Ophthalmic Pathologic Findings in Infantile Traumatic Brain Injury
Douglas J. Cameron
Vaughn M. Emerson
Autopsy examination of the eyes is essential in cases of documented or suspected head injury in infants and children because retinal hemorrhages are an important element of the clinical syndromes associated with infantile head injury (i.e., “shaken baby syndrome” or “shaken-impact baby syndrome”). The purpose of this chapter is to assist pathologists in identifying and reporting ocular findings in fatal cases of suspected inflicted injuries in children.
The association between subdural hemorrhage and multiple long-bone fractures was first reported by Caffey in 1946, who noted the connection between these injuries and the nonaccidental nature of the infliction of these injuries.1 He reported a series of six patients, 13 months of age or younger, with chronic subdural hematoma and with radiologic evidence of 23 fractures and four contusions of the long bones. In none of his cases could the skeletal lesions be reasonably attributed to the stated history of injury, and no evidence was present of generalized or localized skeletal disease that could predispose to pathologic fractures. In a follow-up report 26 years later emphasizing the potential long-term effects of permanent brain damage and mental retardation, he referred to the syndrome as parent-infant stress syndrome, battered baby syndrome, and whiplash-shaking and jerking syndrome.2 Subsequently, the term “whiplash-shaken infant syndrome” was introduced in the early 1970s.3, 4 This term encompassed the combination of subdural hemorrhages, retinal hemorrhages, and metaphyseal fractures and allegedly was caused by the violent shaking of the infant without impact to the head. In the 1980s, with the realization that physical impact was essential for the craniocerebral injury to take place, the term “shaken-impact syndrome”5, 6, 7 was proposed. The terminology of the syndrome has evolved with better understanding of the social characteristics, distribution of affected tissues, and pathophysiology of the injuries. In 1998, the term “shaken baby” was used, 8 and in 2001, the term “inflicted traumatic brain injury” was established by Keenan because of the prominence of head trauma and its sequelae in this syndrome.9 The term “inflicted trauma” is used in this chapter to apply to all of the aforementioned characteristics of the varied manifestations of child abuse. However, some authors focus on the inclusion of shaking trauma as a component of inflicted trauma because of the lower likelihood that shaking is accidental compared with impact trauma. Therefore, the term “shaken baby syndrome” refers specifically to the subset of inflicted trauma that includes shaking without excluding other mechanisms of injury.
The purpose of this chapter is to assist pathologists in identifying and reporting ocular findings in fatal cases of suspected inflicted injuries in children. A surgical pathologist may face many difficulties in obtaining, preparing, evaluating, and reporting a specimen in the setting of fatal inflicted trauma. Some of the problems include interpretation of the ophthalmologist’s notations in the clinical record, lack of familiarity with critical anatomic features of the eye of a child and the characteristics of artifacts of the infant eye, infrequently used methods of specimen procurement (enucleation) and preparation, and lack of familiarity with macroscopic patterns of abnormalities and microscopic characteristics of this particular type of trauma. In cases of suspected inflicted trauma, the diagnosis needs to be precise and informative, and the “Comment” section of the pathology report should recognize appropriate limitations.
EPIDEMIOLOGY
The incidence of child abuse is estimated to be 12.8 to 24.6 children per 100, 00010, 11 and results in death in approximately 20% of cases.12 Abusive head trauma is the most common cause of death from physical injury and is the most common cause of traumatic death in children younger than 12 months.13, 14 Up to 50% of survivors of abusive head trauma have permanent neurologic sequelae, including seizures, mental retardation, and blindness.14, 15 Ocular findings are present clinically in approximately 40% of known cases of child abuse16, 17 and by autopsy in approximately 70% of deaths resulting from child abuse.18, 19, 20 Furthermore, ocular findings are the presenting feature in 4% to 6%of cases of child abuse.16, 17
CLINICAL EYE FINDINGS IN INFLICTED HEAD TRAUMA
Examining the eyes of an injured infant, usually in an emergency room or an intensive care unit, surrounded by anxious hospital staff and relatives, is extremely difficult for all concerned. Historical information acquired by the ophthalmologist often includes the reported nature and circumstances of the injury and historical ocular information, which may include but is not limited to birth history (risk of retinopathy of prematurity and birth trauma), prior ocular trauma, ocular inflammation or ocular neoplasia (retinoblastoma), and general medical history, including previous injuries, inflammatory conditions (sepsis), or neoplasia (leukemia).
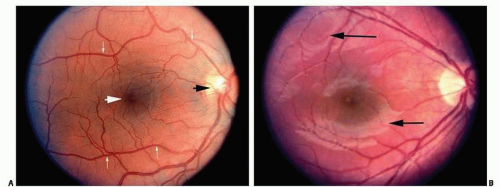
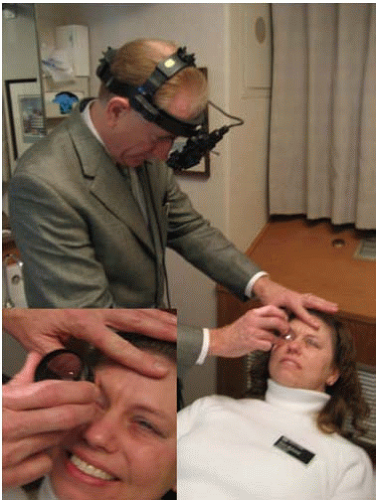
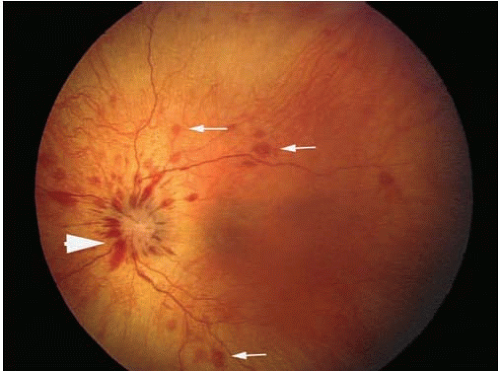
The critical ophthalmologic findings in cases of inflicted head injury are located in the posterior pole of the eye (the retina posterior to the equator of the eye) and the optic nerve (Figs. 17.1A and B). The soft tissues of the anterior orbit and the anterior portions of the eye are usually not clinically affected by shaking but may be affected by blunt force injury in certain circumstances. An ophthalmologist can perform a thorough and efficient examination of the eye, particularly the posterior segment, by using an indirect ophthalmoscope, which yields a magnified image of the retina (Fig. 17.2). Retinal hemorrhage is clinically present in 81% of children with inflicted trauma confessed by the perpetrator and in 94% of children hospitalized with subdural hemorrhage without external signs of head trauma.21 Hemorrhage is seen as reddish opacification of the retina and may be localized (Fig. 17.3) or diffuse (Fig. 17.4). Because the image is seen with both eyes of the examiner, the physician can determine the relative depth of the hemorrhage and changes of the internal contour of the retina and optic disc. The examination requires pharmacologic dilation of the pupil. Medial opacities (e.g., corneal edema or scarring, anterior chamber hemorrhage, cataract, and vitreous hemorrhage) may interfere with a clear view of the retina, requiring other techniques, such as ultrasonography, to further characterize the status of the eye. Retinal photography is frequently used to document the ophthalmoscopic findings for medical and legal purposes.
Many abbreviations and technical terms describing clinical findings may be included in the report by the ophthalmologist (Fig. 17.5). Visual acuity (VA) is an estimate of visual function. “Fix and follow” (FF) indicates that the child appears to fixate on and follow an object (usually a pen light or other object to attract the child’s interest); “hand movements” (HM) indicates that the child can reproducibly indicate the direction of movements of large objects; “counts fingers” (CF) indicates that the child can count the number of fingers the examiner is displaying. Older children may be able to be tested with pictures of standard sizes (Teller cards), or even standard Snellen letters.
Direct communication with the ophthalmologist or an ophthalmologist familiar with clinical ophthalmic notation may be necessary to clarify findings, abbreviations, and historical perspective.
ESSENTIAL ANATOMY OF THE EYE
The eye is a hollow collagenous sphere containing fluid (a hydraulic system), refractive media (an optic system), complex arteries and veins (a vascular system), and peripheral nerves and a tract of the brain (a neural system) (Fig. 17.6).
The cornea and sclera, both of which are composed of type I collagen, form the eyewall. The eyewall anterior-posterior length, between the anterior surface of the cornea and the photoreceptor outer segments of the retina, determines the optical characteristics of the eye. An eye that is optically or physically too long is nearsighted (myopic). An eye that is optically or physically too short is farsighted (hyperopic). Aqueous, the liquid component of the eye, is produced by a portion of the anterior vascular layer of the eye (the ciliary body). The function of aqueous is to nourish the critical internal structures of the eye, and the balance of aqueous formation and drainage from the eye determines the intraocular pressure. The crystalline lens is positioned posterior to the iris and serves to focus light on the retina. Opacification of the crystalline lens (cataract) interferes with the passage of light from the cornea to the retina.
Two independent vascular systems are present in the eye: the retinal and choroidal circulations. The terminal branches of the
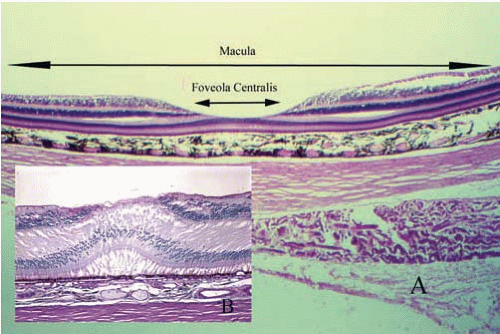
central retinal artery are visible by clinical examination. The ophthalmic artery is the first major branch of the internal carotid artery. It enters the orbit through the optic canal, along with the optic nerve. The central retinal artery is the first branch of the ophthalmic artery and penetrates the optic nerve approximately 12 mm posterior to the sclera, the posterior extent of the embryonic fetal fissure. The central retinal artery system provides nutrition only to the internal half of the neurosensory retina. The central retinal artery branches into nasal and temporal systems at the optic disc (Fig. 17.1). The temporal vessels are the most prominent and extend in an arcuate manner above and below the most sensitive area of the retina (fovea centralis). The area between the superior and inferior temporal arcades is the macula (macula lutea = yellow spot). No retinal vessels are present in the fovea centralis (a capillary-free zone). As with the remainder of the photoreceptors and outer plexiform layer, nutrition for this critical area of the retina is supplied only by diffusion from the choroidal vessels. Collaterals between the choroidal system and the retinal system are not normally present. The retinal arterioles are end arterioles. Tight junctions of the vascular endothelial cells of the retina form one component of the blood-retinal barrier, which is analogous to the blood-brain barrier.
central retinal artery are visible by clinical examination. The ophthalmic artery is the first major branch of the internal carotid artery. It enters the orbit through the optic canal, along with the optic nerve. The central retinal artery is the first branch of the ophthalmic artery and penetrates the optic nerve approximately 12 mm posterior to the sclera, the posterior extent of the embryonic fetal fissure. The central retinal artery system provides nutrition only to the internal half of the neurosensory retina. The central retinal artery branches into nasal and temporal systems at the optic disc (Fig. 17.1). The temporal vessels are the most prominent and extend in an arcuate manner above and below the most sensitive area of the retina (fovea centralis). The area between the superior and inferior temporal arcades is the macula (macula lutea = yellow spot). No retinal vessels are present in the fovea centralis (a capillary-free zone). As with the remainder of the photoreceptors and outer plexiform layer, nutrition for this critical area of the retina is supplied only by diffusion from the choroidal vessels. Collaterals between the choroidal system and the retinal system are not normally present. The retinal arterioles are end arterioles. Tight junctions of the vascular endothelial cells of the retina form one component of the blood-retinal barrier, which is analogous to the blood-brain barrier.
The choroidal circulation is the larger of the two vascular systems and is contained in a layer between the sclera and the retina. This system supplies oxygen to the outer one-third to one-half of the retina. This arterial supply originates from the short posterior ciliary vessels that penetrate the posterior sclera. The veins drain to four to six emissary vessels (vortex veins) that extend from the posterior surface of the sclera into the venous system of the orbit. No valves are present in the veins of the eye or orbit. Lymphatic channels are present only in the conjunctiva and eyelids and are not present within the eye or in the orbit posterior to the orbital septum.
Branches of the trigeminal nerve (cranial nerve V) provide a dense plexus of sensory nerves to the anterior half of the cornea. The posterior cornea and the remaining intraocular structures are not innervated by any sensory fibers and are anesthetic. Sympathetic and parasympathetic fibers that control pupillary tone are supplied via the oculomotor nerve (cranial nerve III).
During embryologic development of the eye, outpouchings (optic vesicles) of the neural tube extend toward the surface ectoderm and induce formation of the eye. Invagination of the optic vesicle forms the optic cup that will ultimately become two distinct anatomic and functional layers of the posterior segment of the eye. The outer layer becomes a monolayer of pigmented cells (retinal pigment epithelium [RPE]) that extend from the pupil to the optic disc in all meridians. The functions of the RPE include supporting the metabolic activity of the photoreceptor cells. RPE melanin pigment granules are distinct in that they have a much larger diameter than those found in cutaneous melanocytes and have an ovoid shape rather than a spherical dustlike shape. This type of melanin granule is formed only during embryonic life and is not renewable. The high concentration of melanin serves to absorb light that passes through the retina to prevent reflection of this light off the choroid and sclera. Tight junctions between the cells of the RPE form the second component of the blood-retina barrier. The RPE rests on a multipartite acellular layer (Bruch membrane) that separates the blood vessels of the choroid from the retina.
The retinoblasts of the primitive neurosensory retina first differentiate into ganglion cells that migrate to the inner part of the retina and form axons. The axons migrate posteriorly to a hiatus in the posterior sclera (optic canal) to form the optic nerve. Most of these axons form synapses in the lateral geniculate nucleus of the thalamus. Subsequently, photoreceptors (rods and cones) differentiate in the outer retina. Signal processing cells (bipolar, horizontal, and amacrine) then differentiate in the central retina to connect the photoreceptors with the appropriate ganglion cells. Glial cells are also present for structural and metabolic support. In the region of the fovea centralis, the inner layers of the retina (ganglion cells, bipolar cells, horizontal cells, and amacrine cells) are displaced peripherally, leaving only photoreceptors in the center of the fovea (Fig. 17.7).
There is no anatomic connection between the neurosensory retina and the RPE. The retina is held in place by physiologic forces (diffusion gradients) between the vitreous and the choroid. The retina can detach from the RPE by the interposition of fluid (serous retinal detachment), pulling on the retina causing tenting (traction retinal detachment), or the formation of a hole in the neurosensory retina (rhegmatogenous retinal detachment).
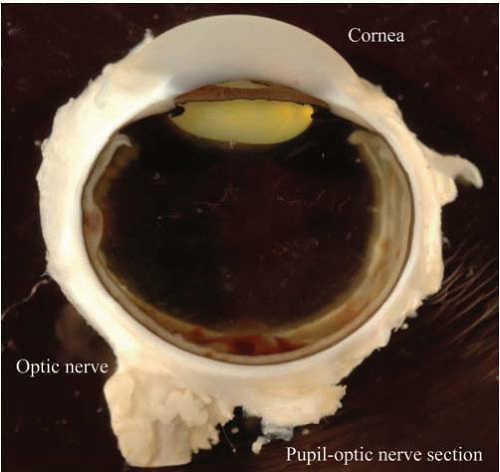
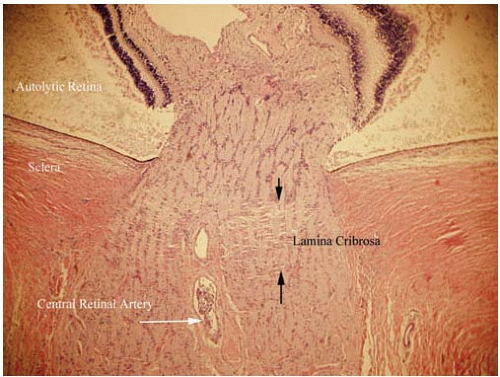
The axons of the ganglion cells exit through the optic canal of the posterior sclera. However, because the pressure systems of the eye and the optic nerve must remain separate, the axons penetrate a sieve-like structure contiguous with the posterior sclera, the lamina cribrosa. The lamina cribrosa maintains the hydraulic competence of the intraocular pressure (Fig. 17.8).
The portion of the ganglion cell axons that lie between the neurosensory retina and the lamina cribrosa is the optic disc. Swelling of these fibers and their supporting glial tissue causes edema of the optic disc or papilledema. Atrophy of these optic nerve fibers is seen in the later stages of many optic nerve diseases, including glaucoma. A portion of the optic nerve posterior to the lamina cribrosa is supplied by the central retinal artery and dural vessels. However, the optic disc vasculature is supplied by a separate group of short posterior ciliary arteries, which enter the sclera near the insertion of the optic nerve dura. A complex of vessels within the sclera constitutes the Circle of Zinn-Haller, which supplies the tissues of the optic disc. The relatively high metabolic demand of the optic nerve at the optic disc and the watershed zone of vasculature makes this tissue particularly susceptible to ischemic damage
from inflammatory (e.g., giant cell arteritis) or degenerative (e.g., atherosclerotic) vascular damage.
from inflammatory (e.g., giant cell arteritis) or degenerative (e.g., atherosclerotic) vascular damage.
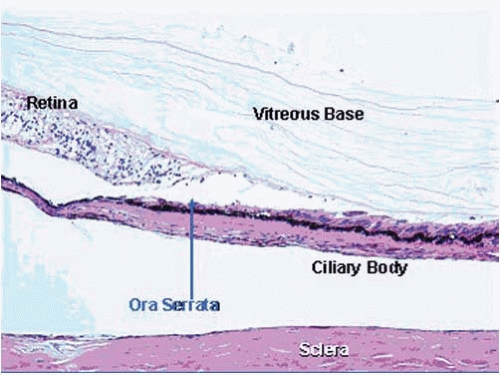
The vitreous is a transparent structure produced by the inner layers of the peripheral neuroepithelium (pars plana and peripheral retina) and is composed primarily of collagen type II, hyaluronic acid, and water (Fig. 17.9). The vitreous comprises approximately 80% of the volume and mass of the entire eye. The vitreous does not contribute in any significant manner to the optical properties of the eye, unless opacified by hemorrhage or inflammatory debris. The vitreous is essential, however, in acting as a biochemical “sink” to stabilize the complex biochemistry of the eye. Postmortem vitreous may be a repository for toxins (e.g., barbiturate) or abnormal accumulation of biochemicalmetabolic products (e.g., glucose). The vitreous normally contains few cells (hyalocytes). The extracellularmatrix of the vitreous is condensed peripherally, particularly where it comes in contact with the internal limiting membrane of the retina. The internal limiting membrane is an extracellular basementmembrane-like structure that constitutes the innermost architectural support of the neurosensory retina. The internal limiting membrane is also intimately associated with the superficial vessels of the retina. There are no specialized anatomic attachments between the vitreous and the retina. The vitreous is most firmly attached at the vitreous base, which is at its site of formation in the area of the ora serrata (the junction between the peripheral retina and the posterior ciliary body). Over time, the nonaqueous portion of the vitreous condenses (vitreous syneresis). The vitreous spontaneously pulls free of the internal limitingmembrane of the retina. Dense protein condensates in the vitreous cortex may cast a shadow on the retina that is perceived as a “floater.”
The dura of the intraorbital portion of the optic nerve is contiguous with the sclera and continues posteriorly through the orbital soft tissues to the orbital apex, where it attaches to the periosteum of the optic canal of the sphenoid bone. The subdural space of the intraorbital optic nerve does not connect with the subdural space of the cerebrum, but the subarachnoid of the optic nerve is contiguous with the subarachnoid space of the brain. The retro-orbital optic nerve is largely supplied by delicate vessels of the pia, as in the cerebral cortex elsewhere.
ESSENTIAL HISTOLOGY OF THE EYE
The cornea is composed primarily of extracellular matrix bordered anteriorly by a homogeneous stratified, nonkeratinized squamous epithelium that is contiguous with the less homogeneous nonkeratinized squamous epithelium of the conjunctiva. The conjunctival epithelium differs from that of the cornea in that it contains goblet cells. The single-layered endothelium (mesothelium rather than vascular endothelium) covers the posterior surface of the cornea and extends to the junction of the cornea and iris (the anterior chamber angle) as trabecular meshwork endothelium. In the region of the cornea, endothelial cells produce a thick basement membrane (Descemet membrane). In contrast to the cornea, the extracellular matrix of the sclera is more randomly organized (hence opaque), contains fewer cells, and is not lined by epithelial cells or endothelial cells on either surface. The anterior chamber is bounded by the cornea and iris and contains aqueous fluid. The trabecular meshwork, located in the periphery of the anterior chamber, is the drainage structure of the eye for aqueous (Fig. 17.10). The trabecular meshwork structures extend from the termination of Descemet membrane (Schwalbe line) to the scleral spur, which is the site of insertion into the sclera of the most external layer of the smooth muscle of the ciliary body (Fig. 17.11). The iris is attached to the ciliary body and not the sclera.
The lens is attached to the ciliary processes by delicate acellular zonules. The lens is bounded by a basement membrane (the lens capsule) containing anucleate lens “fibers.” The epithelial cells of the crystalline lens are located only in the anterior hemisphere.
The uveal tract extends from the iris to the optic nerve. Vessels in the iris are associated with a thick adventitia. The vessels in the pars plicata of the ciliary body form vascular arcades in the ciliary processes that participate in aqueous formation. Over time, these ciliary processes become less prominent, and the vessels contained in the processes become more sclerotic. The choroid is the portion of the uveal tract adjacent to the retina and consists of larger vessels near the sclera and a broad complex of capillary lobules (choriocapillaris) just external to Bruch membrane. The lumen of the choriocapillaris
diminishes in caliber from posterior to anterior. The uveal tract terminates posteriorly at the scleral canal. Bruch membrane is composed of the basement membrane of the choriocapillaris, extracellular matrix elastin fibers, and the basement membrane of the retinal pigment epithelium. The character and content of Bruch membrane differ from the posterior to the anterior eye (Fig. 17.12). The RPE is a cellular monolayer containing individually identifiable oval melanin granules. At an ultrastructural level, the apices of the RPE cells interdigitate with the photoreceptor outer segments.
diminishes in caliber from posterior to anterior. The uveal tract terminates posteriorly at the scleral canal. Bruch membrane is composed of the basement membrane of the choriocapillaris, extracellular matrix elastin fibers, and the basement membrane of the retinal pigment epithelium. The character and content of Bruch membrane differ from the posterior to the anterior eye (Fig. 17.12). The RPE is a cellular monolayer containing individually identifiable oval melanin granules. At an ultrastructural level, the apices of the RPE cells interdigitate with the photoreceptor outer segments.
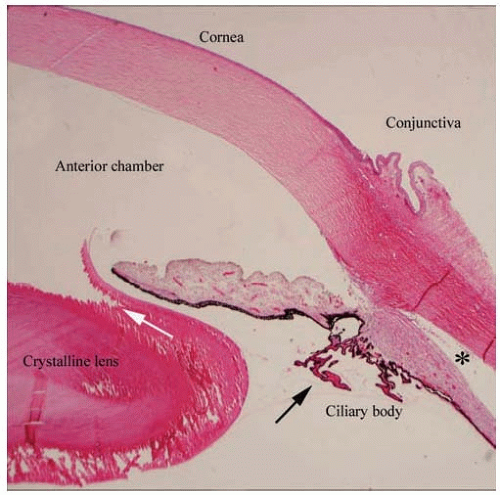 FIG. 17.10. The anterior segment of the eye consists of the cornea, anterior chamber, anterior chamber angle, iris, posterior chamber, and ciliary body. The cornea has a mature histologic appearance, including artifactual separation of the collagenous fibers within the corneal stroma. The fractured appearance of the lens is an artifact due to sectioning (white arrow). The ciliary processes appear long and delicate in young patients (black arrow). The separation of the ciliary body musculature from the overlying sclera (*) is an artifact of tissue preparation. (Stain: hematoxylin-eosin, original magnification × 20.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|