Peripheral Nerves
Principal Signs and Symptoms of Peripheral Nerve Disease
Disorders affecting mixed peripheral nerves cause various symptoms and signs corresponding, in anatomic distribution, to regions supplied by each nerve. To make a correct topographic diagnosis of peripheral nerve lesions, the clinician must thoroughly know the area of the sensory supply of each nerve, the muscles it innervates, and any muscle stretch reflex subserved by the nerve [377]. Certain nerves are purely motor, some are purely sensory, and others are mixed. The symptoms and signs of a peripheral nerve lesion include disturbances as detailed in the following text.
Sensory Disturbances
With the division of a sensory nerve, all modalities of cutaneous sensibility are lost only over the area exclusively supplied by that nerve (the autonomous zone). This zone is surrounded by an intermediate zone, which is the area of the nerve’s territory overlapped by the sensory supply areas of the adjacent nerves. The full extent (autonomous plus intermediate) of the nerve’s distribution constitutes the maximal zone. In clinical diagnosis, the autonomous zone of sensory loss for each nerve must be specifically sought to make an accurate topographic localization. In general, with peripheral nerve lesions, the area of light touch sensory loss is greater than the area of pinprick sensory loss.
Pain and paresthesias may also help in localizing a peripheral nerve lesion, but these subjective sensations frequently radiate beyond the distribution of the damaged nerve (e.g., proximal arm pain in the carpal tunnel syndrome). Some patients describe pain that is evoked by nonnoxious stimulation of the skin innervated by a damaged nerve (allodynia).
Motor Disturbances
Interruption of the motor fibers in a motor or mixed nerve leads to lower motor neuron paresis or paralysis of the muscles innervated by that nerve. Atrophy of specific muscle groups and characteristic deformities follow. The muscle or muscle groups involved may become flaccid (hypotonic), with decreased resistance to passive motion. This hypotonia may be the result of weakness preventing voluntary activity [382].
The actions of agonist muscles, which have the same or similar mechanical effects on a joint, and antagonist muscles, which have the opposite effect, should be considered in testing the strength of a particular muscle. The action of a powerful agonist may conceal weakness in a smaller muscle (e.g., the pectoralis may compensate for subscapular muscle weakness). Also, certain muscles may appear weak because their action requires the support of the paralyzed muscles (e.g., finger abduction by the dorsal interossei may seem weak when a radial nerve palsy prevents fixation of the wrist). A nerve often supplies several muscles with a similar action, and a lesion of that nerve results in weakness of the muscle group.
Disturbances of Muscle Stretch Reflexes
As a consequence of sensorimotor loss, the muscle stretch reflex subserved by each damaged nerve is decreased or absent.
Vasomotor, Sudomotor, and Trophic Disturbances
The skin subserved by the affected nerve may become thin and scaly. The nails may become curved, with retardation of nail and hair growth in the affected area. The affected area of the skin may become dry and inelastic and may cease to sweat. Because the analgesic cutaneous area is liable to injury, ulcers may develop.
Although ancillary procedures (e.g., electromyography and nerve stimulation studies, muscle and nerve biopsy, sweat tests) greatly aid in topographic diagnosis, the following discussion stresses only the bedside diagnosis and localization of individual peripheral nerve abnormalities.
Mononeuropathy Multiplex
Mononeuropathy multiplex (multifocal mononeuropathy) refers to the involvement of several isolated nerves. The nerves involved are often widely separated (e.g., right median and left femoral nerve). These multiple neuropathies result in sensory and motor disturbances that are confined to the affected individual nerves. Mononeuropathy multiplex is usually due to a disseminated vasculitis that affects individual nerves (e.g., vasculopathy in diabetes mellitus or polyarteritis nodosa).
Polyneuropathy
In polyneuropathy, the essential feature is the impairment of function of many peripheral nerves simultaneously, resulting in a symmetric, usually distal, loss of function. The characteristic features include muscle weakness with or without atrophy, sensory disturbances, autonomic and trophic changes, and hyporeflexia or areflexia. In general, the legs are affected before the arms. Polyneuropathy may be caused by different processes and may be mainly sensory (e.g., amyloidosis, paraneoplastic, leprosy), motor (e.g., Guillain–Barré syndrome, porphyria, lead intoxication), or both sensory and motor.
The loss of sensation in peripheral polyneuropathies may involve all modalities of sensation, but because nerve fibers of a specific caliber may be preferentially involved in the pathologic process, sensory impairment may be restricted to a certain form of sensation (dissociation of sensory loss). Preferential loss of pain and temperature perception may be seen in type I hereditary sensory neuropathy, amyloid neuropathy, Tangier disease, and in some cases of diabetic neuropathy. With these neuropathies, smaller-diameter nerve fibers conveying pain and temperature sensation are preferentially involved. A selective loss of touch pressure, two-point discrimination, and joint position sense (conveyed by larger myelinated fibers) with spared pain and temperature sensibility may occur with Friedreich’s ataxia, vitamin B12 deficiency, and the Guillain–Barré syndrome.
The pattern of sensory and motor deficits in many polyneuropathies (e.g., diabetic polyneuropathy) develops according to axonal length, with sensory changes initially occurring at sites most distal from dorsal root ganglia cells [309]. When the sensory abnormality in the limbs extends proximally to 35 to 50 cm from the dorsal root ganglia, there is also a region of sensory loss over the anterior torso in accordance with the length of axons traversing the body wall. This sensory abnormality is wider in the lower abdomen and tends to be narrower in the thoracic region because of the longer, more oblique course of the sensory fibers to the lower abdomen and the shorter course of the nerves traveling along the ribs. When nerves <20 to 24 cm in length become involved, a “beanie cap” of sensory change over the scalp vertex occurs owing to the distal involvement of the ophthalmic branches of the trigeminal nerves. In extreme sensory neuropathies, only the shortest (<12 cm) nerve fibers are spared, so that there is sensory loss over the entire body except for a band of intact sensation over the posterior midline from the occiput to the sacral region [309].
The axonal length principle may also be applied to motor neuropathies where often the intrinsic foot muscles are initially affected, followed by the peroneal innervated muscles, and then by gastrocnemius-soleus involvement [309]. The anterior tibial compartment is affected before the posterior tibial compartment because the former’s nerve supply is longer than the latter by more than 10 cm. When all muscle groups below the knee are affected, intrinsic hand muscle involvement will develop. Patients with peripheral motor neuropathies often have greater motor weakness of ankle dorsiflexors than foot plantar flexors on a biomechanical as well as a physiologic basis [47]. In fact in patients with greater weakness of ankle plantar flexors than dorsiflexors, who are able to walk on their heels but not on their toes, intraspinal disease (e.g., conus-cauda equina tumor, spinal muscular atrophy, spinal stenosis, carcinomatous meningitis) rather than peripheral polyneuropathy should be suspected [47].
In some neuropathies (e.g., Tangier disease), the short axons are preferentially involved, and hence the sensory loss starts proximally and progresses distally, sometimes to the point where the entire body, except for the hands and regions below the knees, shows sensory impairment. Porphyric neuropathy may also start proximally, with paresis affecting the proximal arms, proximal legs, distal arms, and then distal legs (in that order). The muscle stretch reflexes in porphyria may be completely lost except for preserved ankle jerks, and the sensory loss may involve only the trunk, face, and proximal arms and thighs.
In some other neuropathies, the initial involvement and subsequent progression of clinical deficits may not be determined by the axonal length. For example, in lepromatous leprosy neuropathy, sensory loss occurs initially over the areas of the body having cooler surface temperatures (e.g., the tip of the nose, the malar areas of the cheeks) [308] because the proliferation of Mycobacterium leprae is greater in cooler tissues.
Lesions of Individual Nerves
Although peripheral nerves originate in plexuses (discussed in Chapter 3), it is easier to understand the course of peripheral nerves before reviewing the anatomy of the plexuses, than to understand the symptomatology of plexus lesions without the benefit of knowing peripheral innervation. For this reason, the authors have chosen to place this chapter first. Diagrams of the origin of each nerve in the plexus can be found in Chapter 3.
Nerve fibers do not randomly intertwine as they progress distally in nerve bundles. Evidence indicates that specific axons remain grouped together, particularly through their distal course, as they travel toward their destination [359]. Adjacent muscles and, similarly, adjacent sensory dermatomes are innervated by nerve fascicles that remain together within the nerve bundle. Therefore, ulnar neuropathies clinically “localized” to the wrist may actually be occurring at the elbow because of the preferential involvement of certain fascicles, whereas a proximal sciatic neuropathy sometimes masquerades as a peroneal neuropathy at the knee. These anatomic arrangements must always be taken into account when localizing pathologic processes [360].
Dorsal Scapular Nerve (C4–C5)
ANATOMY
The dorsal scapular nerve (a purely motor nerve) arises mainly from the C5 spinal nerve within the substance of the scalenus medius muscle. The nerve courses downward behind the brachial plexus deep to the levator scapulae muscle (which it supplies) and terminates by piercing the deep surfaces of the rhomboids (major and minor). The rhomboids normally elevate and adduct the medial border of the scapula (they are antagonists of the serratus anterior) and, along with the levator scapulae, rotate the scapula so that the inferior angle moves medially. The rhomboids are tested by having the patient press his or her elbow backward against resistance while the hand is on the hip [377].
NERVE LESIONS
Because the dorsal scapular nerve derives from the proximal plexus, affection of this nerve in an upper brachial plexopathy suggests a proximal lesion. The nerve may also be entrapped within the substance of the scalenus medius muscle. Isolated lesions of this nerve may occur in body builders [241]. A dorsal scapular nerve lesion results in the lateral displacement of the vertebral border of the scapula, which is rotated, with the inferior angle displaced laterally. Rhomboid atrophy is concealed by the overlying trapezius muscle. Rhomboid paresis is evident if the elbow can be pressed back only weakly against resistance (keeping the hand on the hip). Weakness is also evident when the patient attempts to push the palm backward against resistance with the arm folded behind the back.
Subclavian Nerve (C5–C6)
This purely motor nerve emerges from the upper trunk of the brachial plexus and descends in the posterior cervical triangle to innervate the subclavian muscle. This muscle depresses and medially draws the lateral end of the clavicle. Lesions of the subclavian nerve cause no important clinical disturbances.
Long Thoracic Nerve (C5–C7)
ANATOMY
This purely motor nerve (Fig. 2.1) arises from the C5–C7 roots shortly after they emerge from the intervertebral foramina. After passing through the scalenus muscle, the two upper roots are joined by a contribution from the C7 root. The nerve runs posterior to the brachial plexus and inferiorly behind the clavicle and then crosses the outer border of the first rib to reach the serratus anterior muscle. The nerve further descends along the lateral thoracic wall, sending individual filaments to the muscle slips of the serratus.
The serratus anterior muscle fixes and stabilizes the scapula against the chest wall and is tested by observing for scapular winging (the vertebral border of the scapula stands away from the thorax, forming a “wing”) while the patient pushes extended arms against a fixed object (e.g., a wall) [377].
NERVE LESIONS
The long thoracic nerve lies superficially in the supraclavicular region where it is subject to trauma [115]. Therefore, it is injured most frequently as a result of pressure on the shoulder (e.g., sudden trauma or carrying heavy objects on the shoulder—rucksack paralysis) [273]. Direct trauma to the shoulder or the lateral thoracic wall while playing football, or during a fall, or an auto accident may compress the nerve. Chiropractic manipulation of the cervical spine may also cause nerve injury [264]. Work-related or athletic activities, especially those involving repetitive stressful movements of the shoulder or those in which the arm is in an outstretched overhead position, may cause stretch or traction injury [319]. The overhead arm positioning may be a factor in the development of isolated long thoracic neuropathy during surgery with general anesthesia [179]. In a review of 197 cases of long thoracic neuropathy, 32 cases were iatrogenic owing to local invasive procedures such as first rib resections, mastectomies with axillary node dissection, thoracotomy, scalenectomies, infraclavicular plexus anesthesia, and chest tube insertion [179]. The nerve may be injured in up to 10% of patients undergoing radical mastectomies and in approximately 1% of patients undergoing simple mastectomies [96]. Isolated long thoracic nerve palsy may also occur as a manifestation of neuralgic amyotrophy (Parsonage–Turner syndrome) [101] or, rarely, after radiation therapy for breast cancer [286]. Also, familial long thoracic nerve palsy may be a major manifestation of familial brachial plexus neuropathy [275], and painful long thoracic neuropathy has been described as the sole manifestation of Lyme disease [245].
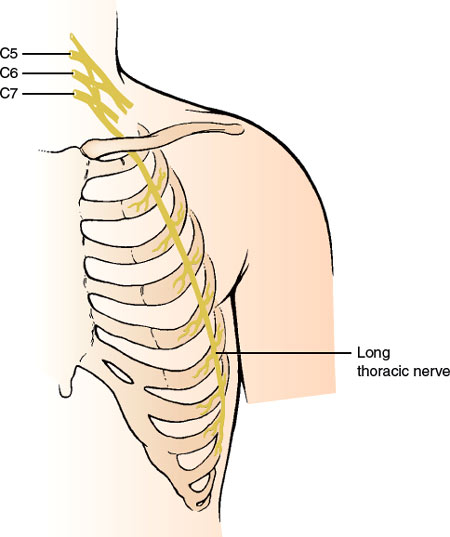
FIG. 2.1. The long thoracic nerve.
Nerve paralysis usually causes no deformity of the scapula when the arm is at rest. If, however, the patient is asked to push the arm forward against resistance or hold the arms up in front of the body, the scapula becomes winged (winged scapula or scapula alata), especially in its lower two-thirds region. The patient often complains of weakness of the shoulder and fatigue on raising the arm above the head. With injuries caused by excessive nerve stretch during physical activities, the sharp pain in the shoulder may radiate to the neck and upper arm.
Suprascapular Nerve (C5–C6)
ANATOMY
This purely motor nerve (Fig. 2.2) is a branch of the upper trunk of the brachial plexus. The nerve passes downward beneath the trapezius to the upper border of the scapula, where it passes through the suprascapular notch. This notch is bridged by the superior transverse scapular ligament, forming an osseofibrous foramen through which the nerve passes to enter the supraspinous area beneath the supraspinatus muscle. The nerve gives off branches to the supraspinatus and to the capsule of the shoulder joint and then courses around the free lateral border of the spine of the scapula to supply the infraspinatus muscle. Although the suprascapular nerve is said to have no cutaneous branches, rare cases have provided evidence for a cutaneous branch of the suprascapular nerve, as described in cadaveric studies [142].
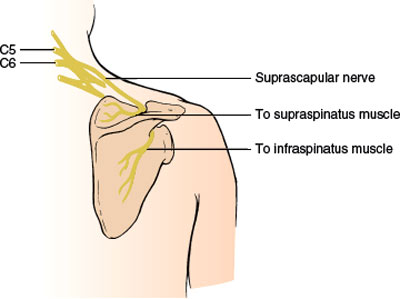
FIG. 2.2. The suprascapular nerve.
The supraspinatus muscle normally abducts the humerus (mainly the first 15 degrees of abduction), whereas the infraspinatus muscle is mainly an external rotator of the upper arm.
NERVE LESIONS
The nerve may be injured in proximal upper brachial plexopathies and is also subject to damage in the supraclavicular region, especially with acute forceful depression of the shoulder and its dislocation. Fractures of the suprascapular notch or excessive callus formation after scapular fracture may compress the nerve. The nerve may also be injured after repair of rotator cuff tears [412]. Repetitive forced cross-body adductions of the arm or repetitive motions involving the scapulothoracic and shoulder joints may also injure the nerve. Suprascapular nerve injury has even been reported after driving for 2 hours speaking on a mobile telephone with the phone cradled between the ear and shoulder, probably by the nerve being compressed by the hard edge of the phone (mobile telephone user’s shoulder droop) [152]. Other etiologies of nerve injury include occupational overuse, sports-related injuries (especially sports involving overhead motions such as tennis, weight lifting, canoeing, and volleyball), direct nerve trauma, and ganglion cysts [187, 195].
Entrapment lesions (e.g., ganglia, spinoglenoid cyst, etc.) may occur in the suprascapular foramen [13,60,208,229,292,325]. This entrapment causes shoulder pain, which is aggravated by shoulder girdle movements, weakness, and eventual atrophy in the two spinati muscles. The pain is deep, located along the superior border of the scapula often extending toward the shoulder joint, occasionally radiating into the arm, and is made worse by movements that adduct the scapula or rotate the head away from the involved shoulder. Supraspinatus paresis results in weakness of arm abduction, whereas infraspinatus paresis results in impaired external rotations at the shoulder joint. Suprascapular involvement may also occur with neuralgic amyotrophy [101].
Harbaugh et al. described a patient with suprascapular nerve entrapment at the suprascapular notch presenting with right shoulder pain and atrophy with weakness of the right supra- and infraspinatus muscles [142]. The patient was noted to also have an area of numbness involving the right upper lateral shoulder. Although the suprascapular nerve is said to have no cutaneous branches, this case provides evidence for a cutaneous branch of the suprascapular nerve [142].
The branch of the infraspinatus muscle may be damaged in isolation by the entrapment of the suprascapular nerve at the spinoglenoid notch by a hypertrophied inferior transverse scapular ligament or a ganglion [7,191,208]. This results in shoulder pain, which is elicited by the external rotation of the shoulder joint, associated with weakness and wasting of the infraspinatus. Lesions at the suprascapular notch may also cause isolated infraspinatus weakness by involving only the portion of the nerve destined to innervate the infraspinatus muscle [41,191,351]. A lesion of the suprascapular nerve at the glenoid notch, causing isolated infraspinatus paresis and atrophy, may occur as a professional hazard in volleyball players [244]. Isolated infraspinatus paresis may also result from a nerve injury in body builders [44,241].
Subscapular Nerves (C5–C7)
ANATOMY
These purely motor nerves arise as branches of the posterior cord of the brachial plexus. The upper subscapular nerves supply the subscapularis, whereas the lower subscapular nerves supply the teres major.
NERVE LESIONS
Subscapular nerve palsies usually occur with posterior cord brachial plexus lesions. The arm is somewhat externally rotated, with some paresis of internal rotation, although the latissimus dorsi and pectoralis major muscles are usually able to compensate well for this paresis. The patient may complain of difficulty in scratching the lower back.
Thoracodorsal Nerve (C6–C8)
ANATOMY
This purely motor nerve, also known as the nerve to the latissimus dorsi, is a branch of the posterior cord of the brachial plexus and usually emerges from the plexus in a close association with the subscapular nerves. The nerve runs along the posterior axillary wall to reach and innervate the deep surface of the latissimus dorsi muscle. This muscle (along with the teres major) adducts and internally rotates the arm and depresses the raised arm. It is best tested by having the patient adduct the horizontally raised upper arm against resistance or by palpating the muscle bellies when the patient coughs [377].
NERVE LESIONS
Lesions of this nerve usually occur with damage to the posterior cord or proximal parts of the brachial plexus. Nerve lesions cause little deformity or atrophy, but proximal arm adduction is compromised. A combined movement comprising extension, adduction, and internal rotation, in which the dorsum of the hand is placed on the opposite buttock, readily reveals latissimus paresis. Isolated thoracodorsal nerve injury has been described in body builders [44,241].
Anterior Thoracic Nerves (C5–T1)
ANATOMY
The anterior thoracic nerves, purely motor nerves, (also called the pectoral nerves) are divided into the lateral anterior thoracic nerve (C5–C7), a branch from the anterior divisions of the upper and middle trunks of the brachial plexus, and the medial anterior thoracic nerve (C8–T1), a branch of the proximal section of the medial cord of the plexus. After these nerves descend posteriorly to the clavicle, the lateral nerve supplies the clavicular and upper sternocostal portions of the pectoralis major, and the medial division supplies the lower sternocostal portion of this muscle and the pectoralis minor.
The pectoralis major is an adductor and medial rotator of the humerus. It is tested by having the patient hold the arm in front of the body. The two portions can be seen and palpated when the patient resists attempts by the examiner to force the arm laterally [377].
NERVE LESIONS
Lesions of these nerves are of relatively little localizing importance except in corroborating brachial plexus damage. Adduction and medial rotation of the upper arm are weak, and the patient notices difficulty in using the arm in climbing. Isolated injury to the lateral anterior thoracic nerve as the result of a seat-belt injury may result in atrophy of the clavicular head of the pectoralis major [221]. Weight lifters may develop medial anterior thoracic nerve injury in isolation or in association with thoracodorsal nerve injury [44,241]. Bilateral medial anterior thoracic neuropathies in a weight lifter were thought to be due to concomitant pectoralis minor hypertrophy causing intramuscular entrapment of the nerves [302].
Gardetto et al. reported two cases of isolated damage to a muscle branch of the lateral pectoral nerve [117]. In both these patients, focal muscle atrophy developed gradually after the initiation of training schedules to increase the cross-section of the major pectoral muscle. The authors therefore assumed that compression injury to the nerve by repetitive muscle contractions may have been of pathogenic relevance. Anatomical studies of this region showed that the nerve branches of the lateral pectoral nerve, having to pierce through a connective tissue septum that is thicker by a few millimeters, may be subjected to an additional risk of compression [117].
Axillary Nerve (C5–C6)
ANATOMY
The axillary (circumflex) nerve (Fig. 2.3), a mixed nerve, arises as one of the terminal branches of the posterior cord of the brachial plexus from spinal segments C5 and C6. It lies first on the lateral side of the radial nerve, then courses laterally and backward to pass just below the shoulder joint. It then goes through the quadrilateral space, an anatomical compartment bounded inferiorly by the teres major muscle, medially by the long head of the triceps muscle, laterally by the surgical neck of the humerus, and superiorly by the teres minor muscle and subscapularis muscle. The nerve descends on the subscapularis muscle behind the axillary artery and then winds around the surgical head of the humerus, accompanied by the posterior circumflex humeral artery. It passes deep into the deltoid and teres minor muscles, supplying both. It sends sensory branches to the capsule of the shoulder joint (articular branch) and to the skin of the upper lateral aspect of the arm superficial to the deltoid muscle (lateral cutaneous nerve of the arm).
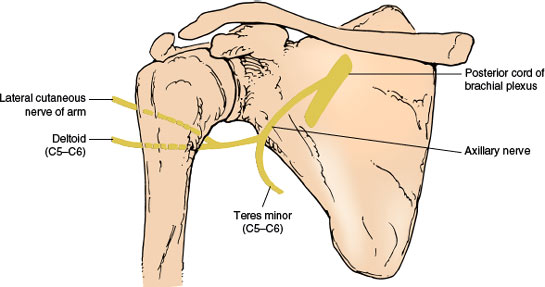
FIG. 2.3. The axillary nerve.
The teres minor muscle is a lateral rotator of the shoulder joint. The central part of the deltoid muscle is tested by having the patient abduct the upper arm against resistance (15–90 degrees), the anterior part by elevating the arm forward against resistance (up to 90 degrees), and the posterior part by having the patient retract the abducted upper arm against resistance.
NERVE LESIONS
Lesions of the posterior cord of the brachial plexus affect this nerve as well as the radial nerve (e.g., crutch paralysis). Trauma is the most common cause of axillary neuropathy. The axillary nerve is thought to be most vulnerable to traction injury of the brachial plexus because it is tethered to the deltoid muscle very close to the shoulder [39,153,267]. The nerve is most often injured as it winds around the lateral aspect of the humerus in a relatively exposed position (e.g., with fracture and dislocation of the humerus), when the shoulder joint is dislocated or when the scapula is fractured [15]. The axillary nerve may also be injured during reduction of a shoulder dislocation [398]. Axillary neuropathy may occur secondary to intramuscular injection high in the posterior aspect of the shoulder [170], after sleeping in a prone position with the arm raised above the head, following laparotomy (attributed to suspending the forearm from the anesthesia screen to gain better abdominal exposure), with neuralgic amyotrophy [101] or by direct nerve injury in athletes [267,271]. The nerve may be injured directly or entrapped by a fibrous band in the quadrilateral space [113,231]. Other etiologies of axillary injury in the quadrilateral space include trauma in athletes (especially volleyball players, baseball pitchers, and tennis players), the use of prosthetic devices for the upper arm using a “figure of eight” type of suspension, and hypertrophy of contiguous muscles [267]. As the posterior humeral circumflex artery also runs in the quadrilateral space, subclavian angiography in the quadrilateral syndrome may reveal occlusion of this vessel when the arm is abducted and externally rotated [59].
Nerve injuries usually cause a sensorimotor nerve palsy, but a purely motor nerve palsy is possible with nerve lesions at the humeral head. A purely sensory loss with no motor deficits is also possible [45]. For example, isolated damage to the sensory branch after arthroscopic surgery has been described [324]. In nerve lesions, the deltoid muscle becomes atrophic, causing a flattening or concavity of the shoulder contour. Teres minor paresis is usually not demonstrable on clinical examination because other muscles can perform its functions. Deltoid paralysis results in difficulty in abducting the arm, but other muscles of the shoulder girdle can compensate this function. An axillary cutaneous sensory defect is located on the outer aspect of the upper arm and is maximal on the patch of skin above the deltoid attachment.
Musculocutaneous Nerve (C5–C7)
ANATOMY
The musculocutaneous nerve, a mixed nerve (Fig. 2.4) arises from the lateral cord of the brachial plexus and proceeds obliquely downward between the axillary artery and the median nerve. The nerve pierces the coracobrachialis muscle and descends further between the biceps and brachialis muscles (it supplies all these three muscles). It may rarely innervate the pronator teres muscle (most often a median-innervated muscle). The nerve then continues distally as the lateral cutaneous nerve of the forearm after it pierces the deep fascia over the anterior elbow. The coracobrachialis muscle is a forward elevator of the arm, the brachialis (which occasionally also receives innervation from the radial nerve) is an elbow flexor, and the biceps is an elbow flexor and forearm supinator (especially when the elbow is flexed at 90 degrees). The biceps is tested by having the patient flex the supinated arm against resistance [377]. The biceps reflex is subserved by the musculocutaneous nerve.
The autonomous zone of the lateral cutaneous nerve of the forearm (a narrow band along the radial forearm) shows sensory loss with musculocutaneous nerve lesions. This zone of cutaneous sensory loss may extend from the elbow to the wrist and cover the entire lateral forearm from the dorsal to the ventral midline.
NERVE LESIONS
Nerve damage may result from lesions of the lateral cord of the brachial plexus. Proximal humeral lesions (e.g., fractures, osteochondroma) or shoulder dislocations may injure or compress the nerve [172]. Because the nerve is deep and is protected between the entry site into the coracobrachialis and the elbow, lesions are relatively uncommon here. A predominantly motor musculocutaneous neuropathy may develop after strenuous exercise of the upper extremity, probably due to nerve entrapment or stretch of the nerve where it passes through the coracobrachialis muscle in the upper arm [225]. Motor and sensory neuropathy may develop in this location after weight lifting, strenuous physical activity, rowing, football throwing, general anesthesia, or sleep [48,159,189,315] and has been described to occur with repetitive carrying of a heavy, rolled object by the shoulder with the object held in place by the arm curled around the object (carpet carrier’s palsy) [315]. Isolated biceps weakness may occur with distal motor nerve injury [44]. A purely sensory syndrome may result when the lateral cutaneous nerve of the forearm is damaged in the cubital fossa or forearm. This sensory branch may be injured by compression [258], venipuncture [40], or cutdown procedures because it lies directly under the median cubital vein in the center of the cubital fossa. Typically, patients notice pain in the proximal forearm, often aggravated by elbow extension, and paresthesias along the radial aspect of the forearm. A pure sensory neuropathy has also been observed in windsurfers who flex the upper extremity slightly at the elbow with the hand gripped over the boom [163]; this has also been observed with nerve compression from a handbag (handbag paresthesia) [140] and with nerve compression by the biceps aponeurosis [80].
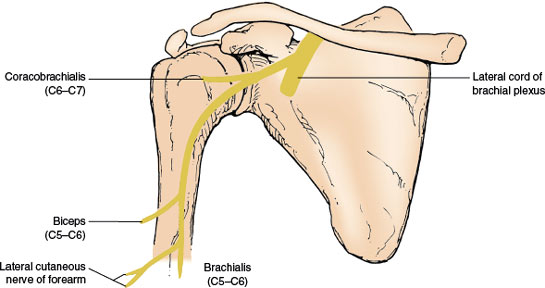
FIG. 2.4. The musculocutaneous nerve.
With lesions of the musculocutaneous nerve, atrophy of the biceps and brachialis results in wasting of the ventral aspect of the upper arm. Loss of coracobrachialis function is difficult to detect clinically. With biceps weakness, flexion of the elbow is weak (especially when the forearm is supine), and the biceps reflex is lost.
Median Nerve (C6–T1)
ANATOMY
The mixed median nerve (Fig. 2.5) is formed in the axilla by the joining of the lateral cord of the brachial plexus (spinal segments C6–C7) with the medial cord (spinal segments C8–T1). The nerve then descends down the medial side of the arm in a close association with the brachial artery to the cubital fossa. From there, the median nerve enters the forearm between the two heads of the pronator teres muscle and gives off the anterior interosseous nerve, after which it dips under the sublimis bridge. It then courses deep to the flexor retinaculum at the wrist (carpal tunnel) to reach the hand. At a variable distance above the flexor retinaculum, the median nerve provides a palmar cutaneous branch, which crosses the flexor retinaculum either subcutaneously or through the superficial ligament fibers to supply the skin over the thenar eminence and proximal palm on the radial aspect of the hand. The median nerve passes through the carpal tunnel accompanied by the flexor tendons of the digits and emerges to divide into its terminal branches. These terminal branches include branches to the thenar muscles and the palmar digital nerves, which innervate the skin of the palmar aspect of the thumb, the second, third, and half of the fourth finger; the palm overlying the corresponding metacarpophalangeal joints; and the posterior middle and distal phalanges of the second, third, and half of the fourth finger.
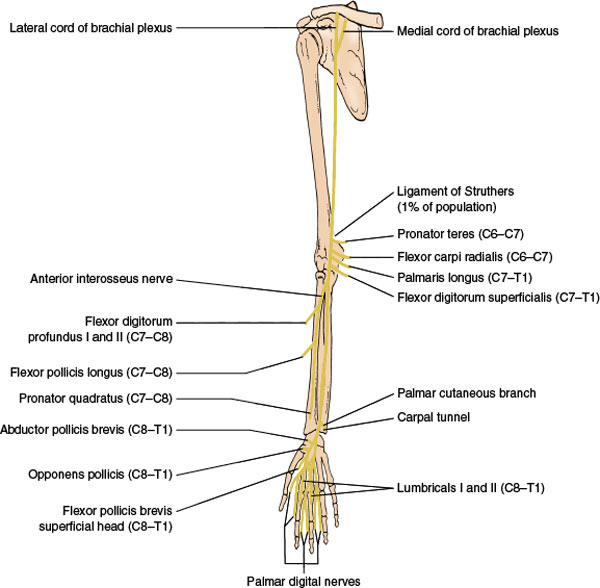
FIG. 2.5. The median nerve.
The median nerve gives off no muscular branches until it reaches the elbow. As it passes between the heads of the pronator teres muscle, it supplies the following muscles:
1. Pronator teres (C6–C7), a forearm pronator. It is tested by having the patient pronate the forearm against resistance. This muscle may rarely be innervated by the musculocutaneous nerve.
2. Flexor carpi radialis (C6–C7), a radial flexor of the hand. This is tested by having the patient flex and abduct the hand at the wrist against resistance.
3. Palmaris longus (C7–T1), a flexor of the wrist.
4. Flexor digitorum superficialis (C7–T1), a flexor of the middle phalanges of the second, third, fourth, and fifth fingers. It is tested by having the patient flex the finger at the interphalangeal joint against resistance, with the proximal phalanx fixed.
After it passes between the two heads of the pronator teres, the median nerve gives off the purely motor anterior interosseus nerve, which innervates the following muscles:
1. Flexor pollicis longus (C7–C8), a flexor of the terminal phalanx of the thumb, it is tested by having the patient flex the distal phalanx of the thumb against resistance, while the proximal phalanx is fixed.
2. Flexor digitorum profundus I and II (C7–C8), a flexor of the terminal phalanges of the second and third fingers. It is tested by having the patient flex the distal phalanx of the second and third fingers against resistance, with the middle phalanx fixed.
3. Pronator quadratus (C7–C8), a forearm pronator.
At the distal end of the carpal tunnel, the median nerve divides into its terminal branches. The motor branches innervate the first and second lumbricals and the thenar muscles, which include the following:
1. Abductor pollicis brevis (C8–T1), an abductor of the metacarpal of the thumb. It is tested by having the patient abduct the thumb at right angles to the palm against resistance.
2. Opponens pollicis (C8–T1), a muscle that brings the metacarpal of the thumb into opposition. It is tested by having the patient touch the base of the little finger with the thumb against resistance.
3. Superficial head of the flexor pollicis brevis (C8–T1), a flexor of the proximal phalanx of the thumb.
4. Lumbricals I and II (C8–T1), flexors of the proximal and extensors of the two distal phalanges of the second and third fingers. They are tested by having the patient extend the finger at the proximal interphalangeal joint against resistance, with the metacarpophalangeal joint fixed and hyperextended.
The finger flexor reflex (C8–T1) is in part innervated by the median nerve.
It is important to be aware of potential variations in the innervation of the intrinsic hand muscles. Anomalous communications in the hand are sometimes referred to as Riche-Cannieu anastomoses [136] and are thought to involve communications between the motor branch of the median nerve and the deep ulnar nerve branch in the radial aspect of the hand. For example, the adductor pollicis and first dorsal interosseous muscles may be exclusively supplied by the median nerve (in 2% and 1% of individuals, respectively) and are therefore involved in median nerve lesions and spared in ulnar lesions. Also, the abductor pollicis brevis and the flexor pollicis brevis may be exclusively supplied by the ulnar nerve in 2% of individuals.
NERVE LESIONS
Lesions in the Axilla and Upper Arm. Median neuropathies in the axilla are often associated with damage to the ulnar and radial nerves (triad neuropathy) or brachial plexus. Etiologies include crutch compression, sleep paralysis, penetrating trauma, shoulder dislocation, vascular malformations, and sheath hemorrhage [303]. In the upper arm, the nerve may be damaged by penetrating trauma, humerus fractures, sleep paralysis, and arteriovenous fistulas [383]. The median nerve may be compressed in the upper arm by an anomalous course of the brachialis muscle [246]. Median lesions in the axilla or upper arm result in paresis or paralysis of all the muscles innervated by the median nerve, with a sensory loss in the distribution of both the palmar cutaneous and palmar digital branches. There is atrophy of the thenar eminence, affecting especially the abductor pollicis brevis and the opponens pollicis. Because of this atrophy, with recession of the metacarpal bones of the thumb to the plane of the other metacarpal bones, the hand takes on an abnormal appearance called simian hand or ape hand. This appearance results from the unopposed action of the extensor pollicis longus (radial nerve) and the adductor pollicis (ulnar nerve). Because the second finger cannot be flexed and the third finger can be flexed only partially, when the person attempts to make a fist, these fingers remain extended. The hand then takes on the appearance of that of a clergyman offering benediction (benediction hand). Because all the median muscles are affected, there are pareses of forearm pronation, radial wrist flexion, distal flexion of the thumb, palmar abduction, and opposition of the thumb and flexion of the second and, to a lesser extent, the third fingers.
Lesions at the Elbow. Median nerve compression at the elbow may be due to tumors and other masses, Struthers’ ligament or other anomalous ligaments, supracondylar spurs, entrapment within the two heads of the pronator teres muscle, a large brachialis muscle, the overlying bicipital aponeurosis, venous varix, and bone or ligament injuries (e.g., elbow fracture or dislocation) [75,78,413]. Vascular causes include catheterization, thrombosis, anomalies such as a persistent median artery, an anomalously enlarged ulnar artery, or a false aneurysm of the brachial artery [141,143,284,299,413]. Motor and sensory signs and symptoms are as described earlier in Lesions in the Axilla and Upper Arm but elbow lesions may also cause an anterior interosseous or pseudoanterior interosseous syndrome (see subsequent text).
Lesions at the Ligament of Struthers. In approximately 1% of the population, an anomalous spur of the bone occurs 3 to 5 cm above the medial epicondyle on the anteromedial humerus. A fibrous tunnel may be formed by a ligament (ligament of Struthers) that connects this spur to the medial epicondyle. The median nerve may be compressed here by this ligament, causing motor and sensory signs and symptoms as described earlier in Lesions in the Axilla and Upper Arm [18,42]. The ligament may also compress the brachial artery or, rarely, the ulnar nerve [240].
The Pronator Syndrome. The median nerve may be entrapped or constricted where it passes between the two heads of the pronator teres muscle and under the fibrous arch of the flexor digitorum superficialis (pronator syndrome) [134,247]. Nerve injury is especially likely to occur if the nerve passes deep to a hypertrophied pronator teres. This syndrome has the following characteristics:
1. Pain may be present in the proximal forearm, especially on resistance to pronation of the forearm and flexion at the wrist.
2. Tenderness is observed over the pronator teres muscle on the application of deep pressure.
3. Frequently, there is a lack of involvement of the pronator teres, flexor carpi radialis, palmaris longus, and flexor digitorum muscles because nerve branches to these muscles often depart from the median nerve proper before the site of nerve compression.
4. There is sparing of the muscles innervated by the anterior interosseous nerve if this nerve takes a high origin from the median trunk.
5. Paresthesias and sensory loss are observed in the median field of innervation (both palmar and digital cutaneous areas).
6. Atrophy and paresis of the median thenar musculature occur.
7. Tinel’s sign may be present.
The Anterior Interosseous Nerve Syndrome (Kiloh-Nevin Syndrome). Isolated lesions of this nerve [131,134,392] are not uncommon and may be due to strenuous exercise, trauma, a fibrous band constricting the nerve, or an accessory head of the flexor pollicis longus (Gantzer’s muscle) entrapping the nerve [304]. Injury to the nerve may follow cutdown procedure (for catheterization) [109,180,327,393] or venipuncture [312] in the antecubital fossa or supracondylar fractures [75]. The nerve may also be involved as part of the Parsonage–Turner syndrome (neuralgic amyotrophy) [101,293], injured while the patient is in the prone position for spinal surgery [8], affected by vascular anomalies [284], or compressed by bronchogenic carcinoma metastatic to the forearm [272]. Bilateral anterior interosseous nerve syndromes due to cytomegalovirus infection have been described [97]. This purely motor syndrome has several characteristics:
1. Pain is present in the proximal forearm or arm lasting hours to days.
2. Mild paresis of forearm pronation (due to pronator quadratus weakness) is observed. The anterior interosseous nerve may rarely give rise to the nerve of the pronator teres muscle and, therefore, the syndrome may rarely present with weakness of the pronator teres [16].
3. Paresis of flexion of the terminal phalanges of the second and third fingers (due to paresis of the flexor digitorum profundus I and II) is observed.
4. Paresis of flexion of the terminal phalanx of the thumb (due to paresis of the flexor pollicis longus) is observed.
5. There is a characteristic pinch attitude of the hand while attempting to make a full circle by applying the pulp of the thumb to that of the index finger with firm pressure. This results from weakness of the flexor pollicis longus and the flexor digitorum profundus. There is hyperextension of the interphalangeal joint of the thumb, inability to flex the distal phalanges of the thumb and index finger, and proximal approximation of the thumb on the index finger.
6. Normal sensation is observed.
Because the anterior interosseous nerve has no cutaneous representation, this syndrome is often considered a purely motor syndrome. However, sensory fibers of the wrist radiocarpal, radioulnar, intercarpal, and carpometacarpal joints travel in the anterior interosseous nerve [88,392]. Injury to the terminal branch of the anterior interosseous nerve can cause persistent, dull, aching volar wrist pain [88].
A pseudoanterior interosseous nerve syndrome has been described [393], with partial median nerve compromise at the antecubital level. The nerve bundles that form the anterior interosseous nerve are primarily involved, and the motor findings are those described with the anterior interosseous nerve syndrome. The anterior interosseous nerve fascicle within the median lies posteriorly at the elbow and is, therefore, most prone to injury by fractures at this site [359]. Other median-innervated muscles are spared, and the only clinical finding betraying a more proximal median nerve lesion is some median distribution sensory change, as the sensory fibers from the index finger and thumb also lie posteriorly [359]. This syndrome has been noted with supracondylar fracture of the humerus, proximal radial fracture, venipuncture or arterial catheterization, and neuroma.
Median nerve entrapment may occur in the forearm because of an accessory bicipital aponeurosis [350] or because of enlarged communicating veins directly compressing the median nerve [54]. Other etiologies include arteriovenous fistulas in patients on chronic renal dialysis, fractures of the ulna or radius, and tumors [397]. This syndrome is characterized by paresis or paralysis of muscles innervated by the anterior interosseous nerve as well as more proximal median-innervated muscles (e.g., pronator teres and flexor carpi radialis). Sensation is intact. Median nerve damage proximal to the carpal tunnel may occur in wheelchair athletes [58].
The Carpal Tunnel Syndrome. The median nerve is particularly vulnerable to compression as it passes into the hand between the carpal bones and the transverse carpal ligament (carpal tunnel) [81,266,301,356]. The incidence of carpal tunnel syndrome is increased among electronic-parts assemblers, frozen-food processors, musicians, cyclists, wheelchair athletes, and dental hygienists; repetitive wrist movements, vibrating tools, awkward wrist positions, and great force seem to correlate with this disorder [81]. Women are more commonly affected than men. The increased pressure within the carpal tunnel is usually caused by a nonspecific flexor tenosynovitis, but certain conditions such as diabetes mellitus, rheumatoid arthritis, pregnancy [386], amyloidosis, hypothyroidism, acromegaly, renal dialysis, and congenital narrowing of the carpal canal, may predispose to this syndrome. The frequency of carpal tunnel syndrome in computer users is surprisingly similar to that in the general population [357]. A carpal tunnel-like syndrome may also occur with compression of the median nerve in the distal forearm, proximal to the carpal tunnel (e.g., due to a thrombosed aneurysm of the epineural vessels) [52,114]. The carpal tunnel syndrome usually consists of four main symptoms:
1. Bouts of pain or paresthesias in the wrist and hand are observed that are often most severe and troublesome during the hours of sleep and that are relieved by shaking or rubbing the involved hand. Although these symptoms are usually localized to the wrist or median-innervated fingers, they may spread upward into the forearm [135,356]. For example, paresthesias and pain occurred proximal to the wrist in 36.5% of 255 patients in one study [356]. Patients may also report sensory symptoms in the hand outside the median distribution (e.g., in the whole hand, in an ulnar distribution, or in a radial distribution), but sensory signs do not extend beyond the median nerve distribution [135]. The symptoms are bilateral in over half the cases but usually appear first and are more severe in the dominant hand.
2. Paresis and atrophy of the abductor pollicis brevis and opponens pollicis muscles are present. Because the opponens pollicis is occasionally anomalously supplied by the ulnar nerve, this muscle may be spared. In the carpal tunnel syndrome, the lumbricals are often normal because, in the carpal tunnel, fibers that innervate the lumbricals lie more posteriorly than those to the thenar muscles, protecting the lumbrical motor fibers from compression [211].
3. Sensory loss on the radial palm, the palmar aspect of the first three-and-a-half fingers, and the dorsal aspect of the terminal phalanges of the second, third, and half of the fourth fingers is noted. This sensory loss is usually most prominent in the appropriate fingertips. (Because the palmar cutaneous nerve takes its origin proximal to the wrist joint, the sensation on the thenar eminence and proximoradial palm is spared. Therefore, if this area shows a sensory loss, the lesion is proximal to the wrist joint.) Because of the fascicular arrangement of median sensory fibers in the wrist, sensory abnormalities may be limited to one side of a digit or to a web space between two digits, falsely suggesting more distal median sensory branch impairment [359].
4. Increased sensitivity of the damaged nerve fibers to mechanical deformation is observed. Because of this, various clinical tests may help detect a lesion at the carpal tunnel. Light percussion over the median nerve at the volar surface of the wrist may elicit a tingling sensation radiating into the hand in the median distribution (Tinel’s sign). When a blood pressure cuff is applied to the arm and compression is above systolic pressure, median paresthesias and pain may be aggravated (cuff compression test of Gilliatt and Wilson). Flexion of the wrist to 90 degrees for 30 to 60 seconds may aggravate paresthesias and pain (Phalen’s sign) as may hyperextension of the wrist.
Lesions of the Palmar Cutaneous Branch of the Median Nerve. The palmar cutaneous branch arises about 5 cm above the wrist and courses distally on the radial side of the palmaris longus tendon. It passes subcutaneously or through a canal of its own within the transverse carpal ligament and then divides into branches supplying the skin over the thenar eminence. The nerve may be damaged by accidental lacerations and during carpal tunnel surgery. Other causes of neuropathy include compression by an abnormal palmaris longus muscle or by a ganglia and entrapment by scars or fascial bands [10,57,121,334,355]. Damage to the nerve produces pain, paresthesias, and numbness over the thenar eminence. Median nerve compression at the wrist may occur with handcuff compression [130].
Lesions within the Hand. Median nerve entrapment in the palm by the head of the left first metacarpal bone while holding golf clubs may cause a sensory neuropathy with hypesthesia in a median distribution [155]. Injury to the deep palmar branches of the median nerve in the distal carpal canal (e.g., by a palmar ganglion [178]) or at the thenar eminence produces a purely motor syndrome with weakness and wasting of the thenar muscles (abductor pollicis brevis, opponens pollicis, and superficial head of the flexor pollicis brevis) with no sensory abnormalities. Selective acute demyelination of the recurrent thenar motor branch of the median nerve owing to vibrations from a sander (vibration-induced median neuropathy) may cause weakness in the abductor pollicis brevis without sensory loss [176].
Lesions of the Palmar Digital Branches of the Median Nerve. The palmar digital nerves are the terminal branches of the median and ulnar nerves (Fig. 2.6). The median nerve divides into a number of branches after it emerges from the distal end of the carpal tunnel. Usually, it divides into two major branches, each of which then divides into the common digital nerves, which in turn divides into two proper digital branches. The two palmar digital branches to the thumb and to the lateral side of the second digit usually arise from the lateral terminal division of the median nerve. The other digital branches from the median nerve arise from the medial division and supply digital branches to the lateral three-and-a-half digits, but the entire fourth digit may be innervated by the median or ulnar nerve.
Lesions of a common palmar digital nerve in the hand cause sensory symptoms and loss usually involving the adjacent sides of two fingers, depending on the nerve distribution. Damage to a proper digital branch produces sensory loss and paresthesias restricted to the side of the finger. Etiologies include trauma (e.g., lacerations, finger dislocations, and fractures), compression from musical instruments (e.g., flutist’s neuropathy), tendon sheath cysts and tumors, tenosynovitis, nerve ischemia (e.g., in diabetes), and nerve tumors [26,79,296].
Digital neuropathy is a pure sensory neuropathy of a digital nerve. It may be caused by acute or chronic local trauma or pressure, or accompany systemic illnesses such as rheumatoid disease, leprosy, Raynaud disease, dysproteinemia, or diabetes mellitus [137]. Digital neuropathy of the median and ulnar nerves may be caused by Dupuytren contracture [137]. Dupuytren tissue usually affects the palmar fascia, superficial to the digital nerves, and it may rarely affect the spiral cord in the digits. A spiral cord may cause sensory loss due to impingement of digital nerves or Dupuytren tissue may compress the palmar digital nerves against the relatively inelastic deep transverse metacarpal ligament.
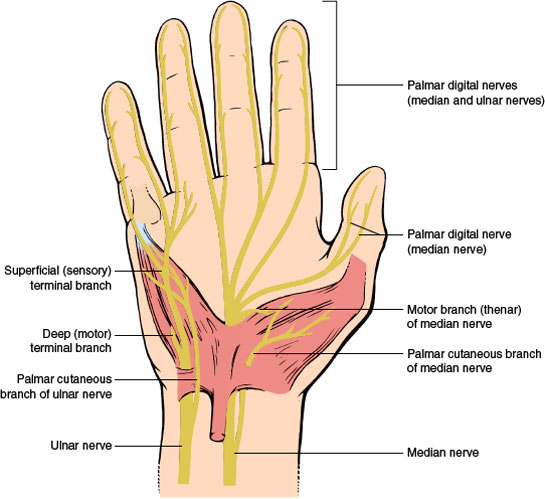
FIG. 2.6. Palmar view of the right hand showing the course of palmar digital branches of the median and ulnar nerves.









