found that the prevalence of an AHI greater than 15/hr in women on hormone replacement therapy (HRT) was approximately half that of the nonusers.32 There was a higher prevalence of SDB in non-HRT users even when other factors such as age and BMI were considered. The risk was especially high in the 50- to 59-year-old group. Thus, it appears that postmenopausal women are at increased risk of developing OSA if they are not on HRT treatment. One study found that OSA is more common in African American than in white populations.33 However, this finding was not present in another study that controlled for differences in age and BMI.27 An investigation by Redline and associates34 found an increase in the risk of having OSA greater in African Americans than in whites only for those younger than age 25 years. Ip and coworkers35 found a similar prevalence of OSA in a Chinese population as in whites. The fact that OSA is common in Asian areas where obesity is much less common has led to the hypothesis that craniofacial characteristics of the Asian population might predispose to OSA.36 Whereas increasing BMI was still associated with an increased prevalence of OSA in the study of Chinese patients, the association was not as strong as that typically seen in white populations. The lack of obesity in Asian patients should certainly not discourage evaluation for possible sleep apnea. Analysis of data for the Wisconsin sleep cohort data by Wetter and colleagues37 found that current cigarette smokers are at a greater risk for sleep apnea than never smokers. Heavy smokers were at the greatest risk. Of interest, former smokers did not have an increased risk. Therefore, smoking cessation should be considered in all patients with OSA. Most studies of acute ingestion of alcohol in patients with snoring or OSA have found an increase in the AHI.38,39 Alcohol does suppress REM sleep and, in this sense, could reduce the longer events associated with REM sleep during the early part of the night. However, more severe desaturations and presumably longer events do occur in some patients in the early portion of the night if bedtime alcohol is consumed.38 Block and associates40 found effects of alcohol on breathing in normal men but not women. Stradling and coworkers20 found alcohol consumption to be associated with the presence of OSA in a group of middle-aged men. However, definite epidemiologic evidence for a worsening of OSA with chronic alcohol consumptions is lacking. It seems likely that consumption of alcohol (or abstinence from alcohol) could significantly change the AHI in individual patients. Alcohol could also prolong respiratory events. In a study of the effect of alcohol on the arousal response to mask occlusion in normal subjects, ethanol ingestion delayed the time to arousal during non–rapid eye movement (NREM) sleep.41 Most occlusions were preformed in the early part of the night when the alcohol level would have been higher. Considering the very common use of alcohol, relatively little is known about its effects on breathing during sleep. Hypothyroidism has been thought to be associated with sleep apnea.42–44 However, there are no large cohort studies evaluating the prevalence of sleep apnea in hypothyroid subjects versus euthyroid patients. Pelttari and coworkers43 examined 26 patients with hypothyroidism and 188 euthyroid control subjects, finding that 50% of hypothyroid patients and 29% of control subjects had significant respiratory events. If hypothyroidism is found in a patient with OSA, continued effective treatment is indicated until restoration of the euthyroid condition.44 Even then, one would need a sleep study to demonstrate that treatment of OSA was not needed. Whereas some physicians order thyroid function tests on all OSA patients, this is not cost effective. Winkelman and colleagues45 reviewed the results of 255 consecutive patients suspected of having OSA in whom thyroid function studies were ordered. Hypothyroidism was present in only 1.6% of patients and the frequency did not differ between those documented to have OSA and those who did not. Thyroid testing is recommended only for high-risk groups (women > 60 yr) and if there are clinical signs or symptoms suggesting possible hypothyroidism.46 Postmenopausal women with OSA or OSA patients without predisposing OSA risk factors might warrant thyroid studies. The reason hypothyroidism exacerbates OSA is unclear and possibly multifactorial. Upper airway muscle myopathy, narrowing of the upper airway by mucoprotein deposition in the tongue (macroglossia), and abnormalities in ventilatory control are possible mechanisms. Growth hormone excess resulting in acromegaly is also associated with sleep apnea. Grunstein and associates46 noted that 60% of unselected patients with acromegaly had sleep apnea. Weiss and coworkers47 found that 75% of a group with acromegaly had OSA. Independent predictors of OSA included increased activity of acromegaly (higher growth hormone), older age, and an increased neck circumference. Potential pathophysiologic mechanisms of the association between acromegaly and OSA include macroglossia and increased muscle mass of the upper airway. Patients with acromegaly may have central as well as obstructive apnea.46 Therefore, alterations in ventilatory control may also play a role. Patients with acromegaly without sleep apnea may also have daytime sleepiness as a direct manifestation of growth hormone excess. The daytime sleepiness may improve after effective treatment of the acromegaly.48 Recent clinical guidelines for the evaluation, management, and long-term care of OSA in adults recommended that high-risk populations for OSA be questioned in detail concerning symptoms of OSA.49 However, certain basic sleep questions were recommended for all patients as part of a general history and physical examination (Table 15–2). The comprehensive sleep examination questions the patient concerning the typical manifestations of OSA. A number of populations with a high prevalence of OSA have been identified including patients with refractory hypertension, congestive heart failure, and recent or past cerebrovascular accident or transient ischemic attack (see Table 15–2). TABLE 15–2 Recommendations for Evaluation of General Medical Patients and Populations at High Risk for Obstructive Sleep Apnea BMI = body mass index; CVA = cerebrovascular accident; OSA = obstructive sleep apnea. From Epstein LJ, Kristo D, Strollo PJ, et al: Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263–276. The patient or bed partner frequently reports excessive daytime sleepiness, loud habitual snoring, gasping/choking or witnessed apnea, personality change, morning headache, or nonrestorative sleep (Box 15–2). Patients may also report a progression of symptoms with recent weight gain or nasal congestion. The Epworth Sleepiness Scale (ESS), a subjective estimate of the propensity to doze off in eight situations, is often (but not invariably) increased (see Chapter 14). The range of the scale is 0 to 24 with greater than 10 indicating excessive daytime sleepiness.50 Data in Table 15–3 taken from one of the first descriptions of the ESS show that the score increases with severity of OSA (the AHI), but there was a wide range of scores at any AHI range.50 Other studies have confirmed that the correlation between the AHI and subjective or objective sleepiness, although statistically significant, is low (correlation coefficient in the range of 0.4–0.5).51,52 The wide variability in symptoms is likely due in part to different individual susceptibility to sleep fragmentation or other factors contributing to symptoms of daytime sleepiness such as medications or reduced sleep time. A number of population-based studies have found that a substantial percentage of patients with OSA do not report daytime sleepiness, and the absence of sleepiness should not discourage a further evaluation. However, patients with more severe OSA do have, on average, greater daytime sleepiness (Fig. 15–2). It is important to remember that two patients with the same AHI can have vastly different degrees of daytime sleepiness or arterial oxygen desaturation. Severe desaturation, even in the absence of daytime sleepiness, could put the patient at risk for a number of adverse cardiovascular consequences of sleep apnea. TABLE 15–3 Epworth Sleepiness Scale Scores in Mild, Moderate, and Severe Obstructive Sleep Apnea From Johns MW: Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest 1993;103:30–36. A number of investigations have studied the effect of gender on presenting symptoms of OSA.53–55 In general, men and women report many of the same symptoms. However, women may report more insomnia and less witnessed apnea. Women with OSA are also more likely to complain of depression, morning headache, awakenings, and fatigue.55 It is important to remember that patients with central sleep apnea may present with many of the same symptoms as OSA. Historical elements that may alert the clinician to possible central apnea include congestive heart failure or the use of potent narcotics. Patients with congestive heart failure can have either OSA, Cheyne-Stokes breathing, or a combination of OSA and central sleep apnea. Patients with narcotics often have central apneas at baseline or when undergoing a positive airway pressure (PAP) titration (see Chapter 21). The physical examination of patients with suspected OSA should target abnormalities known to be associated with the syndrome (Box 15–3). These include measurement of BMI and systemic blood pressure as well as careful examination of the nose, ears, and oropharynx.56–58 Observation of the oropharynx usually reveals a crowded upper airway and examination of the patient’s face in profile may reveal retrognathia (Figs. 15–3 and 15–4). Measurement of neck circumference and observation of signs of right heart failure may also be revealing. A neck size greater than 17 inches in men and 16 inches in women suggests the possibility of OSA. However, a smaller neck circumference does not rule out OSA. The Mallampati (MP) score of the upper airway was developed to predict the risk of difficult endotracheal intubation (see Fig. 15–3). The patient’s oropharynx is examined with tongue protruded. The modified Mallampati score (MMP), also called the Friedman score,56 is similar but the patient simply opens the mouth without saying “ah” or tongue protrusion. Friedman and coworkers56 found that the MMP, tonsil size, and BMI were reliable predictors of OSA. Zonato and colleagues57 found a significant correlation between the AHI and the MMP and BMI. Although retrognathia (see Fig. 15–4) was not correlated with AHI, this abnormality was more frequent in patients with severe OSA as compared with snorers. Nuckton and associates58 analyzed over 30 variables reflecting airway anatomy, body habitus, symptoms, and medical history and found the MMP and MP to be independent predictors of the presence and severity of OSA. The variables associated with an increased risk of OSA in their study included increased neck circumference, witnessed apnea, and hypertension. Laboratory testing in patients with OSA is usually not indicated apart from routine health maintenance unless a particular problem such as hypothyroidism is suspected. In patients with severe nocturnal hypoxemia, polycythemia (increased hematocrit) may be present. An unexplained elevation in the serum CO2 (composed primarily of HCO3) on electrolyte testing is suggestive of chronic compensation for hypercapnia (in the absence of evidence of causes of metabolic alkalosis).59,60 Pulmonary function testing, chest radiography, and arterial blood gas testing are indicated in patients with a low awake SaO2 or suspected hypoventilation to eliminate pulmonary causes of impaired gas exchange.60 A number of clinical indices and questionnaires have been developed to predict the presence of OSA based on symptoms, signs, and measurements. Although they have some success, they are neither satisfactorily sensitive nor specific enough to be a substitute for objective documentation of the presence of OSA by a sleep study. An adaptation of a prediction rule developed by Flemons and coworkers24,61 used an adjusted neck circumference (Table 15–4) to classify patients as low, moderate, or high probability. The population in which the prediction value was developed was predominantly male, hypopneas were defined as a reduction in airflow associated with a 3% or greater desaturation, and the presence of OSA was defined by an AHI greater than 10/hr. Netzer and colleagues62 studied the utility of the Berlin Questionnaire (Appendix 15–1) to predict whether patients were high or low risk for having OSA. The questionnaire consists of three categories: category 1 concerns snoring and witnessed apnea, category 2 concerns being sleepy/tired/fatigued more than three or four times a week or nodding off while driving a vehicle, and category 3 concerns the presence of hypertension. After questionnaire completion, patients were studied by PM. The Berlin Questionnaire identified patients with an AHI ≥ 5/hr (based on assignment to the high-risk group) with a sensitivity of 0.86 and a specificity of 0.77. The STOP-BANG (snoring, tired, observed apnea, [blood] pressure, body mass index, age, neck circumference, gender) Questionnaire is a screening tool (Appendix 15–2) that has been used for preoperative evaluation to detect sleep apnea. A study of 2467 patients found sensitivities of 84%, 92%, and 100% for AHI cutoffs of greater than 5/hr, greater than 15/hr, and greater than 30/hr.63,64 TABLE 15–4 Prediction of Obstructive Sleep Apnea From Flemons WW: Obstructive sleep apnea. N Engl J Med 2002;347:498–504. Attended PSG is the gold standard to determine whether OSA is present and to classify the severity.65 Figure 15–5 presents an obstructive apnea during REM sleep. Box 15–4 lists the classic PSG findings in patients with OSA. The abnormality in the sleep architecture varies with severity. Patients with severe OSA usually have a high arousal index, increased wake after sleep onset (WASO) and stage N1 sleep, and decreases in stage N3 or stage R sleep. A typical classification of OSA severity based on the AHI (or RDI in some sleep centers) is 5 to fewer than 15/hr mild, 15 to 30 moderate, and greater than 30/hr severe OSA. Whereas the AHI (RDI) is the most widely used index for classification of severity, it is also important to characterize the severity of arterial oxygen desaturation. A widely accepted standard for the characterization of the severity of desaturation does not exist. It is common to present the number of desaturations (usually defined as a drop in the SaO2 > 4%), the lowest SaO2, the average SaO2 at desaturation, and the time below various saturations. For example, a commonly used metric is the time at or below an SaO2 of 88%.
Obstructive Sleep Apnea Syndromes
Definitions, Epidemiology, Diagnosis, and Variants
History And Definitions
Diagnostic Criteria
Epidemiology Of Osa
Prevalence and Progression
Risk Factors
RISK FACTOR
EVIDENCE
Obesity—present in roughly 70% of OSA
+++
Male sex
+++
Aging
++
Postmenopausal state
++
Black race
+ (some studies)
Alcohol
++
Smoking
+
Obesity
Male Sex
Age
Postmenopausal Status
Ethnicity
Smoking
Alcohol Intake
Hypothyroidism
Acromegaly
Diagnosis Of Osa
HIGH-RISK PATIENTS FOR OSA
ROUTINE QUESTIONS AND OBSERVATIONS
COMPREHENSIVE SLEEP EXAMINATION QUESTIONS
OSA Symptoms and Key Historical Points
MEAN AHI (MEAN ± SD)
TOTAL NUMBER OF SUBJECTS
ESS (MEAN ± SD)
RANGE OF ESS
Mild OSA (AHI > 5–15)
8.8 ± 2.3
22
9.5 ± 3.3
4–16
Moderate OSA (AHI > 15–30)
21.1 ± 4.0
20
11.5 ± 4.2
5–20
Severe OSA (AHI > 30)
49.5 ± 9.6
13
16.0 ± 4.4
8–23
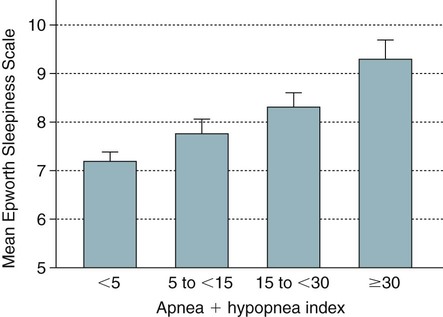
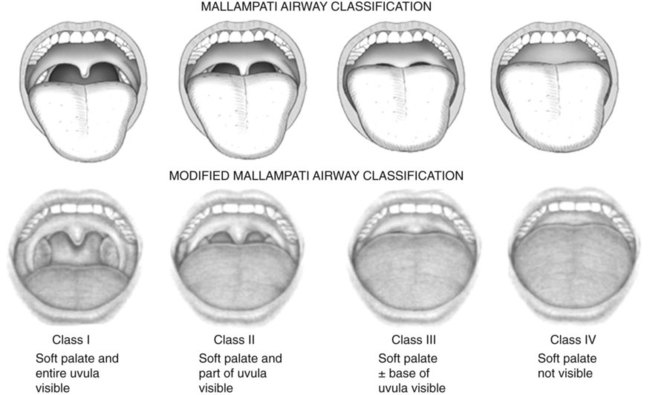
Physical Examination

Laboratory Testing in OSA
Prediction of the Presence of OSA
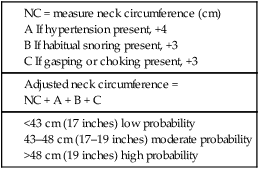
Diagnostic Testing for Suspected Sleep Apnea