INTRODUCTION
There are many case reports and case series describing the development of a movement disorder (MD) following peripheral trauma (see for a review (1)). These so-called peripherally induced MDs (PMDs) are defined as MDs that are triggered by trauma to the cranial or peripheral nerves, or soft tissues (2). In a recently performed review, which focused on trauma-induced extremity-related PMDs, dystonia emerged as the most frequently reported type (72%), followed by tremor (25%), myoclonus (13%), spasms (11%), painful limbs and moving toes or fingers (6%), and less frequently, parkinsonism, chorea, and tics (together 4%) (1).
In the current chapter, we will first discuss whether PMDs actually exist—that is, if they are indeed peripherally induced, since there is a longstanding debate on this topic in the field of MDs. In the subsequent paragraphs, we review the occurrence, phenomenology, diagnosis, course, and treatment of the most commonly encountered PMDs, including hemifacial spasm (HFS), painful limb moving extremities syndrome (PLME), peripherally induced dystonia, peripherally induced tremor, MDs occurring in the context of complex regional pain syndrome (CRPS), and post traumatic cervical dystonia. In addition, a group of rarer PMDs, including jumpy stumps and belly dancer’s dyskinesia, will be addressed at the end of the chapter.
DO PMDS EXIST?
One of the difficulties in studying PMDs is their relative rarity, which impedes the application of epidemiologic studies to this group of MDs. Another difficulty in performing research in this field is that it is unclear which time interval between trauma and ensuing MD must be taken into account, and how severe the trauma must be to be acceptable in the context of a potential causal relationship. This shortcoming led Cardoso and Jankovic (3) to propose diagnostic criteria for PMDs (Table 52.1). Though the authors recognized some limitations of these criteria (4), others have emphasized that they are too arbitrary and lack scientific basis (5,6). Nevertheless, the use of these criteria may facilitate research by bringing some uniformity in a field that would otherwise use very heterogeneous inclusion criteria.
| Diagnostic Criteria for Peripherally Induced Movement Disorders |
1 | The trauma must be severe enough to cause local symptoms for at least 2 wk or require medical evaluation within 2 wk after trauma. |
2 | The onset of the MD is within days or months (up to 1 y) after the injury. |
3 | The onset of the MD is anatomically related to the site of the injury. |
A potential causal relation between trauma and MD is not generally accepted (2,4,6). Proponents and opponents offered a range of arguments and counter arguments to support their view (Table 52.2). The enumeration clearly illustrates that consensus in this field is still far away.
HEMIFACIAL SPASM
One of the best examples of a PMD in humans is HFS. This condition is characterized by unilateral involuntary paroxysmal clonic or tonic contractions of one or more muscle groups innervated by the facial nerve. In HFS, the contractions usually start in the orbicularis oculi muscle and are followed by a gradual spread to the other muscles of the upper and lower face, including the platysma. HFS can be associated with minimal weakness of the orbicularis oculi or orbicularis oris muscles on the involved side. A typical feature of HFS observed during spasms is the so-called other Babinski sign or the brow-lift sign: a unilateral contraction of the frontalis muscle causing eyebrow elevation is associated with concurrent contraction of the ipsilateral orbicularis oculi muscle, resulting in eyelid closure (Video 52.1) (7). Another common finding in HFS is synkinesias, in which subtle voluntary contractions of the orbicularis oculi muscle trigger simultaneous contraction in the orbicularis oris muscle or other facial muscles, and vice versa (Video 52.1). It may be difficult to clinically detect synkinesias in HFS. In these cases, the demonstration of lateral spread responses can be helpful: stimulation of the supraorbital nerve produces a synkinetic contraction of other muscles innervated by the facial nerve, whereas during stimulation of the uninvolved side a contraction of the orbicularis oculi muscle alone is recorded (8,9). In contrast to synkinesias occurring as phenomena of aberrant regeneration after Bell’s palsy, synkinesias in HFS are more variable with more pronounced presentation during and shortly after a spasm (8). HFS may occur bilaterally, but this is rare; only 3% of consecutive cases with HFS had bilateral involvement (10).
Primary HFS is commonly attributed to vascular compression of the facial nerve at the root-exit zone (11). Common vessels implicated in HFS are the anterior inferior cerebellar artery (43%), posterior inferior cerebellar artery (31%), vertebral artery (23%), and veins (3%) (11). Multiple offending vessels were found in 38% of cases (11). Others have questioned the causative role of vascular compression in HFS, since neurovascular compression has been reported on the asymptomatic side of cases with HFS and in control subjects (12). However, vascular compression may occur at different sites of the facial nerve, and these sites differ in their vulnerability to develop HFS. Using magnetic resonance imaging (MRI) with 3D constructive interference, one study addressed this issue and found that vascular contacts beyond the root-entry zone are irrelevant in causing HFS and also reported that no vascular compression at the root-entry zone of the facial nerve of the unaffected side could be demonstrated (13). The root-entry zone of the facial nerve has distinct features that may contribute to an increased vulnerability during compression (14). At this site, the nerve is ensheathed by only an arachnoidal membrane, while epineurium and connective tissue septa traversing the individual fascicles are absent (14). Further, in the root-entry zone, the origin of myelination changes from central (oligodendroglial cells) to peripheral (Schwann cells) (15). Concerning the development of spasms, two main hypotheses have been forwarded. First, compression of the facial nerve by a blood vessel injures the myelin sheath at the root exit of the facial nerve. In turn, this damage facilitates transaxonal transfer of impulses (ephaptic transmission) and spontaneous development of neural impulses (ectopic excitations), collectively explaining synkinesias and the development of spontaneous activity in facial muscles (15–17). Second, HFS is induced by hyperexcitability of the facial motor nucleus (15,17). Support for this hypothesis is provided by findings of blink reflex excitability (elicitation of a larger R2 response) and enhanced F waves, and by shorter latencies and a decreased amplitude of the motor-evoked potentials on the affected side in patients with HFS (18–20). Further, one other study on blink reflexes showed that hyperexcitability of the facial motor nucleus likely is mediated by trigeminal afferent inputs (21).
| Arguments in the Debate on the Causal Relationship between Trauma and Movement Disorder |
Proponents | Counter Arguments |
There are broadly accepted examples of PMDs, such as HFS (2). | The fact that a relation between trauma and MD is identified in some examples does not necessarily mean that this rule applies to other nerves or body sites. |
Family members of patients with peripherally induced dystonia showed similar abnormalities of motor cortex excitability as affected patients (139), which was taken as evidence of increased intrinsic susceptibility (2). | The presence of preexisting cortical abnormalities may in fact argue against a causal relation with trauma, since it indicates that the MD might have occurred anyway and that the relationship with trauma is purely coincidental. |
Increased cortical excitability, a physiologic hallmark of typical (mobile) dystonia, was also observed in patients with fixed dystonia, of whom the majority developed the dystonia following a trauma to the same limb (81). | The presence of preexisting cortical abnormalities may in fact argue against a causal relation with trauma, since it indicates that the MD might have occurred anyway and that the relationship with trauma is purely coincidental. |
Opponents | Counter Arguments |
A causal relation is difficult to prove, since it inevitably involves circular reasoning, i.e., if one searches for patients who developed a MD after a trauma, then this does not prove that the trauma caused the MD if such patients are identified. | The first and foremost requirement involves the temporal order of events, where cause (here: trauma) must precede consequence (here: MD), which is fulfilled in the case of a PMD. |
Given the high frequency of trauma, more PMDs would be expected than are actually observed (the “denominator problem”), and the fact that the condition is so rare may therefore indicate that the association is coincidental. | The prevalence of a disorder can never be used as an argument against its existence, since there are many diseases with a very low prevalence and there may be differences between individuals in intrinsic susceptibility to develop the condition (5). |
The few case–control studies that have been performed so far did not unequivocally identify associations between trauma and PMDs. | There are only a few case–control studies in this area, and insufficient sample size and suboptimal methodology may have prevented the identification of—especially infrequent—exposures such as trauma (106,107,140). |
A clear pathophysiologic explanation for PMDs is lacking. | There are many existing and well-accepted conditions for which the pathophysiologic underpinnings have thus far not been identified. |
Bell’s palsy and other injuries of the facial nerve are the second most common cause of so-called secondary HFS, collectively constituting 20% of HFS (22). In contrast to cases with primary HFS (see above), most patients with secondary HFS report initial involvement of the upper and lower facial muscles simultaneously (22).
Although population-based data on HFS are lacking, findings from secondary/tertiary referral centers suggest that HFS is more common in women (3:1) and in patients of Asian origin (22,23). The role of hypertension in HFS is controversial (22).
The simultaneous occurrence of unilateral spasms of upper and lower facial muscles in combination with the variable presence of synkinesias and the brow-lift sign generally characterize a typical HFS. In less-typical cases, HFS mimics, including blepharospasm, facial myokymia, facial dyskinesia, tics, hemimasticatory spasm, focal epilepsy, facial myorhythmia, or functional movements involving the face, should be considered in the differential diagnosis (22). Routine MRI is sufficient in all cases of HFS to exclude rare space-occupying causes compressing the facial nerve (aneurysms, arteriovenous malformations, tumors in the cerebellopontine angle) and pontine demyelinating and vascular lesions (23).
Although a systematic review showed a paucity of good-quality controlled data on the efficacy of botulinum toxin, the majority of cases with HFS are managed successfully (76%–100% success rates) with repeated botulinum toxin injections (11). There are no studies that have compared the efficacy of botulinum toxin with surgical microvascular decompression, which is also associated with high (90%) remission rates (24–26). However, the latter approach is generally reserved for those cases who fail to obtain a sufficient response on botulinum toxin. The high remission rates after vascular decompression support the peripheral cause of HFS (24–26). Moreover, it has been suggested that failure of surgical microvascular decompression should question the presence of another vascular conflict causing HFS (27).
PAINFUL LIMBS/MOVING EXTREMITIES SYNDROME
PLME was first reported by Spillane et al. (28) and is characterized by pain in usually one limb accompanied by involuntary repetitive, nonrhythmic movements of at least the digits (Videos 52.2 and 52.3) (29–31). Rarely, painless variants have been reported (“painless limb and moving toes”) (32). The pain is often of neuropathic origin (29–31), precedes the involuntary movements by days to years (30), and is experienced to be more distressing than the movements (29,31). The onset of pain generally is unilateral but may spread to the contralateral side (30). The distribution of the pain is never limited to a peripheral or segmental dermatomal pattern (30). The limb movements may be continuous or intermittent and are characterized by involuntary spontaneous flexion–extension and/or abduction–adduction movements of the digits with occasional involvement of the foot or hand (29–31). Each digit may move independently from the other. PLMEs usually disappear when the patient is asleep (29,30). The movements may be momentarily suppressed by voluntary activity (29–31). PLME predominantly occurs in the lower limbs, but variants of the syndrome have been described for the hand, shoulder, and tongue (32–34). On electromyographic recordings, the movements are characterized as unpredictable, irregular bursts of activity lasting 80 to 2,000 ms with normal motor units and normal recruitment patterns (29,30).
PLME can be idiopathic, but peripheral tissue and nerve conditions like nerve injury, root injury, or polyneuropathy, and soft and bony tissue damage are the most common precipitant factors of the syndrome (29–31). Rarely, the syndrome has been reported in conditions affecting the spinal cord and brain (35–38). The etiology of PLME remains unknown, but the clinical characteristics of both the pain and the movements in this condition favor a central origin for this syndrome (29–31). The fact that most cases of PMLE are associated with peripheral nerve lesions may suggest that disturbances of afferent input may impair central sensory processing and sensorimotor integration (29–31). Analogous to CRPS, this may explain the development of neuropathic pain and the abnormal movements (30).
In the largest case series, most patients were middle-aged and female (66%) (29–31). Depending on the history and findings of the neurologic exam, additional investigations, including electromyography and MRI of the spinal cord and brain, can be applied and may reveal potential clues of a peripheral or central cause of PMLE.
Though one case series reported that gabapentin and pregabalin offered the best pain control (29), other case series revealed that the treatment of PLME is notoriously difficult (30). Recently, some cases have been reported showing an improvement of pain and movements in response to botulinum toxin (39,40). The majority of larger case series, however, report that treatment of the abnormal movements is generally disappointing (30,31).
PERIPHERALLY INDUCED DYSTONIA
Fixed dystonia is the most frequently reported PMD (1,41–43) and is associated with pain in the affected extremity in the majority of cases (1,43). Fixed dystonia in the upper extremity is usually characterized by flexion postures of the fingers and wrist, and in the lower extremity by a plantar flexion/inversion posture, usually accompanied by clawing of the toes (4,43,44). In a about one-third of patients, a more mobile form of dystonia is observed, while a combination of posturing and spasms is present in one-fifth of cases (1).
The most common precipitant is soft tissue injury, followed by nerve entrapment, with an identifiable nerve lesion in 36% (1). The median time between trauma and development of the MD was 14 days, with an interquartile range of 2 to 150 days. The associated pain and other sensory abnormalities of the affected body part in the majority of cases with peripherally induced dystonia may suggest that altered afferent input leads to spinal or supraspinal reorganization, and subsequently influences motor processing (1,42).
The incidence and prevalence of peripherally induced dystonia are unknown. In a systematic review of the literature that used a time window of 40 years, 299 patients were identified (1). The prognosis of this type of dystonia is generally considered poor (43); follow-up of the same group of patients after a mean of 7.6 years showed that less than 25% improved and that about one-third deteriorated further (45).
Diagnosis is based on clinical examination and ruling out other causes of dystonia (46) using blood tests, nerve conduction, and imaging studies (computed tomography [CT], MRI) of the spinal cord and brain.
Peripherally induced dystonia is difficult to manage, and treatment is usually unsuccessful (4,42). Muscle relaxants may provide some relief in a few patients, while anticholinergics are usually ineffective. In a few patients, remissions were shown by a multidisciplinary approach, encompassing physiotherapy and psychotherapy (43). In general, the response to botulinum toxin is poor, which may be due to associated contractures. Three cases responded immediately (i.e., within minutes) to botulinum toxin, whereas it normally takes at least 72 hours to achieve some effect, which indicates that in a number of patients, a psychogenic cause may underlie this type of dystonia (47).
PERIPHERALLY INDUCED TREMOR
Peripherally induced tremors are usually postural or kinetic tremors (3,48,49), but may occasionally present as resting tremor in the case of peripherally induced parkinsonism. Spread of tremor to other body sites was described in almost 75% of cases (3). The phenotypical characteristics of peripherally induced tremor resemble those of essential tremor, parkinsonian tremor, and dystonic tremor (50,51). Features indicative of psychogenic tremor are commonly not reported in these series (3). Tremor can present as a single feature, but frequently occurs in combination with other MDs (1,51). The time interval between the trauma and the MD may range from 1 day to 9 months (51), and pain may precede the development of the tremor (3). Local injury to the nerve was identified in 11% (1). Tremor, particularly postural, has also been reported in inflammatory polyneuropathy associated with immunoglobulin M paraproteinemia and hereditary forms of polyneuropathy as Charcot–Marie–Tooth disease and Kennedy syndrome (52).
The incidence of peripherally induced tremor is low, but exact data are lacking, since knowledge only comes from case reports.
Diagnosis is based on history and clinical examination, for which several clinical tests are suggested (53). Additional neurophysiologic testing may provide information on the phenomenology of the tremor and can be helpful to reveal features of functional tremor (54). Peripherally induced tremor is diagnosed when other causes have been excluded and a relationship with trauma is evident.
Peripherally induced tremor is resistant to therapy (3,4,55). A few cases responded to propranolol (3,48,50), whereas in others botulinum toxin may provide (temporary) relief (3,44).
COMPLEX REGIONAL PAIN SYNDROME
CRPS is a pain disorder that usually develops following surgery or trauma to a limb (56). Besides pain, CRPS is characterized by various combinations of sensory, autonomic, trophic, and motor abnormalities (56). Motor disturbances in CRPS involve weakness, bradykinesia, dystonia, tremor, myoclonus, and decreased active range of motion (5,57–59), although it can be argued that the latter rather reflects a trophic than a motor disturbance.
Tonic or fixed dystonia is the most frequently observed MD in CRPS, and is characterized by loss of voluntary muscle control and sustained abnormal postures, from which return to a neutral position is not possible, or only with great difficulty (1,43). In the arm, the dominant pattern involves flexion of the fingers, wrists, and elbows, while in the legs plantar flexion of the toes and plantar flexion and inversion of the ankle are most frequently found (Fig. 52.1) (60). CRPS-related dystonia is more common in women and may spread to other limbs (1,56,61), a risk that increases with the number of limbs that are already dystonic (61).
Compared to non-CRPS patients, dystonia in CRPS more often involves a fixed type (91%). Myoclonus (involuntary, brief, jerk-like contractions of a muscle or muscles) and action and postural tremors are occasionally seen in CRPS (59,62–64), but rarely are the predominating MD. The median delay between trauma and MD is 61 days (interquartile range 5–243) (1).
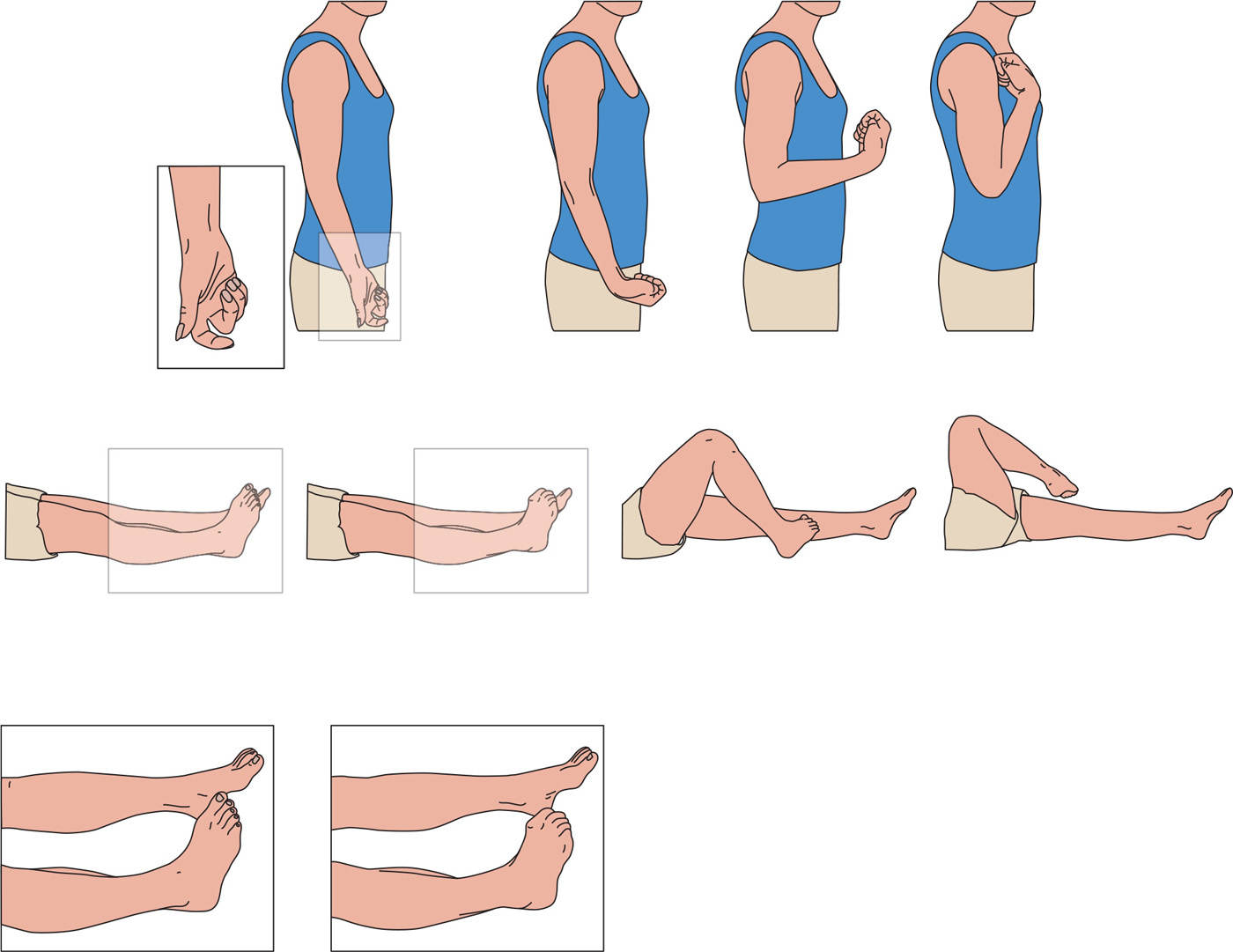
Figure 52.1. Dystonic postures in CRPS. Most common postures in arm and leg in CRPS-related dystonia arranged to the severity from left to right. (From Munts AG, et al. Fixed dystonia in complex regional pain syndrome: a descriptive and computational modeling approach. BMC Neurol 2011;11:53.)








