Chapter 12 Pediatric Neurophysiologic Evaluation
Pediatric neurophysiologic studies are an integral part of the diagnostic evaluation of the infant or child with suspected brain dysfunction; electroencephalography (EEG), polysomnography, evoked responses, and computerized neurophysiological analyses comprise four general diagnostic modalities. Pediatric neurologists must apply proper instrumentation and recording techniques to obtain reliable pediatric neurophysiologic studies in an EEG laboratory or at a child’s bedside. With proper knowledge of these techniques, the clinician can better appreciate the clinical relevance of these studies. Neurophysiological evaluations take on heightened diagnostic and prognostic significance when compared with neuroimaging studies. As a research tool, neurophysiological studies constitute a powerful measure of functional neuronal organization and maturation, particularly when applying quantitative measures during resting, stressful, or disease states. Functional-structural comparisons between neurophysiology and neuroimaging yield far greater information about neurologic health and disease. Genotype-phenotype comparisons are now being investigated that will redefine neurophysiological studies as endophenotypes to study gene–environment interactions [Scher and Loparo, 2009].
In the first section of this chapter, recording techniques unique to children are introduced. As outlined in the second section, maturational changes in arousal states of children are emphasized in considering normal or age-specific patterns. Third, abnormal EEG patterns are discussed in the context of common pediatric neurologic disorders [Blume, 2002; Ebersole and Pedley, 2003; Niedermeyer and Lopes da Silva, 2005]. A fourth section is devoted to computer applications of neurophysiological studies for clinical and research purposes.
The neurologist should consistently follow analytic steps in the interpretation of neurophysiologic studies. Proper interpretative skills ultimately require two essential didactic elements that are beyond the scope of this discussion: presentation of multiple examples of the same neurophysiologic phenomena, and use of typical and atypical clinical situations in which common and complex neurophysiologic patterns are observed. Several excellent reviews supplement this discussion and can assist the physician with the evaluation of children who exhibit these phenomena [Binnie, 2003; Blume, 2002; Cracco and Cracco, 1986; Daube, 2002; Ebersole and Pedley, 2003; Mizrahi and Kellaway, 2003; Niedermeyer and Lopes da Silva, 2005; Scher, 2005a; Spehlmann, 2001].
Utility of Pediatric Neurophysiological Studies
Neurophysiological interpretation can assist in the diagnosis and prognosis of specific neurologic conditions. Recognizing specific situations in which the EEG findings are helpful allows efficient and effective use of this comparatively low-cost and safe tool [Drazkowski, 2003]. However, there is little advanced evidence-based medicine in the literature on the subject of pediatric clinical neurophysiology. In a review published by Medline and Cochrane [Fowle and Binnie, 2000], there is a lack of evidence-based medicine concerning the efficacy of EEG interpretation, as well as the standardization of pediatric neurophysiological training. Only retrospective studies have assessed the efficacy of EEG results in the evaluation of neurologic conditions for children, uniformly reporting that interictal EEG findings may be overused during the evaluation of various pediatric brain disorders [Aydin et al., 2003], whereas normal EEG findings are highly predictable in children with nonepileptic conditions [Jan, 2002]. Although the physician should not ignore multivariable analyses in which EEG is used to differentiate risks, diagnostic decision-making still rests on clinical effectiveness data, rather than EEG diagnoses [Gilbert and Buncher, 2000]. The clinician must consider the clinical settings of the outpatient clinic, emergency department, or intensive care unit in which the indications and diagnostic yield of the EEG will vary [Alehan et al., 2001; Varelas et al., 2003], based on the selection bias of the pediatric groups chosen for study. EEG can also be useful as an independent prognostic indicator of outcome for a general patient population, conveying information about the general level of illness and the cost of caring for patients.
The standard of the EEG services and the accuracy of the EEG interpretation must not be overlooked when recommendations and management of pediatric neurological problems are being developed. The inappropriate use or incorrect interpretation of the EEG, or both, is the most common reason for misdiagnosing common conditions such as epilepsy [Tan et al., 2008].
Training in Clinical Neurophysiology
Although the neurology trainee receives basic clinical neurophysiology instruction during residency, it is recognized that neurologists with more training and experience in clinical neurophysiology are also needed, in part because of the increased complexity of the specialty and the demand for high-quality care for people with complex neurologic diseases. Accredited training programs in clinical neurophysiology and individual certification in the subspecialty of clinical neurophysiology have been available since 1993 [Burns et al., 2000]. Standards of practice in clinical electroencephalopathy have been published regarding recommendations for qualifications of both electroencephalographers and technologists, laboratory organization, and appropriate instrumentation. An adequate duration of training with appropriate curriculum requirements has been described. Future opportunities for the clinical neurophysiology subspecialist will lead to concentrations in specific areas of neurophysiology, such as for epilepsy, sleep medicine, and neurointensive care. Pediatric neurologists must also satisfy these training requirements, with special emphasis on the unique aspects of developmental clinical neurophysiology for office and hospital settings, including neonatal and pediatric intensive care units and pediatric epilepsy monitoring units. In addition to the more thorough training requirements for pediatric neurologists, practice parameters have been more rigorously enumerated to develop strategies for patient management that are based on evidence-based medicine. Suggested parameters include the use of EEG more selectively, such as for children with specific neurologic conditions such as development delay, cerebral palsy, or headache [Ashwal et al., 2004; Lewis et al., 2002; Shevell et al., 2003], or in specific clinical settings such as intraoperative monitoring [Husain, 2009]. Revised guidelines require re-evaluations to improve the use of EEG methodology and training across disciplines, such as for the diagnosis of epilepsy [Flink et al., 2002; Tan et al., 2008].
Instrumentation and Recording Techniques
Each EEG channel measures the electropotential difference between two points on the scalp under the assigned electrodes. Ten electrodes are required as a minimum number for adequate EEG recordings [AES, 1986]. A greater area of cerebral activity can be measured with more EEG channels. EEG laboratories devoted to pediatric studies should use 16–24 channels. In the neonate, it may be more practical to apply fewer electrodes, as discussed later in this chapter. The International 10-20 System of electrode placement is the standard method of electrode application, and it routinely requires the placement of 22 electrodes on the head. The basis of the 10-20 system is the measurement of four standard points on the head: nasion, inion, and left and right preauricular points [Holmes, 1987; Niedermeyer and Lopes da Silva, 2005]. Electrodes are spaced at 10 or 20 percent of the total distance between pairs of skull landmarks; scalp locations correspond to anatomic landmarks. Electrode names correspond to the underlying brain regions over which they are positioned. Electrodes with odd numbers correspond to regions in the left hemisphere, and even numbers refer to areas in the right hemisphere. Midline electrodes have a zero (z) as a suffix. Additional electrodes may be used to localize electrical activity. Readjustment of the electrode placements may be required for preterm neonates and for patients with skull deformities.
Polarity Localization
Proper electrode placement and montage choices allow accurate comparisons between homologous brain regions. In this way, the neurologist can localize EEG data on an anatomic basis and can standardize regional or hemispheric findings over multiple EEG recordings. An understanding of the principles of polarity is essential in using the localization technique [Ebersole and Pedley, 2003].
Inputs for two electrodes for one EEG channel are generated by two differential amplifiers, called input terminal one and input terminal two. With a differential amplifier, the output is proportional to the voltage difference between these two input terminals. The direction of the pen deflection depends on differences in polarity of this voltage difference. By convention, when input terminal one is relatively more negative, there is an upward deflection. Other possibilities include a downward deflection if input terminal one is relatively more positive. The reverse situation pertains to input terminal two; when input terminal two is relatively more negative, there is a downward deflection [Cooper et al., 1980].
Instrumental Control Settings
Filter Settings
High- and low-frequency filters are required to facilitate accurate representation of the appropriate range of frequency-specific waveforms of cerebral origin. Adjustment of these filters should be made judiciously so that genuine brain-generated activities are not eliminated along with undesirable, noncerebral artifactual signals. High-frequency filters are set at a standard 70 Hz so as not to blunt the appearance of fast frequencies, including abnormal spikes and sharp waves. Occasionally, lower settings may be needed when excessive fast-frequency artifacts exist, such as 60-cycle interference from other electrical devices. Low-frequency limits, commonly referred to as time constants, routinely vary between 0.3 and 0.1 seconds. For situations when slow-frequency waveforms need to be analyzed or eliminated, longer or shorter time constants, respectively, can be chosen. This procedure is particularly important in EEG recordings of neonates and young infants in whom slower frequency activities need to be clearly represented. The use of DC recordings has also been advocated to study both maturation and disease states [Vanhatalo et al., 2005]. Conversely, high-frequency oscillations also have been studied to improve prediction of cognitive function, maturation, and brain pathology [Ben-Ari et al., 2007; Herrmann et al., 2009].
Artifact Recognition
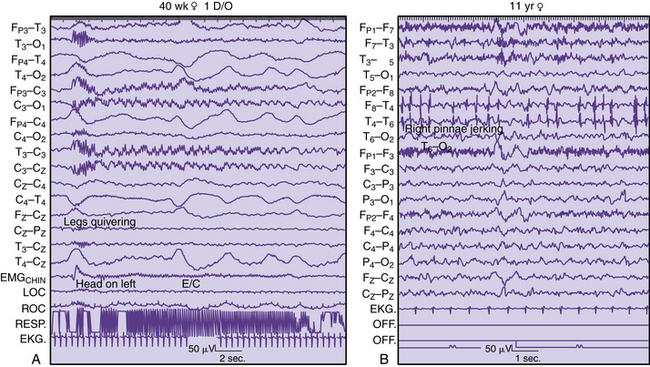
As with adults [Richey, 1976], physiologic and nonphysiologic artifacts must be properly identified in pediatric recordings because either may interfere with the interpretation of cerebral activity. Three basic forms of artifacts have been classified: instrumental, external, and physiologic. Accurate descriptions by the technologist can assist the neurologist in the diagnosis of a neurologic disorder in adults [Klass and Reiher, 1968], children [Blume, 2002], and neonates [Scher, 1985] (Figure 12-1). Such artifacts may help define normal and abnormal sleep and waking behaviors. The technologist should try to identify the source of any artifacts, initially to reproduce them and subsequently to eliminate artifacts that were identified during the recording.
Recording Setting
A minority of children may require sedation. The need for sedation can be substantially reduced by adequate preparation of the child, including the creation of a less-threatening environment in which to perform the study. One report described a decreased proportion (32 to 2 percent) of 513 children who needed sedation over a 4-year period when appropriate preparation was provided [Olson et al., 2001]. Chloral hydrate (50–80 mg/kg; maximum dose, 2 g) has historically been the most commonly used sedative, but should be administered only to a child who has not been recently fed and cannot tolerate the study in the natural state. Rectal administration may be advantageous for the encephalopathic or extremely uncooperative child who requires the study [Thoresen et al., 1997]. Psychotropic or sedative drugs should be avoided because they produce more profound behavioral and EEG effects. Infant recordings should be obtained around a scheduled feeding or nap. At least 90 minutes should be scheduled for electrode application, EEG recording, and clean-up. With some exceptions, the parents’ presence during the recording session should be discouraged. A child-life specialist or similarly trained individual can provide developmentally appropriate and child-friendly support. The technologist should accurately record the pertinent clinical evaluation of the child that explains the reason for obtaining the EEG study. Information from parents or the medical record should include the child’s age; gestational and postconceptional ages should be included for neonates. The ordering physician should be identified, and the written request for an EEG study should clearly state the clinical problem that prompted the EEG request.
Neurophysiological Basis for Electroencephalography
Electrical activity generated by the brain can be classified into three broad categories. Spontaneous and evoked electrical activity detected on the scalp as oscillatory potentials is discussed primarily in this section. Electrical potentials generated by single neurons recorded by microelectrodes are a third type that is briefly mentioned in the context of the neurophysiologic basis of EEG studies. Informative reviews of the neurophysics of cerebral electrical fields are available in other sources [Ebersole and Pedley, 2003; Niedermeyer and Lopes Silva, 2005; Nunez, 1981; Olejniczak, 2006].
Depolarization, represented by an action potential, travels down the axon to synaptic clefts, where chemical transmitters are released. Presynaptic membrane release of neurotransmitters begins the process of cell-to-cell communication, with activation of the postsynaptic membrane by depolarization or hyperpolarization. Depolarization by specific neurotransmitters causes an inward flux of sodium, creating a voltage gradient that is called the excitatory postsynaptic potential. Hyperpolarization by other neurotransmitters causes an outward flow of sodium, contributing to an inhibitory postsynaptic potential. Summation of excitatory postsynaptic potentials and inhibitory postsynaptic potentials within a region involving a number of synapses determines the degree of postsynaptic membrane depolarization or hyperpolarization. If excitatory postsynaptic potentials predominate, depolarization will lead to an action potential. If inhibitory postsynaptic potentials predominate, hyperpolarization will follow, preventing the occurrence of an action potential. Although synaptic potentials are of lower voltage than action potentials, their longer time course and larger membrane surface area contribute primarily to scalp-detected EEG activity. Figure 12-2 illustrates the generation of extracellular voltage fields from graded synaptic activity, depicting the relationship between polarity of surface potentials and the site of dendritic postsynaptic potentials [Ebersole and Pedley, 2003]. EEG fields are primarily generated by the large, vertically oriented pyramidal neurons located in cortical layers III, V, and VI. Because of the attenuating properties of the skull, spatial summation of cortical activity is critical for producing a voltage field recordable from the scalp (Figure 12-3).
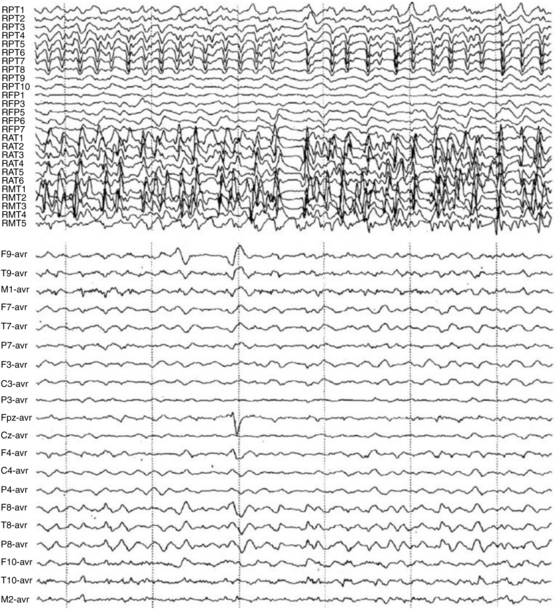
Fig. 12-2 Simultaneous intracranial and scalp EEG recording of a temporal lobe seizure.
(From Olejniczak P, Neurophysiologic basis of EEG. J Clin Neurophysiol 2006;23(3):187.)
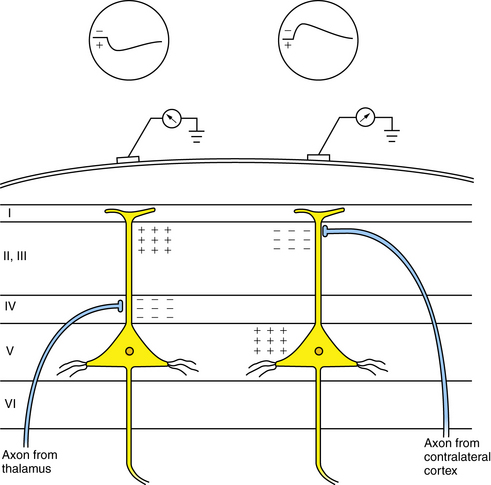
Fig. 12-3 Generation of extracellular voltage fields from graded synaptic activity.
Relationship between polarity of surface potentials and site of dendritic postsynaptic potentials.
(From Olejniczak P, Neurophysiologic basis of EEG. J Clin Neurophysiol 2006;23(3):188.)
Figure 12-4 depicts a schematic cross-section of a brain, illustrating four representative cortical EEG sources. Certain radial fields are negative since the negative maximum voltage is maximal directly above them. Alternatively, tangential fields produce both positive and negative voltage maxima [Ebersole and Pedley, 2003]. During the excitatory postsynaptic potential or inhibitory postsynaptic potential, there is a current flow in a loop orientation with opposite polarities at each end of the cell’s axonal or dendritic processes. Because most neurons are oriented radially to the cortical surface, these current flows follow a radial direction in and out, as detected by surface electrodes. The polarity, as recorded on the surface, reflects an excitatory or inhibitory current flow, depending on the nature of the summated synaptic potentials and on the site of the synapse. Virtually all electrical activity recorded on the scalp is generated by current flow across the synaptic membranes of cortical neurons.
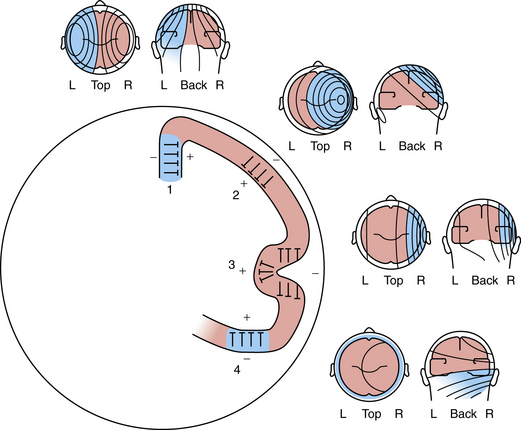
Fig. 12-4 Schematic of a brain cross-section, illustrating four representative cortical EEG sources.
(From Olejniczak P, Neurophysiologic basis of EEG. J Clin Neurophysiol 2006;23(3):188.)
The main sources of EEG derive from the cerebral cortex and form three-dimensional potential fields, which can be recorded as two-dimensional potential fields in the function of voltage versus time. It takes a combined synchronous electrical activity of approximately 108 neurons and their synaptic connections in a cortical area of 6 cm2 to create visible EEG. The area of cortex required for the generation of interictal spikes may be as large as 20 cm2 [Olejniczak, 2006].
Subcortical activity does not substantially contribute to the generation of current flow and electric fields. Instead, diencephalic structures, such as the thalamus, exert a modulatory effect on cortical rhythms. The dorsal thalamus is considered the chief subcortical EEG rhythm generator, synchronizing populations of neocortical neurons as voltage generators. In normal conditions both thalamic and cortical regions interact to produce synchrony of cortical postsynaptic potentials during wakefulness and sleep. The facultative pacemaker theory assumes that the thalamocortical relay neurons send fibers to the cortex, as well as giving off branches that turn back and end on thalamic inhibitory interneurons (biofeedback servomechanism). The nucleus reticularis hypothesis attributes the cells of the nucleus reticularis thalami to release the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) in rhythmic bursts of depolarization directed to the neurons of the dorsal thalamus and rostral brainstem (Figure 12-5) [Olejniczak, 2006].
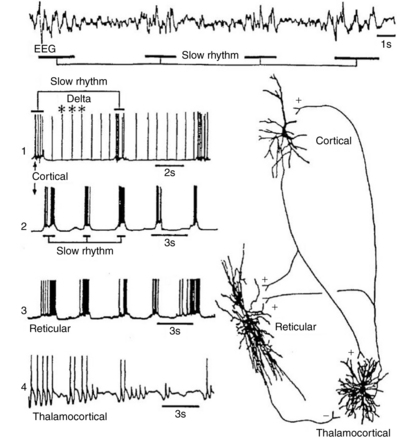
Fig. 12-5 Relationship between EEG slow waves and thalamic intracellular potentials.
(From Olejniczak P, Neurophysiologic basis of EEG. J Clin Neurophysiol 2006;23(3):188.)
Unlike sleep spindles, which require synaptic activities to establish the rhythmic oscillations, delta oscillation is an intrinsic rhythm that depends on potassium fluxes at voltage-dependent ion channels of cortical and thalamic neurons. Desynchronization of the EEG is the interruption of its rhythmical activity. It occurs with activation of ascending cholinergic projections of the basal forebrain and brainstem projections from the raphe nuclei and locus ceruleus. Rhythmic activity is interrupted both by direct effects on cortical neurons and indirectly by thalamic neurons. At the cellular level, desynchronization is accompanied by a transition from a burst firing pattern to a more continuous or single spike pattern. Desynchronization is enhanced by behavioral arousal and suppressed by non-rapid eye movement (REM) sleep. Certain abnormal synchronous patterns, such as alpha coma, occur in the setting of widespread injury to the ascending neuronal networks that otherwise produce arousal and desynchronization [Olejniczak, 2006].
Glial cells consist of a soma and fibers. Glial cells intermingle with the neuronal structures and are electrically coupled, building up an extended functional network. Glial cells also exhibit a membrane potential. Unlike neurons, glial cells do not generate postsynaptic action potentials. Their resting potential is exclusively based on potassium-outward current through leakage channels. The membrane potential value is close to the potassium equilibrium potential. With a decrease in extracellular potassium concentration, glial cells depolarize and repolarize, respectively. Glial cells are functionally linked by way of the extracellular potassium concentration. Neuronal action potentials are associated with an outflow of potassium ions. With an increase in the repetition rate of neuronal action potentials, extracellular potassium concentration increases, resulting in depolarization of glial cells adjacent to active neurons [Somjen and Trachtenbers, 1979].
Potential Fields and Neuronal Networks
Each neuronal arrangement generates opened and closed fields. In open fields, one electrode largely integrates potentials of the neuronal population, whereas the other electrode sees only the positive or negative field, permitting the recording of the field potential. In a closed field, external electrodes do not appear to see potential differences because currents within the pool compensate for each other [Niedermeyer and Lopes da Silva, 2005]. Several types of field potentials can be differentiated, depending on the time constant of the amplifying recording device. Amplified EEG recordings at a time constant of 1 second or less can be contrasted with an infinite time constant using a direct current (DC) amplifier, permitting additional recording of baseline shifts and wavelike potentials without filtering of slow-frequency activities.
Neurophysiologic Basis of Abnormal Electrical Patterns
Interictal spike discharges, the hallmark of the epileptic neuron in experimental models of epilepsy, involve a specific type of membrane depolarization, referred to as a paroxysmal depolarization shift. There are general similarities between the neuronal events underlying this interictal epileptogenic process [Jeffreys, 1990]. In hippocampal and neocortical slice recordings taken after penicillin application [Ayala et al., 1973], high-voltage discharges appear in the EEG recording and are associated with an intracellularly recorded paroxysmal depolarization shift. These phenomena appear in intracellular recordings of experimental animals and human cortices. The depolarization is of relatively high voltage (10–15 μV) and long duration (100–200 msec), and it is associated with a burst of spike discharges. The event produces a train of action potentials that conduct away from the neuron along the axon. The interictal paroxysmal depolarization shift is followed by a period of hyperpolarization. Over time, this depolarization can summate to form a prolonged depolarization with a persistent loss of hyperpolarization, which is recorded on the surface as continuous spike discharges coinciding with the tonic phase of a generated tonic-clonic seizure. Subsequently, large inhibitory potentials alternate with recurrent rhythmic depolarization, which coincides with the clonic phase of the seizure. Despite the higher threshold necessary to achieve depolarization and the longer refractory period observed in immature animals [Prince and Gutnick, 1972], an understanding of paroxysmal depolarization shift is crucial to an appreciation of maturational aspects of epileptogenesis.
Based on these experimental observations, several hypotheses have been advanced to explain the mechanism by which normal neuronal activities convert to those of abnormal interictal discharges [Prince, 1985]. One mechanism involves intrinsic membrane abnormalities that promote burst-generating properties in individual neurons by an imbalance of calcium and sodium ion movements [Schwartzkroin and Prince, 1978]. Another mechanism involves the loss of inhibition by a group of neurons because of the absence of the inhibitory neurotransmitter, GABA [Prince, 1985]. A third mechanism of summated excitatory postsynaptic potentials helps explain how large groups of neurons generate depolarization shifts [Prince, 1978].
Although overshadowed by factors influencing seizure initiation, seizure termination is a critical step in the return to the interictal state [Lado and Moshe, 2008]. Understanding the mechanisms contributing to seizure termination could potentially identify novel anti-ictal and antiepileptogenic drugs, as well as highlighting the pathophysiology contributing to both seizure initiation and termination.
Proposed mechanisms unfortunately do not yet fully explain the transformation from an interictal to an ictal event. Other mechanisms have been proposed based on additional experimental studies, including changes in the ionic microenvironment that result in enhanced neuronal excitability, axonal burst generation, and cholinergic alteration of neuronal properties [Prince, 1985]. A systems biology approach from membranes to synapses to networks and finally circuits may better elucidate pathways to normal or abnormal neurobehavior [Grillner et al., 2005].
Metabolic factors related to glucose or electrolyte imbalance or noncerebral factors, such as trauma or fever, also facilitate the onset of seizures, particularly in children. Additional biochemical and anatomic alterations within a seizure focus may further promote seizure generation by neuronal destruction or glial proliferation [Aird et al., 1984].
Abnormal Suppression or Slowing of Electroencephalographic Activity
In addition to the presence of spike or sharp-wave discharges for the diagnosis of seizure disorders, other types of abnormal EEG patterns coincide with different neuropathologic substrates [Ebersole and Pedley, 2003]. Unfortunately, little information exists that correlates altered neuronal properties, such as the mechanisms discussed for epileptogenesis, with specific neuropathologic entities. Instead, clinical interpretations of the following EEG patterns are empirically based on correlative neuropathology.
Local suppression or absence of background rhythmic activity is a strong indication of brain dysfunction, irrespective of age or location in the brain. Complete absence of activity usually implies cortical necrosis that is close to the surface [Goldensohn, 1979a; Goldensohn, 1979b]. However, incomplete depression of background activity is the more common situation. The presence of symmetric suppression of frontal beta activity, central rhythms, dominant alpha activity, or sleep spindles in association with polymorphic delta waves strongly indicates a destructive process in the brain underlying those electrodes (Figure 12-6A) [Arfel and Fischgold, 1961]. EEG recordings of neonates showing asymmetric background activities usually suggest that the brain lesion is in the attenuated location [Scher, 1982].
Continuous polymorphic delta waves constitute a category of EEG abnormality that may suggest a structural brain lesion on an acquired or congenital basis (see Figure 12-6B). Irregular delta waves between 0.5 and 2.5 Hz do not react to eye opening, arousal, or sleep. Correlations with brain lesions in animals [Gloor et al., 1968] and in humans at postmortem examination have been described [Rhee et al., 1975]. Focal slowing may imply focal destructive lesions of white matter by a congenital, vascular, neoplastic, or infectious process. Polymorphic delta waves can commonly appear immediately after a seizure of focal onset and with reversible conditions, such as migraine [Hockaday and Whitty, 1969]; both are common occurrences in children. The combination of bilateral, synchronous rhythmic discharges and polymorphic delta waves occurs in diffuse encephalopathies involving white and gray matter. Certain brain regions, such as the frontal, temporal, and occipital areas, are more likely to express polymorphic delta waves. The association of background rhythm depression and polymorphic delta waves increases the likelihood of destructive lesions, especially tumors. Parasagittal and parietal lesions often are associated with attenuation of rhythms or prominent theta rhythms.
Intermittent rhythmic delta activity is another category of nonepileptiform abnormality with a variety of neuropathologic correlates. Runs of sinusoidal 2.5 Hz are generally reactive to eye opening, hyperventilation, or arousal. Two distinct distributions of rhythmic delta activity over the scalp have been identified. Frontal intermittent delta activity (Figure 12-7A) and occipital rhythmic delta activity (see Figure 12-7B) generally indicate modification of cortically generated EEG activity by midline subcortical structures. The thalamus, reticular activating system, and midbrain can contribute to the dysfunction between subcortical gray matter and cortical neurons [Daly, 1974]. These two types of rhythmic delta activity are largely age-related. Frontal intermittent delta activity usually occurs in adult patients, whereas occipital rhythmic delta activity is found mainly in children. Intermittent rhythmic delta activity does not necessarily indicate that the major pathologic condition is in that region. Occipital rhythmic delta activity and frontal intermittent delta activity have been documented in 53 percent of 145 children with posterior fossa tumors [Martinius et al., 1968]. As a rule, it has little localizing value for supratentorial masses, although it may be accentuated on the side of the tumor [Hess, 1975]. Intermittent rhythmic delta activity is nonspecific and may be observed with systemic metabolic disorders, increased intracranial pressure, encephalitis, and trauma. However, a subsequent report concerning frontal intermittent delta activity described this pattern as rare for children, observed in only 20 of 1500 EEG studies of children between 18 months and 17 years of age. Most patients were awake and showed no encephalopathic signs, but were cognitively impaired. Another 50 percent had a history of epilepsy, with epileptiform activity occurring in 55 percent of the cohort [Watemberg et al., 2003].
Epileptiform discharges can be found in combination with slowing as an indication of a structural abnormality. Focal discharges during a seizure have better localizing value than interictal spikes [Hess, 1975]. In general, slow-growing neoplasms, such as oligodendrogliomas or astrocytomas, produce more epileptiform activity than rapidly growing tumors [Kershman et al., 1949]. Spike discharges are more common after surgery, such as for meningiomas, but neurologists are advised to exercise extreme caution concerning spike discharges as reliable guides to localization of destructive lesions. Imaging studies are far more reliable diagnostic guides for structural abnormalities.
Significance of Normal Variation in Electroencephalography: Maturational Patterns
Guidelines for Interpretation
EEG analysis requires a systematic, orderly process in which a series of steps are followed to reach a proper interpretation [Ebersole and Pedley, 2003]. The rhythmicity of spontaneous EEG signals gives a continuous admixture of scalp-generated oscillatory potentials. EEG frequencies used for clinical studies are classified in four band ranges. Delta activity is less than 4 Hz; theta activity is 4–8 Hz; alpha activity is 8–13 Hz; and beta activity is greater than 13 Hz. While DC (usually below 3 Hz) and gamma-frequencies (above 30 Hz) can be recorded with appropriate filter settings, these rhythms are currently applied only to research recordings. In general, the amplitude of EEG activity is inversely proportional to frequency.
For pediatric EEG studies, the amount of slow activity decreases with increasing age, and the persistence and frequency of slow activity vary in different brain regions. It is important to appreciate the presence and degree of expression of frequencies in various regions at different ages, and other parameters, such as waveform, manner of occurrence (e.g., random, continuous), and amplitude, are essential to visual analysis. By means of this cumulative analysis, using a list of parameters of EEG activity [Ebersole and Pedley, 2003; Kellaway and Crawley, 1964; Lombroso, 1980], the neurologist can attempt to assign normal or abnormal clinical significance to the EEG pattern. Critical information regarding age and state of the patient affects the ability of the neurologist to judge normality in the EEG record. It may require several EEG recordings and the persistence or resolution of abnormal electrical activity relative to the state of the patient to assess normality properly. Several recordings allow an appreciation of the ontogeny, or evolution, of significant age-specific EEG patterns. These guidelines must be repeatedly emphasized as the pediatric neurologist acquires an appreciation of the rich diversity of normal maturational patterns.
Newborn Electroencephalographic Patterns
Neonatal EEG studies have been reported for more than 60 years [Okamato and Kirikae, 1951]. Pioneering investigations by several independent researchers have all contributed information concerning the developmental neurophysiology of the immature brain. Some of these studies predate the development of the tertiary-care neonatal intensive care unit, and the neurologist using EEG recordings had an understandably limited role in the diagnostic assessment and clinical care of the sick neonate.
Improvements in recording equipment and standardization of the recording techniques are available in modern neonatal intensive care units. An internationally accepted system of electrode placement, adapted for the neonate and young infant, permits standardization of recordings between laboratories [AES, 1986]. Development of 16- and 21-channel EEG machines with improved filtering systems and electrode construction minimizes environmental and physiologic sources of artifacts. Synchronized video-EEG studies permit more accurate comparison between electrographic and behavioral changes in the assessment of seizures, movement disorders, and sleep cycles [Dyken et al., 1997; Scher, 2008; Scher and Barmada, 1987a].
Several fundamental principles for neonatal EEG studies serve as an introduction to an understanding of its chief clinical application [Scher, 2005a; Scher, 2008]. There are expected changes in the scalp-generated EEG patterns for neonates of different gestational ages. The experienced encephalographer can approximate the electrical maturity within 2 weeks of the gestational age. Changing electrical patterns reflect the postconceptional age of the neonate independent of birth weight. Maturation of the neonate’s sleep–wake behavior follows maturation of the CNS and is independent of the birth weight. Preterm neonates, when corrected to term postconceptional age, should have EEG patterns and sleep behavior similar to those of term, appropriate-for-gestational-age newborns.
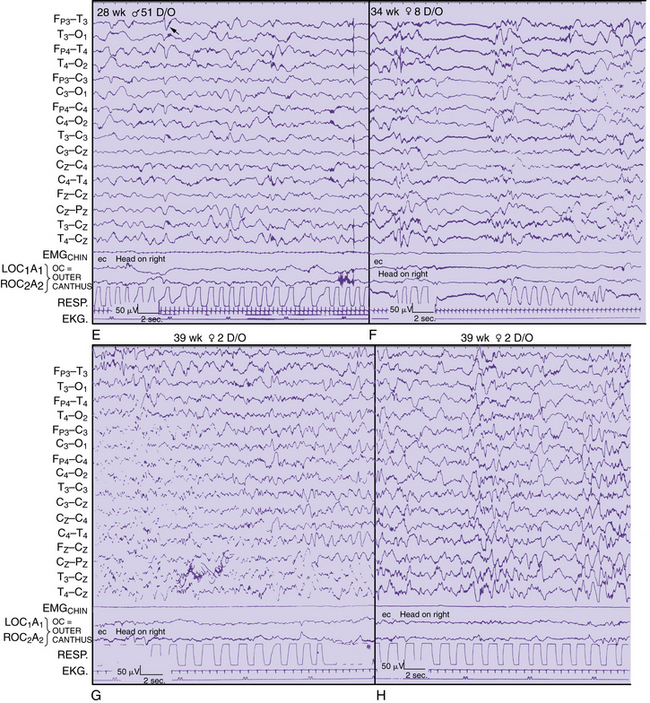
Detailed electrographic and anatomic correlations that compare sulcal-gyral development with evolving electrical patterns have been correlated with clinical information concerning gestational maturity [Scher and Barmada, 1987a] (Figure 12-8 and Figure 12-9). Of 25 infants, 23 had maturational agreement within 2 weeks between electrical patterns and sulcal-gyral measurements of the inferior frontal, superior temporal, and calcarine gyri, and between the cytoarchitecture of various brain regions.
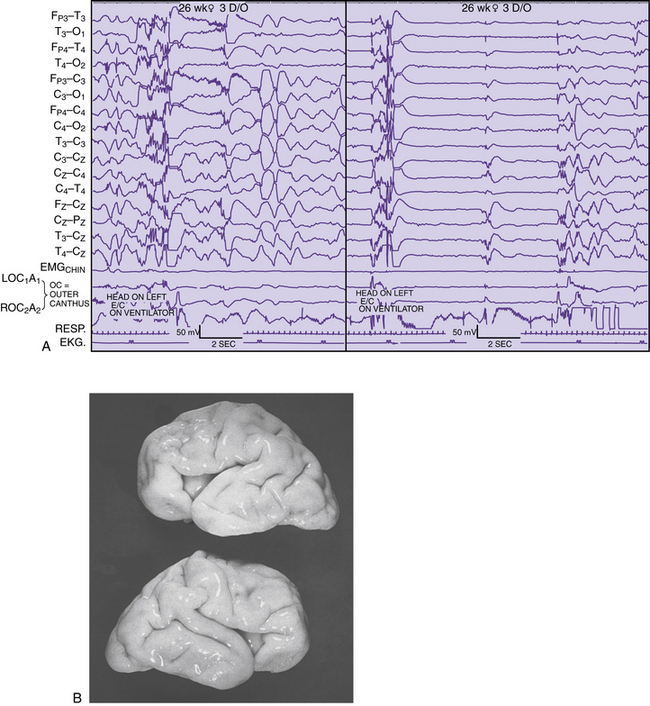
Fig. 12-8 Correlation of EEG and anatomical development in a 26-week-gestation preterm neonate.
(From Scher MS, Ahdab-Barmada M. Gestational age by electrographic, clinical and anatomical criteria. Pediatr Neurol 1987;3:256.)
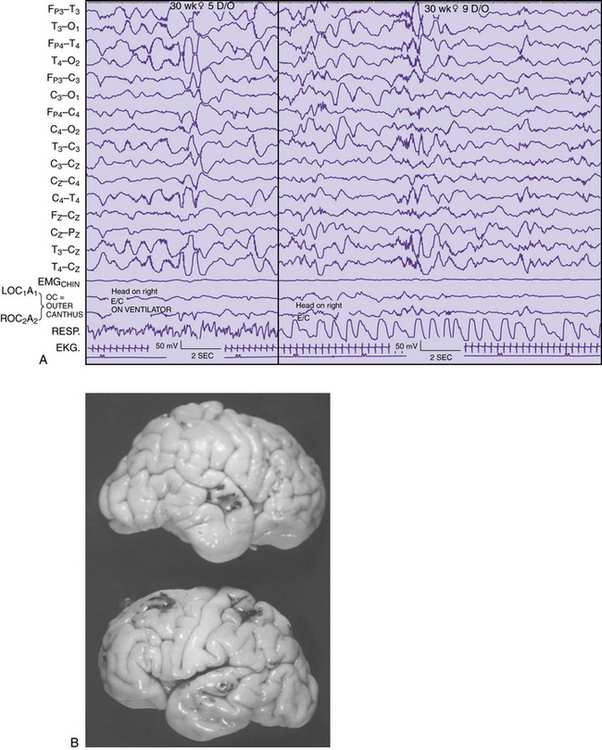
Fig. 12-9. Correlation of EEG and anatomical development in a 30 week-gestation preterm neonate.
(From Scher MS, Ahdab-Barmada M. Gestational age by electrographic, clinical and anatomical criteria. Pediatr Neurol 1987;3:256.)
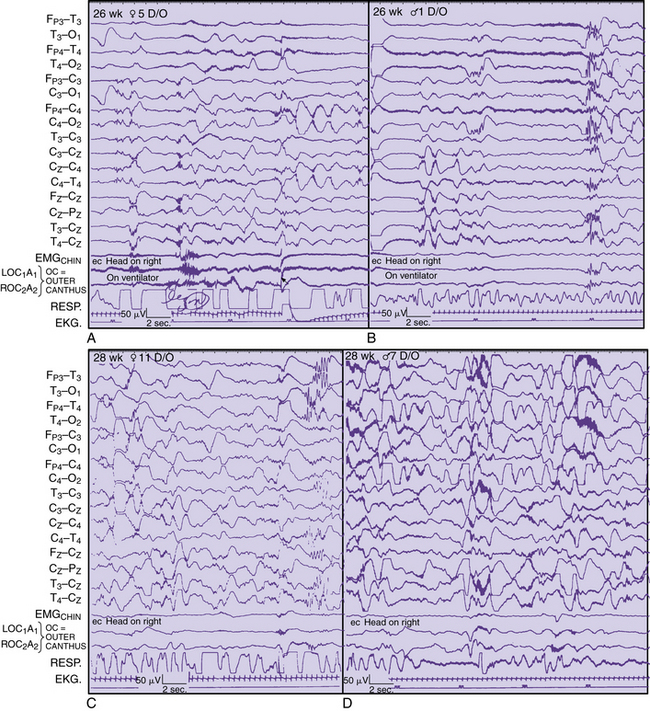
Regional changes in electrical activity clearly occur in the EEG of the premature neonate when recordings are obtained after 24–25 weeks’ estimated gestational age and at weekly intervals [Dreyfus-Brisac, 1979; Mizrahi and Kellaway, 2003; Scher, 2005a; Scher et al., 2003a; Tharp et al., 1981; Torres and Anderson, 1985] (Figure 12-10). Sleep–wake behavior cannot be distinguished for patients younger than 30 weeks of age, but other hemispheric or regional patterns demonstrate predictable, evolving characteristics.
Ontogeny of Electroencephalographic Features
The evolving EEG sleep patterns in the asymptomatic, presumably healthy preterm infant has been the subject of many excellent reviews [Ebersole and Pedley, 2003; Lombroso, 1985; Pope et al., 1992; Scher, 1993a; Scher, 1993b; Torres and Anderson, 1985]. Improvements in neonatal intensive care, however, require periodic revisions to include more preterm infants [Mizrahi and Kellaway, 2003; Scher, 2005a, 2008]. These revisions are particularly relevant for preterm infants younger than 32 weeks’ gestation, for whom substantial improvement in survival rates has occurred over the past decade. However, there is a greater prevalence of late preterm infants (34–37 weeks estimated gestational age [EGA]) who also remain at increased risk for neurodevelopmental deficits [Ramachandrappa and Jain, 2009]. Applying the principles of functional brain maturation using EEG is therefore relevant across the gestational age spectrum. It will always be important to verify which EEG-sleep patterns are within the range of normal by systematic neurodevelopmental assessment of such children at increasing postnatal ages.
In the following sections, regional and hemispheric EEG patterns for preterm and term neonates are arbitrarily assigned within specific gestational age ranges. Temporal, spatial, and state organization of the EEG recordings of cerebral activity during each age range is highlighted with appropriate illustrations. Greater details can be found in recent reviews [Scher, 2008; Scher and Loparo, 2009].
Gestational Age of Younger than 28 Weeks
A pattern of alternating active and sleep periods in preterm infants is characterized as discontinuity, or tracé discontinuity [Ellingson, 1964; Tharp et al., 1981], with alternating longer periods of quiescence and shorter periods of EEG activity. In fact, a 1-hour cyclic rhythm between burst and interburst intervals is present as early as 25–30 weeks EGA and persists up to a term age [Scher et al., 2005c]. None the less, Anderson and colleagues found that 60 percent of EEG recordings consisted of discontinuity [Anderson et al., 1985]. These same investigators found that the average interburst interval for this age range was 8–16 seconds, with the longest interburst intervals occurring between 15 and 88 seconds. Similar ranges for the duration of interburst intervals have been found by other investigators [Benda et al., 1989; Connell et al., 1987; Eyre et al., 1988; Hughes et al., 1983a; Hughes et al., 1983b; Hughes et al., 1987]. This finding sharply contrasts with earlier descriptions of interburst intervals as long as 2 minutes [Lombroso, 1985]. Such differences may be related to the medical condition of the neonate at the time of the recording or the lack of information concerning long-term follow-up. In this age group, EEG activity predominates in the vertex, central, and occipital regions. Bitemporal attenuation is common and may reflect intrahemispheric asynchrony [Scher and Barmada, 1987a]. Underdevelopment of the inferior frontal and superior temporal gyri may also help explain the relative quiescence of activity in this brain region.
EEG activity consists of mixed frequencies, dominated by delta activity in the parasagittal and occipital regions. Occipital delta activity is isolated to one or two waveforms and rarely lasts longer than 5–6 seconds. Faster frequencies in the theta, alpha, and beta ranges are intermixed in multiple brain regions. Diffuse theta bursts are common during continuous or discontinuous periods. Beta/delta complexes are transient patterns that identify preterm infants of various conceptional ages. Random or briefly rhythmic 0.3- to 1.5-Hz delta activity of 50–250 μV has superimposed bursts of low to moderate amplitude, and faster frequencies, with a frequency range of 10–20 Hz [Ebersole and Pedley, 2003]. The amplitude of such activity on bipolar recordings rarely exceeds 60–75 μV. Historically, various terms have been applied to such complexes, including spindle delta bursts, brushes, spindle-like fast waves, and ripples of prematurity. These complexes can be seen as early as 24–26 weeks, largely in the central and midline regions, as well as in the occipital regions.
Runs of monorhythmic alpha or theta activities are independent of delta brushes principally occurring in the occipital regions in the neonate younger than 28 weeks’ gestation. This transient pattern has been described in more detail and can last for 6–10 seconds in an asynchronous or asymmetric manner [Hughes et al., 1990]. Such activity should be distinguished from the more diffuse bursts of moderate- to high-amplitude theta activity that commonly exists at this degree of prematurity (see Figure 12-10A and B).
Intrahemispheric synchrony and interhemispheric synchrony have been variably described by different investigators. Lombroso described synchronous bursts as morphologically similar bursts of activity appearing within 1.5 seconds. He estimated that synchronous periods ranged from only 50 percent of all periods of activity at 31–32 weeks’ postconceptional age to 100 percent at 40–43 weeks’ postconceptional age, with a midpoint of 70–85 percent at 35–36 weeks’ postconceptional age [Lombroso, 1989, 1985]. This finding is in sharp contrast to Anderson’s observations that interhemispheric synchrony had a mean of 93 percent for 1-minute epochs at 27 weeks’ postconceptional age, with a mean of 94 percent at 29–32 weeks’ postconceptional age [Anderson et al., 1985]. A high degree of intrahemispheric synchrony already exists in the preterm infant younger than 30 weeks’ gestation. However, it is also reasonable to consider that more epochs of asynchrony occur as the distance from the midline increases, given the rapid brain growth beyond 30 weeks’ postconceptional age. Rapid growth of temporal and parietal structures in particular may contribute to more frequent episodes of asynchrony [Scher, 2005a]. Measures of asynchrony must include information about postconceptional age and about the EEG state. Asynchrony should be assessed for all background rhythms[Scher, 2008], not just for bursts during discontinuous epochs [Anderson et al., 1985; Lombroso, 1985].
Gestational Age of 28–31 Weeks
Cyclic organization of state remains visually undifferentiated in infants with a gestational age of 28–31 weeks [Lombroso, 1989; Lombroso, 1985], although a rudimentary ultradian rhythm of approximately 1 hour can be discerned with polysomnographic analysis [Scher et al., 2005c]. Also, periods of body and eye movements are more distinctively associated with irregular respirations during continuous periods of EEG activity. Discontinuous epochs still predominate but decrease in duration compared with the premature neonate younger than 28 weeks’ postconceptional age. Interbursts become progressively briefer with a dramatic increase in low to moderate-amplitude faster rhythms primarily in the theta range. By 32 weeks’ postconceptional age, the degree of discontinuity has decreased to 45 percent of the record [Anderson et al., 1985]. Dreyfus-Brisac found an alternating pattern in 24 percent of records at 32–34 weeks’ gestation [Dreyfus-Brisac, 1970]. The average interburst intervals at 29–31 weeks’ postconceptional age ranged between 5 and 14 and between 4 and 11 seconds, respectively, with means of 9 and 7 seconds; whereas the longest interburst periods for these two postconceptional age groups were found to be between 16–57 and 6–41 seconds with means of 36 and 20 seconds, respectively.
Monorhythmic occipital delta patterns with durations of more than 30 seconds are quite abundant at 28–31 weeks. Delta brush patterns continue to be abundant, involving the vertex and central regions along with the occipital and temporal regions [Watanabe et al., 1972]. Sometimes, it may be difficult to differentiate delta brushes in the temporal region, particularly because of superimposition with temporal theta bursts.
Another useful developmental marker is the appearance of rhythmic, 4.5- to 6-Hz activity occurring independently and synchronously in each midtemporal region. Although this activity is occasionally observed in neonates younger than 28 weeks’ postconceptional age, it is expressed maximally between 28 and 32 weeks. In the past, this feature has been described as “temporal sawtooth waves” [Scher, 2005a]. Amplitudes range from about 20 to 200 μV; with maturation, these bursts may reach the alpha frequency. In a study of 436 infants, a parabolic polynomial function described the age incidence of temporal theta activity, strongly reflecting the pattern as a maturational landmark [Hughes et al., 1987]. Temporal theta activity can obtain a maximum incidence of 36 percent at 29–30 weeks, after which it diminishes rapidly. At 32 weeks, only 12 percent of the record exhibits this pattern [Anderson et al., 1985]. This rhythmic theta activity should be distinguished from repetitive, sharp activity in the theta range seen at near-term and term ages in the midline and rolandic regions, particularly during quiet sleep.
The clinical significance of sharp-wave transients in healthy, preterm infants has been poorly documented at early preterm ages. Regional patterns associated with maturation must be considered before assigning spike or sharp-wave criteria to a transient waveform. Sharply contoured delta brushes and temporal or occipital theta bursts may appear epileptiform without clearly satisfying morphologic criteria of an epileptiform discharge (see Figure 12-10C and D).
Sporadic multifocal spikes and sharp waves can be seen at any gestational age [Ellingson, 1964; Harris and Tizard, 1960; Parmelee et al., 1968; Samson-Dollfus, 1955; Scher, 2005a; Thoresen et al., 1997], but few studies include preterm infants younger than 32 weeks’ estimated gestational age. Anderson and associates studied the incidence and location of spikes and sharp waves in 33 preterm infants of 27–32 weeks’ EGA [Anderson et al., 1985]. Both features were uncommon, with spikes less common than sharp waves. Both morphologies were most abundant in frontal and temporal regions, with the frequency increasing from 27 to 32 weeks. Central sharp waves decreased in number over this period, whereas occipital and vertex discharges had the lowest incidence. Unfortunately, only 55 percent of infants were verified as normal on follow-up at 6–8 months of age. Larger preterm populations with longer periods of neurodevelopmental follow-up are needed before assigning clinical significance to sharp waves in asymptomatic preterm infants.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree