Fig. 13.1
Sagittal T1-weighted MRI. Note the Chiari I malformation (long arrow) and cervical syrinx (short arrow)
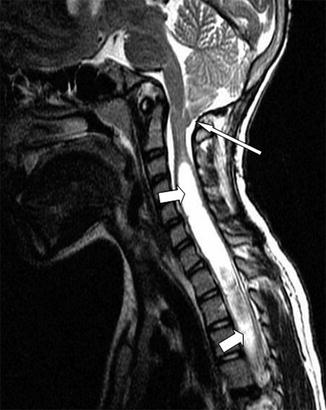
Fig. 13.2
Sagittal T2-weighted MRI. Note the Chiari I malformation (long arrow) and holocord syrinx (short arrows)
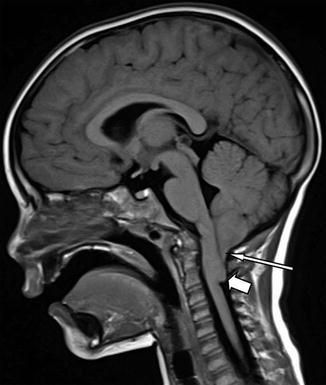
Fig. 13.3
Sagittal T1-weighted MRI. Note the pointed cerebellar tips (long arrow) often seen in the Chiari I malformation and elongation of the brainstem (short arrow)
Patients suspected of having Chiari I malformation should also undergo imaging of the brain, as a small percentage of patients will be found to have associated hydrocephalus , nearly one in ten cases in one large series. Those with untreated hydrocephalus should have this addressed, prior to any decompressive procedure at the craniovertebral junction, and those patients with an already shunted hydrocephalus should have the functioning of the shunt confirmed.
Other radiographic abnormalities are seen in association with the Chiari I malformation, including retroverted odontoid process which was found in 24 % of operative Chiari I patients in one series. Other reported associations include scoliosis (18 %), atlanto-occipital fusion (8 %), Klippel-Feil anomaly (3 %) and basilar invagination (3 %) (Tubbs et al. 2011). Plain flexion-extension radiographs of the cervical spine are helpful in resolving the question of spinal instability and can further delineate the extent of the bony abnormalities. The presence of significant basilar invagination should alert the practitioner to the possible need for an anterior decompression, prior to a posterior decompression (Oakes 1991).
Cine-MRI may be used to assess flow across the foramen magnum. Some authors find it a valuable tool in decision making, whilst others find that its usefulness is dependent upon the software used to process the acquired sequences (Soleau et al. 2008; Ventureyra et al. 2003).
13.2.3 Surgical Intervention
Surgical manoeuvres in the paediatric Chiari I patient include bony decompression of the posterior fossa, usually with exploration and decompression of the fourth ventricular outlet. In most cases this necessitates removal of the posterior arch of C1 as well, with excision of the sub-adjacent fibrous band and duraplasty. Coagulation or partial resection of the herniated tonsils may be necessary, if they obstruct fourth ventricular outflow or in the event that a re-operation is needed due to failure of the syrinx to collapse after the initial procedure.
Controversy exists regarding the necessity for duraplasty in the treatment of Chiari I malformation. A small retrospective study of 11 adult patients, who underwent bone only decompression, with removal of the posterior arch of C1, found that eight of the patients had improvement in their symptoms. Seven of these patients also had syringomyelia, and three had radiological decrease in the size of their cavities and improvement in their symptoms. All three of these patients also had increased posterior fossa volume on post-operative imaging (Munshi et al. 2000). A study of brainstem auditory evoked potentials in 11 paediatric patients, before and after duraplasty, found that no additional improvement was noted following this manoeuvre (Anderson et al. 2003). Intraoperative ultrasound has been employed in an effort to identify patients who might benefit from duraplasty, in addition to bony decompression (McGirt et al. 2008; Yeh et al. 2006). A retrospective study of 256 paediatric patients stratified the success rates of bone only decompression versus duraplasty, according to the extent of tonsillar herniation. In children with tonsillar herniation caudal to C1, suboccipital decompression alone was associated with a twofold increase in the risk of symptom recurrence, when compared with those children who also underwent duraplasty. Children who had tonsillar herniation rostral to C1 had equivalent outcomes when undergoing duraplasty or bone only decompression (McGirt et al. 2008).
A large retrospective review of 500 surgically treated paediatric Chiari I patients, of whom 285 also had syringomyelia, found that 12 % of this latter group had an arachnoid veil occluding the fourth ventricular outlet. Fifteen patients in total required re-operation, 13 for persistent syringes and two for persistent Valsalva-related headache. One of the latter, two improved following re-operation with duraplasty. Of the 13 patients with persistent syrinx, 11 had resolution of their hydromyelia with re-operation and tonsillar coagulation. The two patients that did not improve following re-operation enjoyed some decrease in their syrinx size following subsequent placement of syringopleural shunts (Tubbs et al. 2011).
Some authors have noted an association between duraplasty and a higher complication rates (Navarro et al. 2004). Others have noted a small but significant correlation between duraplasty and longer hospitalisations (Yeh et al. 2006). Other authors report low complication rates but including transient acute hydrocephalus and extra-axial subdural collections (Elton et al. 2002). Other complications include direct vascular or neural injury, bleeding from dural venous sinuses, CSF leak , meningitis and pseudomeningocoele formation. Sagging or slumping of the cerebellum may also be encountered, in the setting of overly generous craniectomy and dural grafting. In this case, a partial cranioplasty, with revisional duraplasty, to create a cerebellar ‘sling’, can be effective in supporting the herniated cerebellum (Holly and Batzdorf 2001). Bone regrowth at the foramen magnum and at the site of the C1 bone removal has been reported in young children and infants (Aoki et al. 1995).
13.2.4 Response to Cervicomedullary Decompression
A review of 49 paediatric patients operated on for Chiari I and syringomyelia observed that just over half had radiological improvement in their syringomyelia during a mean follow-up period of 41 months. The median time until radiological improvement was 14 months following surgery. The same proportion of children enjoyed symptomatic improvement with a median time to improvement of 4 months (Attenello et al. 2008).
A series of 500 operated paediatric patients, of which 57 % had syringomyelia, included a re-operation rate of 3 %, mostly for persistent syrinx. Posterior fossa decompression was sufficient in relieving symptoms in 80 % of patients at the time of the first operation and in 95 % of patients following a second operation. In the 13 patients with persistent syringomyelia, all but two improved with re-exploration alone. The two patients who did not improve following re-operation underwent placement of syringopleural shunts, but given the success rates of re-operation, the authors have almost completely abandoned the practice of syrinx shunting (Tubbs et al. 2011). In this same series, an arachnoid veil was found to be obstructing the outlet of the fourth ventricle in 12 % of patients with syringomyelia. Thirty patients in the series underwent placement of stents, passing from the fourth ventricle into the subarachnoid space. These patients were less likely to have resolution of their syringomyelia, although this may be because patients selected for stent placement had unfavourable anatomical features.
13.3 Chiari II Malformation and Syringomyelia
The Chiari II malformation is characterised by caudal displacement of the cerebellar vermis, brainstem and fourth ventricle below the level of the foramen magnum (Fig. 13.4). It occurs exclusively in association with myelodysplasia. In the United States, the historical prevalence of myelomeningocoele is cited as one to two per 1,000 live births, but the advent of prenatal screening and increased awareness of the need for maternal folic acid supplementation has decreased the prevalence to between 2 and 4.6 cases per 10,000 births (Honein et al. 2001; Piatt 2010), although regional variations in prevalence do exist (Shurtleff 2004). The frequency of syringomyelia in Chiari II patients varies between different reports, from 40 to 80 %, and was documented in 20 out of 26 (3 in 4) autopsy cases of young children born with myelomeningocoele (Piatt 2004). The true incidence may, in fact, be underestimated because some pre-existing but undiagnosed cavities may collapse, following shunting of hydrocephalus in infancy.
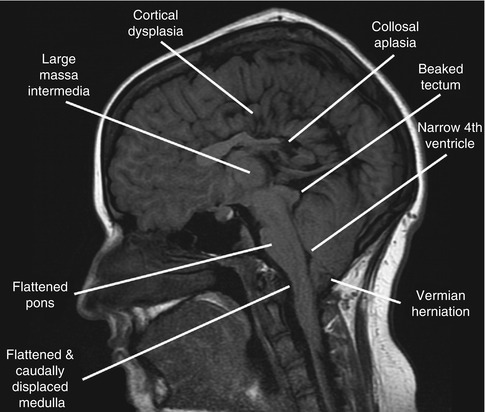

Fig. 13.4
Sagittal T1-weighted MRI. An adult patient with Chiari II malformation
Syringomyelia cavities are often found in the lower cervical and upper thoracic cord and may be missed on routine cranial imaging. Low lumbar syrinx cavities are rare but are frequently associated with rapidly progressive scoliosis and are less likely to respond to ventricular shunting (Piatt 2004). Spinal arachnoid cysts may also be found in association with Chiari II malformation.
Biopsy specimens of the cyst walls, when analysed by electron microscopy, have demonstrated the presence of axons which suggests that some of these cysts are actually formed by dorsal rupture of eccentrically located syringes (Heinz et al. 1992).
There also appears to be an association between untreated or partially treated hydrocephalus and syringomyelia. A study of 18 Chiari II patients with progressive myelopathy, who underwent radionucleotide ventriculography, demonstrated the accumulation of tracer in the syrinx cavities of the 14 patients with untreated hydrocephalus (Batnitzky et al. 1976). These observations underscore the need to confirm the presence of a functioning shunt in Chiari II patients with shunted hydrocephalus that present with syringes.
13.3.1 Presentation of Chiari II
Patients with Chiari II malformations present at birth with myelomeningocoele . The outlook for this population is somewhat restricted, and historically, approximately one third of the neonates with symptoms of brainstem dysfunction did not survive beyond infancy. Survival has, however, increased with prompt ventricular shunting, shunt revision when needed and posterior fossa decompression when appropriate (Talamonti et al. 2007). Overall, some 20–30 % of Chiari II patients will become symptomatic from their hindbrain herniation at some point in their lifetime.
Clinical presentation varies in different age groups. Infants and neonates can present with apnoea , inspiratory stridor, dysphagia , bradycardia and opisthotonus. The dysphagia may be severe enough to necessitate placement of a gastrostomy tube. When present at birth, such symptoms suggest hypoplasia or aplasia of brainstem nuclei and often predict a poor prognosis. More commonly, symptoms begin within the first few months of life and when they are seen in a previously well infant attention should first be given to normalisation of the intracranial pressure through shunt placement or shunt revision if necessary (Holinger et al. 1978; Soleau et al. 2008). Feeding and swallowing difficulties are the most common presenting symptoms for infants at risk and were found in 59–71 % of symptomatic Chiari II patients in one series (Pollack et al. 1992b). Infants may present with weak suck and prolonged feeding times, and they may demonstrate weight loss and failure to thrive, as their physical growth outpaces their ability to meet their nutritional needs. Breathing difficulties are the most dangerous symptom in Chiari patients and have been reported to occur in between 29 and 76 % of patients (Rauzzino and Oakes 1995). Inspiratory stridor is the result of bilateral tenth nerve paresis, with weakness of the vocal cord abductors. It may be caused by direct traction on the tenth nerve or by damage to the dorsal motor nuclei, secondary to micro-haemorrhage or compressive ischemia (Benjamin et al. 2009; Linder and Lindholm 1997; Rauzzino and Oakes 1995). One series observed that all infants began with normal swallowing and feeding that progressively deteriorated (Pollack et al. 1992b). In these cases, they found that feeding problems preceded respiratory difficulties and were a recognisable warning sign. In another publication, the same group found that all neonates that underwent surgical decompression, prior to the development of bilateral vocal cord paralysis , demonstrated some improvement in neurological function following surgery (Pollack et al. 1992a).
Older children more commonly have symptoms of spinal cord compromise, and the syringomyelia associated with Chiari II malformation behaves in a manner similar to that seen in Chiari I patients. Classic presentation is with lower motor neuron features in the arms and upper motor neuron findings in the legs. These include atrophy of the small muscles of the hands , together with disassociated sensory loss. Progressive enlargement of the syrinx cavity leads to loss of the ability to perform fine motor tasks, and fasciculation and loss of tendon reflexes may be evident in the upper limbs. Further expansion of the syrinx may impede descending corticospinal tracts, such that patients who were previously ambulatory may report a history of increasing falls, coming on over months or years.
Syringobulbia , or expansion of the syrinx into the brainstem, will manifest itself as dysfunction in multiple brainstem nuclei, which may wrongly be attributed to the underlying Chiari II malformation. Neurogenic athropathies may also be present. Back pain and scoliosis (see below) may also be the initial presenting complaints.
Changes in bowel or bladder function , however, should prompt imaging to exclude a tethered cord (Rauzzino and Oakes 1995). Headache and posterior cervical neck pain are also common and may be due to the distortion of the descending fibres of the spinal trigeminal tract or the upper cervical roots.
13.3.2 Imaging Chiari II
Radiographic assessment of patients with Chiari II malformation can begin with plain films of the cervical spine. Flexion and extension views can be used to assess for cervical instability . The posterior arch of C1 is incomplete in up to 70 % of cases and may be replaced by a compressive band of periosteal tissue. Full spine radiographs permit assessment of scoliosis, segmentation errors and other occult dysraphisms. Rarely, split cord malformation , with a bony median septum, may be identified on plain films (Rauzzino and Oakes 1995).
CT images are inadequate for studying the pathology of hindbrain herniation, but head CT imaging is helpful in evaluating ventricular size and can provide an objective measurement of the adequacy of ventricular shunting, although surgical exploration remains the ‘gold standard’ for the determination of the adequacy of shunt function.
MRI is the diagnostic tool of choice in evaluating patients with suspected Chiari malformations. The hallmark of the Chiari II malformation is the elongation and caudal displacement of the cerebellar vermis, medulla and lower brainstem, below the foramen magnum. In up to 70 % of Chiari II patients, a medullary kink may be identified. Elongation of the fourth ventricle into the upper cervical cord is also a common finding. The cerebellum of Chiari II patients is smaller than normal and is contained in a hypoplastic posterior fossa with obliterated retrocerebellar CSF spaces (Fig. 13.5). The tentorium cerebelli has a low insertion and can also be hypoplastic, allowing the cerebellum to tower above it. Other frequently observed cerebellar findings include persistent foetal location with extension around the midbrain into the cerebellopontine cisterns and cerebellar dysplasias and heterotopias (Rauzzino and Oakes 1995).
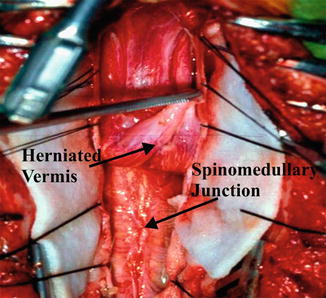

Fig. 13.5
Operative view of the Chiari II malformation
13.3.3 Surgery for Chiari II Malformations
The surgical management of Chiari II malformation generally consists of bony decompression followed by some form of dural expansion manoeuvre. Prior to any posterior fossa decompression, however, patients with questionable shunt function should first undergo shunt revision. This approach can sometimes avoid the need for posterior fossa surgery because reducing the downward pressure on the cerebellum may lead to resolution of symptoms. Obtaining effective ventricular drainage may also lead to collapse of the syrinx, without recourse to major posterior fossa surgery. On the other hand, ignoring an active hydrocephalus and performing a craniovertebral decompression may sometimes lead to a potentially dangerous exacerbation of a hydrocephalus that was previously in a state of hydrodynamic equilibrium .
Reference to pre-operative MRI assists in the localisation of the vermian herniation, a medullary kink, the fourth ventricle and a low-lying torcular Herophilus and transverse sinus. For patients with a large foramen magnum, occipital craniectomy may not be required. If the torcular is low-lying, care must be taken to avoid inadvertent entry into the major venous sinuses, which could lead to dangerous blood loss. Care must be taken to distinguish the medullary kink from the cerebellar vermis, and intraoperative ultrasonography may be useful in this regard (Tubbs and Oakes 2004). In Chiari II patients, the choroid plexus usually retains its embryonic extraventricular location and marks the caudal end of the fourth ventricle.
Unlike with Chiari I malformations, adequate decompression of the Chiari II hindbrain hernia requires that the decompression should extend to the level of the displaced posterior fossa content. The herniated brainstem, unlike the cerebellar tonsils, cannot be delivered upwards or coagulated (Fig. 13.6). The fourth ventricular outlet should be identified and widely opened, and it is often necessary to develop several tissue planes, before the floor of the fourth ventricle can be clearly identified. Misidentification of anatomical landmarks or excessive tissue manipulation can result in damage to at-risk parts of the medulla or lower pons. Dense adhesions and hypervascularity may be encountered at points of compression or traction, making identification and dissection of these structures more difficult.
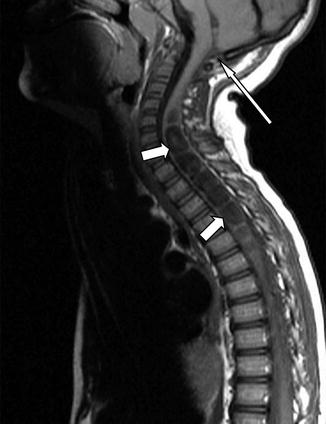

Fig. 13.6
Sagittal T1-weighted MRI of the Chiari 0 malformation. Note the absence of cerebellar tonsillar herniation (long arrow) and large cervicothoracic syrinx (short arrows) that resolved following posterior fossa decompression
13.3.4 Surveillance
Early surgical therapy may prove to be life saving for symptomatic Chiari II patients, when the symptoms are attributable to brainstem dysfunction. For infants and neonates who present with respiratory distress and stridor, the timing of decompression is important. Beyond this age group, approximately 10 % of patients who survive will have late symptoms of brainstem dysfunction (Talamonti et al. 2007). Continued well-child surveillance and family education can therefore help to ensure that late presentations of brainstem compression do not go unrecognised. The importance of having a functioning shunt in this patient population should again be stressed. Obstructive apnoea , which can have a mortality rate as high as 60 %, can be reversed by an optimally functioning shunt (Cochrane et al. 1991; Hesz and Wolraich 1985; Soleau et al. 2008).
The extensive laminectomy required to decompress a Chiari II malformation increases the risk of delayed cervical instability , and the patients should undergo post-operative cervical spine evaluation (Oakes 1991).
13.4 Chiari 0
The term Chiari 0 malformation has been used to describe a subset of patients with syringomyelia and without cerebellar tonsillar ectopia but with observed ‘crowding’ of the posterior fossa (Tubbs et al. 2001; Weprin and Oakes 2001). These patients do not have caudal displacement of their cerebellar tonsils, beyond that which is considered to be within the normal anatomical range (Soleau et al. 2008). Analysis of the posterior fossa in these patients has, however, demonstrated that they have caudal displacement of their brainstems, with a low-lying obex and a hypoplastic posterior fossa (Weprin and Oakes 2001). These patients are considered to have impaired circulation of CSF at the outlet of the fourth ventricle and across the craniovertebral junction. In addition, those that come to surgery are often found to have barriers to free egress of CSF. The first published series described five patients with syringomyelia but without hindbrain herniation. They all underwent posterior fossa decompression that entailed a suboccipital craniectomy, removal of the posterior arch of C1 and duraplasty. Additionally, the patients underwent lysis of adhesions, opening of the fourth ventricular veil (if present) and partial resection of one of the cerebellar tonsils. Four patients had resolution of their symptoms post-operatively, and all went on to display a diminution in the size of their syringes (Iskandar et al. 1998).
13.5 Scoliosis
There is a particularly marked association between scoliosis and syringomyelia in children. One review identified scoliosis in 4 out of 5 syringomyelia patients under 20 years of age, compared with 1 in 6 older patients (Isu et al. 1990). Scoliosis is the presenting feature of syringomyelia in as many as two thirds of children (Isu et al. 1992). Features that alert an orthopaedic surgeon to the possibility of such underling cord pathology include male sex and curves that are convex to the left; idiopathic curves are more commonly convex to the right and have a predilection for girls. The presence of neurological symptoms and signs as well as rapid progression of a curve also suggest a neurogenic aetiology. Extensive cord cavitations, comprising 50 % or more of the cross-sectional diameter of the cord, are more likely to experience progression of their scoliosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







