Most other cases are identified at routine medical examination. Otherwise, age at presentation is related to the severity rather than the site of obstruction, as a result of cardiac failure or occasionally cerebrovascular accident (CVA), aortic dissection, or endocarditis. Adults with coarctation repaired in infancy are more likely to have associated bicuspid aortic valve, subaortic stenosis, ventricular septal defect (VSD), and hypoplastic aortic arch (Warnes et al., 2008). There also is an important association with circle of Willis aneurysms, which can rupture.
Pathophysiology
If the coarctation is proximal to the ductus arteriosus, pulmonary hypertension, congestive failure, and cyanosis of the lower half of the body occur early in life. Before surgery was possible, 45% to 84% of infants found to have coarctation died during their 1st year of life (Campbell, 1970).
Patients with less severe postductal lesions may have no difficulties during childhood. However, they almost always develop premature cardiovascular disease; in the two largest series of autopsied cases seen before the advent of effective surgery, the mean age of death was 34 years (Campbell, 1970). The causes of death reflected the pressure load on the heart and the associated cardiac and cerebral lesions.
In addition to the mechanical stimulus of high BP, coarctation somehow induces structural and functional abnormalities in arterial segments proximal to the constriction that persist after successful repair and thus may contribute to the late development of hypertension and an excessive arteriosclerotic cardiovascular disease (ASCVD) risk (Brili et al., 2005). These abnormalities—which include endothelial dysfunction in the right forearm vessels and impaired elasticity of the carotid artery but not of the femoral artery—are accompanied by increased circulating levels of proinflammatory cytokines and adhesion molecules, all of which can be improved by treatment with an angiotensin converting enzyme inhibitor (ACEI) (Brili et al., 2008). In a rabbit model of coarctation, complete correction of the pressure gradient has absolutely no effect on endothelial dysfunction and abnormal protein expression (decreased smooth muscle myosin and increased nonmuscle myosin) in the walls of the proximal aorta, coronary arteries, or cerebral vessels (Menon et al., 2012). Progressive weight gain and obesity, possibly from restricted exercise, is increasingly recognized after coarct repair and may contribute to the late onset of hypertension and ASCVD risk (Smith-Parrish et al., 2014).
Recognition of Coarctation
Hypertension in the right arm with weak femoral pulses in a young adult strongly suggests coarctation. Patients may complain of exertional headaches and/or intermittent claudication. Detection of a murmur of a bicuspid aortic valve (crescendo–decrescendo murmur of aortic stenosis with or without a diastolic murmur of aortic regurgitation), VSD (harsh systolic murmur), or collaterals (a continuous murmur over the parasternal area and left scapula) may lead to the diagnosis (Warnes et al., 2008). Coarctation is present in 12% of young women with Turner syndrome, which is 400 times higher than in the general population (Wong et al., 2014). With minimal constriction, symptoms may not appear until later in life. Often the heart is large and shows LVH with strain on the electrocardiogram. The chest radiograph can be diagnostic, demonstrating the “three” sign from dilation of the aorta above and below the constriction and notching of the ribs by enlarged collateral vessels (Fig. 14-1) (Quiros-Lopez & Garcia-Alegria, 2007). The diagnosis is now usually made by suprasternal notch echocardiography with color Doppler flow imaging and confirmed by cardiac magnetic resonance imaging (MRI) or computerized tomography (CT) (Fig. 14-1) (Quiros-Lopez & Garcia-Alegria, 2007). Exercise testing with a supine bicycle ergometer is important to determine the responses of BP and echocardiographic Doppler gradient to exercise, which estimate the coarctation gradient (Warnes et al., 2008).
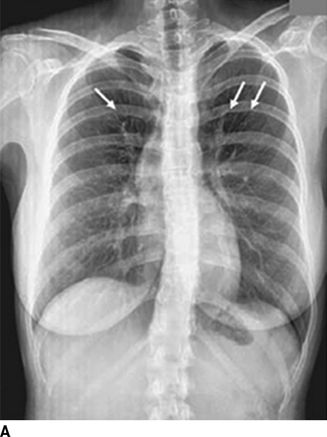
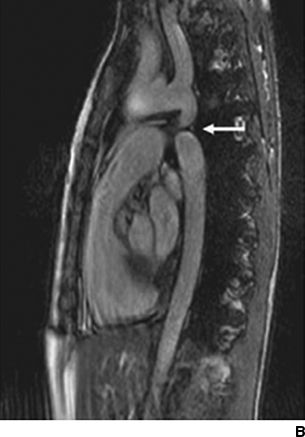
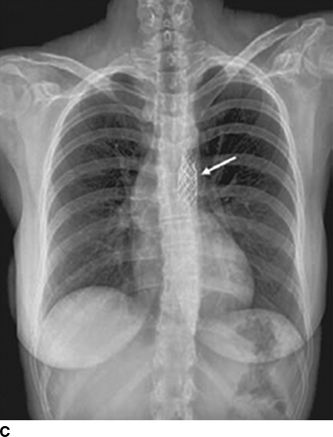
FIGURE 14-1 Coarctation of the aorta in a young woman. A chest radiograph shows classical changes associated with coarctation of the aorta (A, arrows). Magnetic resonance imaging shows constriction of the lumen of the aorta (B, arrow). A chest radiograph shows endovascular repair with stent placement (C, arrow). (From Quiros-Lopez R, Garcia-Alegria J. A medical mystery—High blood pressure. N Engl J Med 2007;356:2630.)
Atypical aortic coarctation in adults most likely represents Takayasu arteritis, or pulseless disease—a giant cell arteritis occurring mainly in young women in their 20s or 30s and causing stenosis and occasionally aneurysm formation of the aorta and its major branches (Fig. 14-2) (Clifford & Hoffman, 2014). While corticosteroids are first-line therapy, early results with anti-TNF biologics, mainly etanercept or infliximab, are promising (Clifford & Hoffman, 2014).
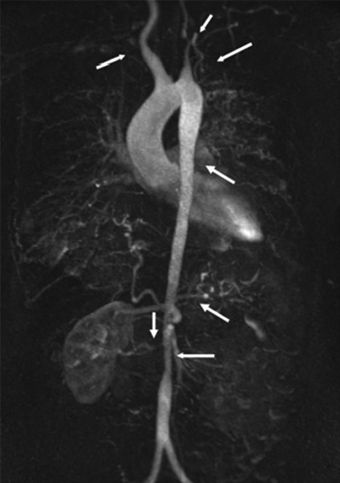
FIGURE 14-2 Vascular lesions in Takayasu arteritis. MR imaging of the entire aorta in a 33-year-old woman with Takayasu arteritis. Arrows indicate multiple stenotic or occluded lesions. The left common carotid and both subclavian arteries are occluded, resulting in antegrade flow through the vertebral arteries. The right renal artery is stenotic and the left is occluded, resulting in an atrophic left kidney. There are also areas of stenosis in the descending and abdominal aorta. Presenting features were syncope, transient ischemic attack, and severe hypertension. (From Clifford A, Hoffman GS. Recent advances in the medical management of Takayasu arteritis: An update on use of biologic therapies. Curr Opin Rheumatol 2014;26(1):7–15.)
Management
Prior to repair, hypertension should be controlled with an ACEI or angiotensin receptor blocker (ARB) as first-line therapy. Add-on therapy may include a beta-blocker if the aortic root is large to minimize the risk of aneurysmal rupture or a vasodilator if there is associated aortic insufficiency that would be exacerbated by bradycardia from beta-blockade. According to the 2008 American College of Cardiology/American Heart Association Guidelines, (Warnes et al., 2008) intervention is recommended if peak-to-peak coarctation gradient is ≥20 mm Hg or the gradient is less than 20 mm Hg in the presence of anatomic imaging evidence of significant coarct with radiologic evidence of significant collateral flow. Early repair is recommended with very low rates of recoarctation being encountered but nonetheless with high rates of late postoperative hypertension and ASCVD. Percutaneous catheter intervention with stenting is indicated for recurrent discrete coarctation whereas surgical repair is indicated for previously repaired coarct with a long recoarctation segment or concomitant hypoplasia of the aortic arch and women of childbearing age to assure removal of the precoarct segment. If repair is delayed until adulthood, there is a greater likelihood of persistent hypertension, which may be severe. Even in those with normal resting BPs, there may be an exaggerated BP response to exercise (Warnes et al., 2008).
Obviously, patients after repair need to be followed at least annually with stress testing, and any degree of resting or exercise-induced hypertension needs to be intensively treated (Gurvitz et al., 2013). Despite improvements in surgical technique and earlier age of operation, long-term survival has not improved as much as expected: up to 75% of patients develop lifelong postoperative hypertension if repaired after age 15 and still 40% if before age 15 (Brown et al., 2013). Thus, lifelong medical follow-up with intensive management of hypertension and ASCVD risk factors is essential.
HORMONAL DISTURBANCES
Hypothyroidism
Hypertension, particularly diastolic, may be more common in hypothyroid patients. Among 40 patients prospectively followed over the time they became hypothyroid after radioiodine therapy for thyrotoxicosis, 16 (40%) developed a diastolic BP higher than 90 mm Hg (Streeten et al., 1988). Hypothyroid patients tend to have a low cardiac output with a decrease in contractility and impaired diastolic relaxation (Danzi & Klein, 2003). To maintain tissue perfusion, peripheral resistance increases, from a combination of increased responsiveness of α-adrenergic receptors, increased levels of sympathetic nervous activity (Fletcher & Weetman, 1998), and aldosterone (Fommei & Iervasi, 2002). These would tend to raise diastolic BPs more than systolic BPs, the usual pattern seen in hypothyroidism (Saito & Saruta, 1994).
Subclinical hypothyroidism, defined as an elevated thyrotropin-stimulating hormone but normal free thyroxine levels, was associated with marginally higher BP in a meta-analysis of 50,147 individuals in 20 studies (Ye et al., 2014). A meta-analysis of 10 population-based studies found a 51% higher relative risk of coronary disease in such patients under age 65 (Gencer et al., 2013).
Hyperthyroidism
An elevated systolic but lowered diastolic BP is usual in patients with hyperthyroidism, associated with a high cardiac output and reduced peripheral resistance. Even after successful therapy, cardiovascular disease (CVD) morbidity persists (Metso et al., 2008).
Hyperparathyroidism
Primary hyperparathyroidism is common, accounting for 80% to 90% of hypercalcemia in asymptomatic outpatients. Calcium elevation is typically mild (10.5 to 11.5 mg/dL) and often noted only after thiazide therapy for hypertension. Only half of those with hyperparathyroidism have high parathyroid hormone (PTH) levels, and the others have inappropriately “normal” PTH levels with high serum calcium (Adami et al., 2002). The incidence of primary hyperparathyroidism is highest among Blacks, followed by Whites, with lower rates for Asians and Hispanics (Yeh et al., 2013). Consensus guidelines on indications for parathyroid surgery have been published (Bilezikian et al., 2009).
Several recent studies indicate that hyperparathyroidism is a cardiac risk factor. Irrespective of the serum level of 25OHD, increased levels of PTH are associated with higher BP in older patients with isolated systolic hypertension (Mateus-Hamdan et al., 2013). The Parathyroid Epidemiology and Audit Research Study (PEARS), an observational cohort study of 2,097 patients with untreated primary hyperparathyroidism (mean age 68, 70% women), found that serum PTH—not serum calcium—was the best predictor of fatal and nonfatal CVD (Yu et al., 2013). Patients with primary hyperparathyroidism are predisposed not only to hypertension but also to hypertensive heart disease, which regresses after parathyroidectomy (Agarwal et al., 2013).
Aldosterone may link elevated PTH with hypertension. Parathyroidectomy lowers serum aldosterone and improves cardiovascular outcomes (Tomaschitz et al., 2014). PTH induces aldosterone secretion both directly by binding to PTH receptors on adrenal zona glomerulosa and indirectly by potentiating Ang II effects. These effects of PTH on aldosterone and the cardiovascular system may warrant parathyroidectomy, even in the elderly (Oltmann et al., 2013). Among patients with primary aldosteronism, elevated serum PTH levels favor the diagnosis of aldosterone producing adenoma rather than bilateral adrenal hyperplasia and thus may be useful for selecting those patients to be submitted to adrenal vein sampling (Rossi et al., 2012).
Vitamin D Deficiency
Chronic vitamin D deficiency increases levels of PTH and can cause secondary hyperparathyroidism, which may increase cardiovascular risk, especially in patients with chronic kidney disease (CKD) (Lavie et al., 2013). Epidemiologic studies, such as the Copenhagen City Heart Study (Brondum-Jacobsen et al., 2012) and the Whitehall study (Tomson et al., 2013), continue to show that lower levels of plasma 25-hydroxyvitamin D associate with nonfatal and fatal CVD events. However, as discussed in Chapter 3, proper randomized clinical trials (RCTs) show that Vitamin D supplementation has no effect on BP either in patients with difficult hypertension and LVH (Witham et al., 2014) or in older patients with ISH (Witham et al., 2013).
Acromegaly
Acromegaly affects 20% of patients with McCune-Albright Syndrome, which refers to the triad of bone (often skull-based) fibrous dysplasia, cafe-au-lait spots, and hyperfunctioning endocrinopathy (Salenave et al., 2014). In a recent series, the mean age of diagnosis of acromegaly was 24 years of age and a pituitary adenoma was seen in over half (Salenave et al., 2014). Hypertension is found in approximately 35% of patients with acromegaly and is a risk factor for their increased rate of mortality (Dekkers et al., 2008).The hypertension is related to a number of factors: Sodium retention, neurogenic vasoconstriction, endothelial dysfunction, and hypertrophic remodeling of resistance arteries (Rizzoni et al., 2004). LVH and impaired systolic function are usual (Bogazzi et al., 2008). Guidelines for management are available (Melmed et al., 2009). When the condition is controlled, hypertension usually improves (Melmed, 2009).
OBSTRUCTIVE SLEEP APNEA
Obstructive sleep apnea (OSA) has been implicated as the most common form of identifiable hypertension in the United States (U.S.), as BP tracks with OSA in epidemiologic studies and OSA is present in 50% of hypertensive patients (Konecny et al., 2014). Still, clear proof of causality is lacking. One challenge is how to clearly separate an independent effect of OSA from associated problems such as obesity and troubled sleep. Most troubling is that continuous positive airway pressure (CPAP) produces a trivial improvement in BP despite a large improvement in OSA (Montesi et al., 2012).
Clinical Features and Diagnosis
OSA should be considered in patients with the clinical features of increasing obesity, loud snoring, fitful sleep, and daytime sleepiness (Table 14-2). Although OSA is common in patients who are morbidly obese, most afflicted are not “pickwickian.” A 10% increase in weight was associated with a sixfold increased risk of developing OSA among subjects initially free of OSA (Peppard et al., 2000b). Virtually all with OSA will snore, but only approximately half of people who snore for more than half the night have sleep apnea (Konecny et al., 2014). The diagnosis can be made by a sleep study at home (Tishler et al., 2003) but with more certainty by overnight polysomnography in a sleep laboratory, with continuous recordings of respiration, electroencephalogram, electromyogram, eye movements, electrocardiogram, O2 saturation, and BP.
TABLE 14-2 Clinical Features of OSA
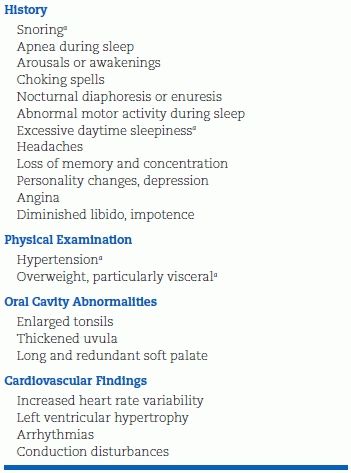
aMost useful in considering diagnosis.
Association with Hypertension
Incidence
Multiple cross-sectional and observational studies have unequivocally shown a higher prevalence and incidence of systemic hypertension in direct proportion to the severity of sleep apnea (Hiestand et al., 2006) (Fig. 14-3). Lavie et al. (2000) found that each apneic event per hour of sleep increased the odds for hypertension by 1%, whereas each 10% decrease in O2 saturation increased the odds by 13%.
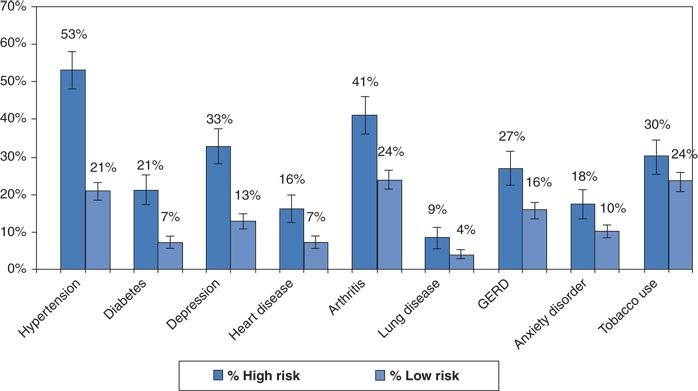
FIGURE 14-3 Prevalence of chronic illness among individuals with either high or low risk for sleep apnea by the Berlin questionnaire. (Data from Hiestand DM, Britz P, Goldman M, et al. Prevalence of symptoms and risk of sleep apnea in the US population: Results from the national sleep foundation sleep in America 2005. Chest 2006;130:780–786.)
A history of snoring, by itself, has been associated with an increased incidence of hypertension. Among 73,000 U.S. female nurses followed for 8 years, the risk of developing hypertension increased by 29% in those who snored occasionally and by 55% in those who snored regularly as compared to those who said they did not snore (Hu et al., 1999). The association was independent of age, body mass index, waist circumference, and other lifestyle factors.
The risk of hypertension is greater for younger subjects than for those older than 60 years (Konecny et al., 2014). Moreover, the prevalence of sleep apnea is even higher both in patients with uncontrolled hypertension and in patients with stroke. Typically, patients with OSA have nondipping BP during sleep and accentuated morning surge of BP when monitored by ambulatory BP monitoring (Amin et al., 2008).
Mechanisms of Hypertension
A number of possible mechanisms for persistent hypertension as a consequence of OSA have been proposed (Konecny et al., 2014). These include: increased carotid chemoreceptor drive both night and day driving sympathetic neural activation, vascular inflammation, increased cortisol, increased erythropoietin, arterial stiffness, and, most recently, central fluid shift during nocturnal recumbency. In support of the last mechanism, graded lower body positive pressure—used experimentally to displace venous blood from the lower body to the cardiopulmonary region—triggered a greater degree of upper airway constriction in patients with resistant hypertension than in those with well-controlled hypertension (Friedman et al., 2013). Moreover, high serum aldosterone levels with low plasma renin activity have been found in over half of patients with OSA, suggesting that aldosterone contributes to OSA by shifting fluid from the plasma to the extracellular space surrounding the airway (Clark et al., 2012).
Treatment
Weight loss—even as little as 10% of body weight—will help over the long term (Peppard et al., 2000a); avoiding the supine position during sleep may help in the short term (Kuhlmann et al., 2009). However, among a group of 60 obese patients with OSA, the use of bariatric surgery compared with conventional weight loss therapy did not result in a greater reduction in apnea–hypopnea index, despite the much greater weight loss with surgery (Dixon et al., 2012). The best relief is by nasal continuous positive airway pressure (CPAP), but dental appliances (mandibular advancement devices) are better tolerated and gaining popularity. In a recent RCT of 126 patients with moderate–severe OSA, CPAP was more efficacious than the mandibular device in reducing the apnea–hypopnea index but compliance was better with the latter (Phillips et al., 2013). However, in that study, neither approach improved BP by 24-hour ambulatory monitoring. A recent meta-analysis including 1,948 patients in 28 trials has confirmed several older studies showing that CPAP only lowers office BP by approximately 3/2 mm Hg (Montesi et al., 2012).
The conclusion is obvious: Neither CPAP nor dental appliances alone will be sufficient to control hypertension in patients with OSA. Proper RCTs are needed to determine if diuretic-based therapy, perhaps with a combination of an aldosterone blocker and a potent thiazide, would be highly effective therapy both for the OSA and the associated hypertension. In the meantime, weight loss and standard antihypertensive therapy are needed to control BP, while CPAP or a dental appliance is needed to improve the OSA and thus daytime sleepiness. By reducing transthoracic transmural pressure, CPAP may reduce the risk of LVH and atrial fibrillation (less atrial stretch) even if it does little to improve BP (Naughton et al., 1995).
NEUROLOGIC DISORDERS
Beyond stroke, a number of seemingly different disorders of the central and peripheral nervous system may cause hypertension. Many may do so by a common mechanism involving sympathetic nervous system discharge from the vasomotor centers in response to an increased intracranial pressure. The rise in systemic pressure is necessary to restore cerebral perfusion.
As noted in Chapters 4 and 7, patients with acute stroke may have transient marked elevations in BP. Rarely, episodic hypertension suggestive of a pheochromocytoma may occur after cerebral infarction (Manger, 2008).
Alzheimer Disease
According to the American Heart Association/American Stroke Association (Gorelick et al., 2011),
“Midlife hypertension ranks as an important modifiable risk factor for late-life cognitive decline, mild cognitive impairment, and vascular dementia. In longitudinal cohort studies, higher systolic BP has been associated with greater late-life cognitive decline, although some studies have reported a J- or U-shaped relation. The data on the role of blood pressure and hypertension in later life are not consistent, leaving open the issue of blood pressure treatment in older people.”
The existing RCTs have produced inconsistent results as to whether antihypertensive therapy reduces cognitive decline, which has never been the primary end point. The most compelling data are from the Syst-Eur trial in which the onset of Alzheimer disease was reduced by 50% with calcium channel blocker (CCB)-based therapy therapy versus placebo (Forette et al., 1998). Provocative observational data from the Cardiovascular Heart Study indicate that cognitive decline in older hypertensives is slowed by centrally acting ACEIs that cross the blood–brain barrier such as ramipril, perindopril, and lisinopril but not by other ACEIs that do not cross such as benazepril, enalapril, and quinapril (Sink et al., 2009). Perindopril treatment showed a positive signal for reduced cognitive decline in PROGRESS (Tzourio et al., 2003) but not in the HYVET-COG study (Peters et al., 2008).
Brain Tumors
Intracranial tumors, especially those arising in the posterior fossa, may cause hypertension (Pallini et al., 1995). In some patients, paroxysmal hypertension and other features that suggest catecholamine excess may point mistakenly to the diagnosis of pheochromocytoma. The problem may be confounded by the increased incidence of neuroectodermal tumors, some within the central nervous system, in patients with pheochromocytoma. Unlike patients with a pheochromocytoma who always have high catechol levels, patients with a brain tumor may have increased catecholamine levels during a paroxysm of hypertension but normal levels at other times (Manger, 2008).
Quadriplegia
Patients with transverse lesions of the cervical spinal cord above the origins of the thoracolumbar sympathetic neurons lose central control of their sympathetic outflow. Stimulation of nerves below the injury, as with bladder or bowel distension, may cause reflex sympathetic activity via the isolated spinal cord, inducing hypertension, sweating, flushing, piloerection, and headache, a syndrome described as autonomic hyperreflexia. Such patients have markedly exaggerated pressor responses to various stimuli (Krum et al., 1992). The hypertension may be severe and persistent enough to cause cerebrovascular accidents (CVAs) and death. An α-blocker effectively controlled the syndrome (Chancellor et al., 1994).
Severe Head Injury
Immediately after severe head injury, the BP may rise because of a hyperdynamic state mediated by excessive sympathetic nervous activity (Simard & Bellefleur, 1989). If the hypertension is persistent and severe, a short-acting β-blocker (e.g., esmolol) should be given. Caution is needed in the use of vasodilators such as hydralazine and nitroprusside, which may increase cerebral blood flow and intracranial pressure. Moreover, hypotension is an even greater threat (Fuller et al., 2014). Both high and low prehospital systolic BP (and heart rate) predict mortality after traumatic brain injury (Reisner et al., 2014).
Other Neurologic Disorders
Hypertension may be seen with:
- Guillain-Barré syndrome (Watson et al., 2014)
- Fatal familial insomnia, a prion disease with severe atrophy of the thalamus (Portaluppi et al., 1994)
- Baroreceptor failure (Heusser et al., 2005)
- Autonomic failure with orthostatic hypotension and supine hypertension, often helped by a bedtime ARB (Arnold et al., 2013).
- Parkinson disease, wherein severe postural hypotension and exercise-induced hypotension (Low et al., 2014) may also be accompanied by nocturnal hypertension, indicating the importance of ambulatory BP monitoring to manage BP in this patient population (Tsukamoto et al., 2013)
FUNCTIONAL SOMATIC DISORDERS
Anxiety and depression are common in the general population and even more prevalent in patients with hypertension or cardiovascular disease (Davies et al., 2004). In the U.S., terrorism is taking its toll. Using health data on a representative national sample collected before 9/11 as a baseline, acute stress response to the terrorist attacks predicted increased reports of physician-diagnosed hypertension and other cardiovascular ailments over 3 years following the attacks (Holman et al., 2008). An anxiety disorder was found in 19.5% of consecutive patients seen in 15 U.S. primary care clinics in 2005 (Kroenke et al., 2007).
Anxious patients are more likely to have white-coat reactions that may persist over many office visits (Pickering & Clemow, 2008). Such patients obviously will be more anxious unless their excessive altering reaction is recognized and their anxiety over their BP relieved.
As common as it is, anxiety and its manifestations are often not recognized as being responsible for a variety of symptoms (Pickering & Clemow, 2008). Because of the common failure to recognize the underlying nature of various functional syndromes (Wessely et al., 1999) (Table 14-3), patients and their physicians often enter into a vicious cycle: More and more testing, often with false-positive results; more and more incorrect “organic” disease diagnoses; more and more ineffective therapy; more and more anxiety; and more and more functional symptoms.
TABLE 14-3 Functional Somatic Syndromes by Specialty
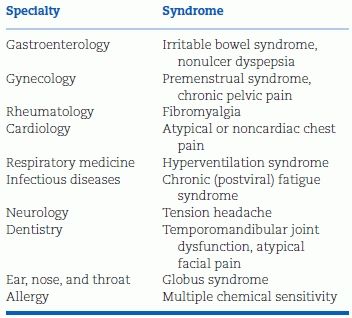
Modified from Wessely S, Nimnuan C, Sharpe N. Functional somatic syndromes: One or many? Lancet 1999;354:936–939.
Anxiety-Induced Hyperventilation
The problem is often encountered with hypertensive patients, either because of their concern over having “the silent killer” or because of their poor response to antihypertensive therapies. In 300 consecutive patients referred to me, usually because of hypertension that was difficult to control, 104 had symptoms attributable to anxiety-induced hyperventilation (Kaplan, 1997) (Fig. 14-4). The symptoms and signs of panic attack encompass all these same manifestations but go beyond them to include fears of falling apart, losing control, or even more acute anxiety and are associated with increased reactivity of vasoconstricting sympathetic nerves (Katon, 2006). Among 351 hypertensive patients randomly selected from one primary care practice in Sheffield, United Kingdom, panic attacks had occurred in 18% during the previous 6 months and in 37% over their lifetime (Davies et al., 1999). The reported diagnosis of hypertension usually antedated the onset of panic attacks. Anxiety and panic attacks were even more common among their patients who had nonspecific intolerance to multiple antihypertensive drugs (Davies et al., 2003). These patients are extremely difficult to help.
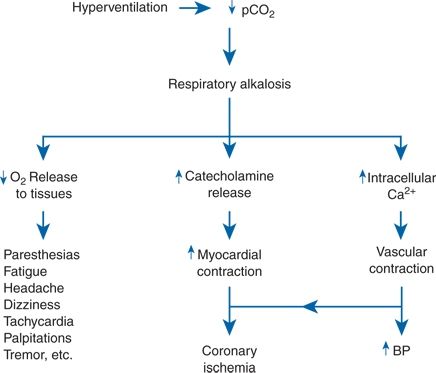
FIGURE 14-4 The mechanisms by which acute hyperventilation may induce various symptoms, coronary ischemia, and a rise in blood pressure (BP). Ca, calcium; pCO2, partial pressure of carbon dioxide.
Many of these patients had been subjected to intensive workup for dizziness, headaches, chest pain, fatigue, and the like (Newman-Toker et al., 2008). When the symptoms are reproduced by voluntary overbreathing and relieved by rebreathing into a paper sack, the patient’s recognition of the mechanism often provides immediate relief and opens the way to the appropriate use of rebreathing exercises, other cognitive therapy, or, if needed, antianxiety medications.
Mechanistic Underpinnings of the Heart/Brain Connection
Depression may not be more common in uncomplicated hypertension (Lenoir et al., 2008). Antidepressants can raise the risk of hypertension (Licht et al., 2009). Hypertension has been linked to anger, with rumination about previous anger-provoking events being both common and a strong predictor of daytime BP and heart rate during ambulatory BP monitoring (Ottaviani et al., 2011).
In patients with panic attacks, brain imaging studies indicate a fundamental down-regulation of the inhibitory neurotransmitters—GABA and serotonin—in patients with panic attacks (Davies et al., 2010). Brain spillover of norepinephrine (NE) is increased and linked to increase NE spillover in the heart and to multiple single unit axon firings per cardiac cycle in muscle sympathetic nerves (Lambert et al., 2011).
ACUTE PHYSICAL STRESS
Hypertension may appear during various acute physical stresses, usually reflecting an intense sympathetic discharge and sometimes the contribution of increased renin–angiotensin from volume contraction.
Surgical Conditions
Perioperative Hypertension
In addition to the reasons mentioned in the coverage of anesthesia and hypertension in Chapter 7, for numerous reasons, hypertension may be a problem during and soon after surgery. Elevated postoperative readings can be related to pain, hypoxia and hypercapnia, and physical and emotional excitement. These causes should be managed rather than treating the elevated BP with antihypertensives.
Marked rises in BP have been measured when pneumoperitoneum is performed for abdominal laparoscopic surgery (Joris et al., 1998). The rise in BP was accompanied by increases in blood catecholamines, cortisol, and vasopressin and was blunted by preoperative clonidine.
Cardiovascular Surgery
Table 14-4 summarizes the causes of hypertension associated with surgery in a temporal fashion (Vuylsteke et al., 2000).
TABLE 14-4 Hypertension Associated with Cardiac Surgery

Modified from Estafanous FG, Tarazi RC. Systemic arterial hypertension associated with cardiac surgery. Am J Cardiol 1980;46:685–694.
Coronary Bypass
Approximately one-third of patients will have hypertension after coronary artery bypass grafting, usually starting within the first 2 hours after surgery and lasting 4 to 6 hours. Immediate therapy may be important to prevent postoperative heart failure or myocardial infarction. In addition to deepening of anesthesia, various parenteral antihypertensives have been used, including nitroprusside and nitroglycerin (Vuylsteke et al., 2000).
Other Cardiac Surgery
Hypertension has been reported, although less frequently, after other cardiac surgery. Virtually all patients who undergo orthotopic heart transplantation develop hypertension (Taegtmeyer et al., 2004) and lose the usual nocturnal fall in BP, likely from a combination of effects, including the effects of immunosuppressive agents (see the section on Immunosuppressive Agents, later in this chapter), impaired low-pressure baroreceptor control from cardiac (afferent) denervation (Scherrer et al., 1990), and the inability to excrete sodium normally (Hoorn et al., 2011). The hypertension may be controlled by combination therapy with an ACEI and a DHP-CCB, with monotherapy of each effective in half of patients (Rockx & Haddad, 2007) and, if needed, a thiazide (to counter inhibition of the thiazide-sensitive Na–Cl transporter by the calcineurin inhibitors), and a central sympatholytic (to counter the sympathetic neural activation).
Carotid Endarterectomy
Postoperative hypertension may be particularly serious in patients with cerebrovascular disease who have carotid endarterectomy (Demirel et al., 2012a), because of altered carotid baroreceptor activity (Demirel et al., 2012b). The acute neurogenic hypertension is worse with eversion carotid endarterectomy, which requires the surgeon to transect the carotid sinus nerve than with the conventional longitudinal incision (Demirel et al., 2012a). Perioperative control of hypertension is important because severe postoperative hypertension can lead to cerebral hyperperfusion syndrome, which is a form of hypertensive encephalopathy often leading to cerebral hemorrhage with a mortality rate of 67% (Stoneham & Thompson, 2009). With unilateral carotid disease, the hypertension typically is self-limited. Short-term BP management most logically should be with labetalol (α,β-blocker) rather than with a vasodilator such as nitroprusside or nifedipine that would further increase cerebral blood flow.
INCREASED INTRAVASCULAR VOLUME
If vascular volume is raised a significant degree over a short period, the renal natriuretic response may not be able to excrete the excess volume, particularly if renal function is also impaired. Cell-free, hemoglobin-based oxygen carriers (HBOCs) cause hypertension by vasoconstriction secondary to scavenging of nitric oxide (NO). Both inhaled NO and intravenous sodium nitrite given before the infusion of HBOC prevent subsequent hypertension in mice and lambs (Yu et al., 2008). The same benefit is seen with direct soluble guanylate cyclase (sGC) activators in rats (Raat et al., 2013). Clinical trials are needed.
Erythropoietin Therapy
Correcting anemia with by erythropoietin (EPO) administration in patients with advanced CKD can exacerbate hypertension. As the hematocrit rises, so do blood viscosity and BP. The mechanism may be more complicated than simply increasing viscosity as EPO may stimulate production of both endothelin and reactive oxygen species (Rancourt et al., 2010). Nearly one-third of patients developed clinically important hypertension (Luft, 2000). This may add to the currently recognized danger of treating the anemia of chronic renal disease (Vaziri & Zhou, 2009), leading to a recent modest decrease in EPO use (Winkelmayer et al., 2014).
Polycythemia and Hyperviscosity
Patients with primary polycythemia are often hypertensive, and some hypertensives have a relative polycythemia that may resolve when the BP is lowered. The hypertension seen in polycythemic states could also reflect increased blood viscosity. Significant falls in BP were seen in 12 hypertensive patients with polycythemia when blood viscosity was reduced without changing the blood volume (Bertinieri et al., 1998).
CHEMICAL AGENTS THAT CAUSE HYPERTENSION
Table 14-5 lists various chemical agents that may cause hypertension, indicating their mechanism if known. Some of these substances, such as sodium-containing antacids, alcohol, insulin, licorice, oral contraceptives, and monoamine oxidase inhibitors, are covered elsewhere in this book because of their frequency or special features.
TABLE 14-5 Hypertension Induced by Chemical Agents

Adapted from Grossman E, Messerli FH. High blood pressure: Aside effect of drugs, poisons, and food. Arch Intern Med 1995;155:450–460.
Caffeine and Coffee
Caffeine is likely the most widely consumed drug in the world, and its use will almost certainly increase with the amazing proliferation of Starbucks and its clones. Coffee consumption causes an acute increase in BP lasting about 3 hours (Mesas et al., 2011) due to central sympathetic activation and vascular adenosine receptor blockade (Vlachopoulos et al., 2007). Although tolerance to this pressor effect has been widely assumed, such tolerance was found in only half of regular consumers (Lovallo et al., 2004). However, habitual coffee intake was not associated with an increased incidence of hypertension in two separate meta-analyses (Mesas et al., 2011; Steffen et al., 2012).
Thus, the effects of caffeine on hypertension may, over the long term, be neutral but, at least acutely, a pressor effect may be noted. Perhaps the wisest course is to order an ambulatory BP monitor or simply have patients check their home BPs before and within an hour after drinking their coffee, tea, or caffeinated soft drink. Those who experience a large pressor effect should be advised to reduce or stop their caffeine consumption, particularly if does not habituate.
Nicotine and Smoking
Among U.S. adults, currently an astounding 32% use one or more tobacco products (Lee et al., 2014). While cigarette smoking rates have stabilized or declined recently in the U.S., both cigar and Hookah (water pipe) smoking are increasing at alarming rates (Lee et al., 2014). Among a representative survey of 3 billion people in 16 low or middle income countries conducted between 2008 and 2010, 49% of men and 11% of women smoked a tobacco product (Giovino et al., 2012). Clearly, the world public health community is waging an uphill battle against Big Tobacco.
The next big epidemic is electronic cigarettes, or e-cigarettes, which deliver nicotine but replace combustible smoke with steam (Fairchild et al., 2014). Thus e-cigarettes represent harm reduction rather than abstinence. However, in a randomized trial of 657 cigarette smokers, after 6 months, verified smoking abstinence was only 7% with nicotine e-cigarettes, 6% with nicotine patches, and 4% with nicotine-free placebo e-cigarettes (Bullen et al., 2013). If they are not very effective smoking tobacco aids, could e-cigarettes backfire by encouraging dual cigarette habits or even serve as a bridge to traditional cigarette smoking (Fiore et al., 2014)?
As described in Chapters 3 and 6, even in chronic smokers, each cigarette induces a pressor response (Mahmud & Feely, 2003). Whereas the peripheral BP returns to near baseline within 15 minutes, pressure within the aorta remains higher. Moreover, the indices of large artery stiffness start higher in the chronic smokers and remain higher than in the nonsmokers. These hemodynamic consequences of smoking have been underestimated for two reasons: First, in the smoke-free environment where patients are seen, the BP is usually measured well after the acute effects are over; second, the arm (peripheral) BP is usually deceptively lower in chronic smokers who have reduced aortic–brachial pressure amplification (Mahmud & Feely, 2003). A similar acute increase of larger artery stiffness has been seen with cigar smoking (Vlachopoulos et al., 2004).
Data on prevalence of persistent hypertension among smokers have not been consistent: Most find them to have higher BP recorded by ambulatory monitoring while they continue to smoke (Oncken et al., 2001), but if the BP is taken while subjects are not smoking, little more hypertension is seen (Halimi et al., 2002). When chronic smokers quit smoking, their BPs tend to rise in large part because of weight gain (Halimi et al., 2002). On the other hand, the data are much clearer that both active cigarette smoking (Hänninen et al., 2014) and passive second-hand smoke (Seki et al., 2010) are a major cause of masked hypertension. Hookah smoking has been identified as a risk factor for hypertension and metabolic syndrome in a Middle Eastern population, where this practice has been endemic for centuries (Shafique et al., 2012).
Smoking has been found to have a profoundly deleterious effect on renal function (Orth & Ritz, 2002), and on cognitive function (Sabia et al., 2008). Moreover, the 2,983 smokers enrolled in the massive Hypertension Optimal Treatment (HOT) trial were the only subgroup to experience an increased risk of major cardiovascular events when given more intensive therapy to achieve a lower BP (Zanchetti et al., 2003). As the authors note, these data “strengthen the need for concerted efforts to persuade patients to quit smoking.” This potentially important finding is yet to be confirmed in an independent study. For the majority of patients who do not quit, the general goal of antihypertensive therapy is less than 135/85 mm Hg for their home BP—which will be higher than their BP in the smoke-free physician’s office.
Alcohol
Alcohol is a two-edged sword: In excess, it is a major cause of social disorder, trauma, and death; in moderation (one drink per day for women, two for men), it is a protector against heart attack, stroke, diabetes, and heart failure (O’Keefe et al., 2014). Part of its diverse roles involves hypertension: In excess, alcohol raises the BP; in moderation, it may be protective against the development of hypertension (see also Chapters 3 and 6).
The Relation to Hypertension
When consumed in amounts equivalent to three usual portions—a usual portion being 12 oz of beer, 4 oz of wine, or 1.5 oz of whiskey which all contain about 12 g of ethanol—alcohol causes an immediate depressor effect and subsequently a pressor action (Rosito et al., 1999). These changes are reflected in the measurements of arterial stiffness by pulse wave velocity (Sasaki et al., 2013).
In large population studies, the incidence of hypertension is increased among those who drink more than three drinks per day (Ohira et al., 2009), either in a linear dose–response relationship or with a threshold wherein smaller quantities are associated with a modest decrease (O’Keefe et al., 2014). The cessation of heavy drinking is usually followed by significant falls in BP (Ohira et al., 2009). The mechanism for the pressor effect of large quantities of ethanol is not well defined but may involve central activation of the sympathetic nervous system and release of corticotropin-releasing hormone (Randin et al., 1995).
Relation to Other Diseases
Light to moderate consumption, i.e., less than three drinks per day, has been shown to provide multiple significant benefits as detailed in Chapter 3.
This litany of benefits must be balanced by the potential for encouragement of alcohol abuse and a high prevalence of excessive drinking among the elderly (O’Connell et al., 2003). Among 553 elderly subjects (mean age 71), heavy drinking was associated with higher diastolic BP by 24 hour ambulatory BP monitoring while light drinking was associated with reduced daytime BP variability (Jaubert et al., 2014).
Gout is more common among even light drinkers (Choi et al., 2004). Light to moderate alcohol consumption of three to six drinks per week was found to cause a small increase in the risk of breast cancer in the Nurses’ Health Study (Chen et al., 2011) and a recent review of the literature (Scoccianti et al., 2014). Moreover, as described in Chapter 3, a genetic mutation may cause some people to be bothered by even small amounts of alcohol (Tseng et al., 2008).
We have no hesitation in allowing hypertensives to drink in moderation, but others do not believe that drinking any amount of alcohol should be recommended by physicians (O’Keefe et al., 2014).
Nonsteroidal Anti-Inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are well known to raise BP, blunt the antihypertensive effect of some antihypertensive agents, and increase the risk of MI and stroke (Patrono & Baigent, 2014). This interference likely reflects an inhibition of prostaglandin-dependent counterregulatory mechanisms in the kidney that have been invoked by the antihypertensive drugs and possibly reduction in endothelial nitric oxide synthase. Reduced generation of NO and inhibition of cyclooxygenase enzymes may induce renal sodium retention and thereby increase the BP and precipitate hypertension, increasing the risk of stroke (Patrono & Baigent, 2014).
A cohort study of 5,710 hypertensive subjects in the French health insurance system found that ACEIs and ARBs were the only classes of drugs requiring intensification of antihypertensive therapy after the addition of a non-steroidal anti-inflammatory drug (NSAID) (Fournier et al., 2012). Moreover, a recent case–control study of the UK Clinical Practice Research Database found that the addition of a NSAID to combination therapy with ACEI or ARB plus a diuretic is associated with a 31% increase in the risk of acute kidney injury, 82% within the 1st month of therapy (Lapi et al., 2013).
The American College of Rheumatology recommends that acetaminophen should be initial therapy for osteoarthritis (Hochberg et al., 2012). If 4,000 mg/day does not provide symptomatic relief, oral NSAIDs are recommended except for patients aged 75 years or older in whom topical rather than systemic NSAIDs are recommended. A recent systematic review found conflicting and inconclusive evidence as to whether acetaminophen increases BP in patients with or without hypertension (Turtle et al., 2013). Clearly, the risk of increased BP and associated CV events is lowest with acetaminophen and low-dose aspirin (81 mg daily), intermediate with non–COX-2-selective NSAIDs including high-dose aspirin, and highest with COX-2-selective NSAIDs (Antman et al., 2007).
Immunosuppressive Agents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








