Fig. 11.1
Typical MR appearances of post-traumatic syringomyelia (Reproduced with kind permission of the Ann Conroy Trust)
It is important to distinguish primary, post-traumatic cysts from true syringomyelia cavities. The former are more common and are seen on magnetic resonance imaging (MRI) scans in half of all spinal cord injury victims (Backe et al. 1991). They are the result of primary damage to the cord and are located at the level of the original injury. They are usually small and rounded or ovoid and do not produce a great deal in the way of cord expansion (Perrouin-Verbe et al. 1998). Post-traumatic syringomyelia, on the other hand, refers to propagating cavities, extending beyond the level of the original trauma. These develop as a secondary, delayed consequence of the original injury.
Post-traumatic syringomyelia is important in two respects. Whereas syringomyelia remains an uncommon condition in the community as a whole, it is very common in the population of spinal cord injury victims. Quoted figures vary, but even early estimates of about 5 % represent a significant incidence , more even than common medical disorders such as diabetes or asthma. Later publications give figures that are higher still, at around 20 %. These are based on both radiological surveys (Perrouin-Verbe et al. 1998; Squier and Lehr 1994; Wang et al. 1996) and post-mortem studies (Squier and Lehr 1994). The differences in these figures reflect the fact that earlier estimates were derived when MR imaging was carried out selectively, as indicated by the onset of new neurological symptoms (El Masry and Biyani 1996). The higher estimates also depend upon a definition of syrinx cavities as being those that extend two or more segments beyond the level of the original injury. If the definition stipulated three segments, then the incidence would fall back to single figures (Pearce 1995). A reasonable working rule, therefore, would be to say that up to one in five of all spinal cord injury victims develop anatomical syringomyelia and that at least 1 in 20 will develop symptoms.
The second point to note is that most victims of spinal cord injury already have to contend, in their day-to-day life, with significant neurological disability. The devastating transformation that spinal cord injury brings about in somebody’s life is very apparent, and it is a cruel irony when the development of a post-traumatic syringomyelia cavity threatens the individual with further loss of independence. Yet most patients cope remarkably well, albeit requiring a good deal of support . They are often the least complaining of all the patients who come to our clinics. Few conditions in neurosurgery challenge our skills, as surgeons and as doctors, to the same extent.
11.2.1 Presentation
Most spinal cord injury patients living in economically privileged societies are currently offered regular surveillance of their neurological and general status. Their supervising units have a low threshold for carrying out MR imaging if problems develop. Indeed, there is a case for offering routine screening for all victims of spinal cord injury, looking specifically for post-traumatic syringomyelia (Sett and Crockard 1991). Syrinx cavities are now being detected with increasing frequency, often in patients with little in the way of new neurological symptoms.
Many patients do display clear indications of having developed a complication of their original spinal cord injury (Table 11.1). In most published series, pain is at the top of the list of presenting symptoms. The differential diagnosis of the symptoms of post-traumatic syringomyelia includes constipation, pressure sores and urinary tract infections, causing sweating attacks and spasms, and ulnar nerve palsies resulting from repeated pressure being placed on the elbows, during transfers. As with all forms of syringomyelia, there is a poor correlation between the size of the cavity and the magnitude of the clinical features.
Table 11.1
Common presenting features of post-traumatic syringomyelia
Symptoms |
Increasing pain |
Hyperhidrosisa |
Increasing spasms |
Loss of sensation |
Loss of trunk control |
Reduced dexterity |
Altered bladder function |
Autonomic dysreflexia |
Signs |
Ascending sensory level |
Focal motor deficits |
Loss of upper limb reflexes |
Horner’s syndrome |
An impressive feature of post-traumatic syringomyelia is the wide variation in the latent interval between the original spinal cord injury and the first onset of symptoms arising from the syrinx. The range extends from a few months to several decades. Attempts have been made to predict which spinal cord victims might go on to develop post-traumatic syringomyelia, and although individual studies have pointed to one factor or another (Table 11.2), there are currently no reliable predictors. Lifelong surveillance remains the only safe option.
Table 11.2
Possible predictors of development of post-traumatic syringomyelia
Level | Cervical vs dorsal spine |
Severity | Complete vs incomplete functional transection |
Displaced vs undisplaced fracture | |
Age | Older vs younger patients |
Management | Conservative vs surgical management |
Fixation with or without bony decompression | |
References |
The generation of symptoms and neurological deficits probably relates, in part at least, to hydraulic pressure within the syrinx, leading to local ischemia, as well as stretching of decussating fibres and long tracts (Milhorat et al. 1997; Young et al. 2000). The magnitude and duration of raised pressure may account for whether or not these clinical features are reversible. Another mechanism is tethering , leading to traction on the cord during normal movement of the spinal column. Release of tethering may, in some cases, be at least as important as collapse of the syrinx, when it comes to gaining some clinical improvement (Ragnarsson et al. 1986). It has also been suggested that leptomeningeal fibrosis may lead to cord ischemia, but it is difficult to substantiate or refute such suggestions.
11.2.2 Syringomyelia Following Minor Trauma
A particular category of lesions is that of cavities detected following relatively minor injuries, such as whiplash or a fall. Victims may complain, often after an interval, of a variety of neurological symptoms, yet there may be no accompanying physical signs. MR imaging is carried out, sooner or later, and the sort of lesion revealed may consist of a short, spindle -shaped intramedullary cavity, without any associated abnormality at the craniovertebral junction and without any obvious obstruction elsewhere in the spinal canal (Fig. 11.2). Not uncommonly, medicolegal experts become involved and discussions centre on whether the cyst was caused by the accident or was pre-existing. Questions arise as to whether it is a true syrinx or something else, such as a glioependymal cyst . The latter are well documented as intracranial lesions but also occur within the spinal cord. Radiologically, they are usually short but relatively plump in size, being quite well defined but not enhancing with contrast injection (Robertson et al. 1991; Saito et al. 2005). Legal debate continues as to whether, irrespective of its nature, the cavity was rendered symptomatic by the injury or was simply an incidental finding, having nothing to do with the claimant’s symptoms. Onset of symptoms immediately after the injury suggests a pre-existing cavity, rendered symptomatic by the event. Delayed onset suggests that the cavity was caused by the trauma (Barnett 1973). To declare that the presence of a syrinx bears no relationship to a prior injury, or to suggest that neurological symptoms do not arise from the lesion, ignores the temporal association between the injury and detection of the syrinx. This legal issues are discussed in more detail in Chap. 18, but it is fair to say that there is little in the literature to guide an expert witness, beyond the fact that any series of post-traumatic syringomyelia cases may well include a significant number where the initial trauma was moderate and where no neurological deficits were evident at the time (Klekamp and Samii 2002; LaHaye and Batzdorf 1988).
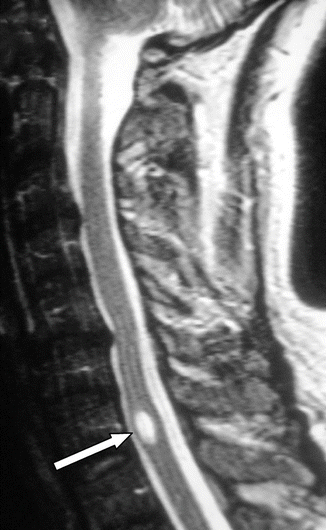

Fig. 11.2
Short, spindle -shaped cavity (arrow). Revealed during the course of investigation for pain and somatic sensory disturbances, following minor trauma, this could represent a small, post-traumatic syrinx or a pre-existing glioependymal cyst , rendered symptomatic by the injury
11.3 Subarachnoid Haemorrhage
Relative to the overall incidence of subarachnoid haemorrhage, the occurrence of spinal arachnoid adhesions, as a complication, is low (Augustijn et al. 1989; Tjandra et al. 1989). The incidence of syrinx formation, as a further sequel, is distinctly rare, although cavities can develop following both intracranial and intraspinal subarachnoid haemorrhage (Siddiqi et al. 2005). What determines why and when this complication occurs is unknown. It may be that, if a patient remains recumbent for any period, after a subarachnoid haemorrhage, blood products pool on the spinal canal.
Clearly, any intradural surgical procedure carried out on the spine will result in some blood and tissue products being shed into the canal. This can result in formation of arachnoid adhesions, potentially resulting in syringomyelia. Once again, such complications are rare (Cusick and Bernardi 1995; Klekamp et al. 1997).
11.4 Contrast Media
Prior to the advent of MR scanning, the principal means of investigating the spinal canal and its contents was by myelography. Originally, this involved the use of oil-based contrast media although, subsequently, water-soluble agents were developed. Once injected into the spinal canal, this oily material would not be absorbed, and in some cases, an arachnoiditic reaction followed. Once these effects were recognised, it became the normal practice to aspirate as much of the contrast medium as possible, after the radiological study was completed. Some patients, nevertheless, developed meningeal fibrosis, with disabling consequences. A curious aspect of the problem was why only a minority of people developed this complication. One suggestion was that the inflammatory response depended upon a synergistic reaction between the contrast medium and something else, such as blood or even powder from surgical gloves.
Although largely a matter of historical interest these days, newly diagnosed cases of syringomyelia still present, occasionally, in somebody who underwent myelography, with an oil-based contrast medium, many years ago (Tabor and Batzdorf 1996).
11.5 Post-tuberculous Syringomyelia
Spinal cord involvement is a recognised complication of tuberculosis, and as with tuberculosis anywhere in the nervous system, different pathological manifestations are seen. These include acute inflammation and oedema of the cord, intramedullary abscesses and intramedullary or intradural granuloma formation (Muthukumar and Sureshkumar 2007). The most common consequence, however, is late adhesive arachnoiditis. Despite this, syringomyelia is a relatively uncommon sequel. Even surgeons who deal with a lot of cases of syringomyelia are unlikely to see many tuberculosis-related syrinxes, and most reports in the literature relate to no more than a handful of patients (Kaynar et al. 2000; Moghtaderi et al. 2006; Schon and Bowler 1990).
Most cases present at an interval, after the underlying infection has been treated. As with post-traumatic syringomyelia, this latent interval can vary widely from case to case. Early presentation has also been described and the assumption is that acute cord inflammation is the underlying mechanism rather than arachnoid adhesions (Daif et al. 1997; Fehlings and Bernstein 1992).
Post-tuberculous adhesions are likely to be extensive, making the creation of a conduit all but impossible in most cases. It is in this type of syringomyelia that direct shunting may be a preferred option.
11.6 Other Infections
Syringomyelia is also recognised as an occasional complication of other infections involving the spinal cord. Organisms which have been reported include Listeria (Nardone et al. 2003), Cryptococcus (McLone and Siqueira 1976), Syphilis (Bulundwe et al. 2000; Mebrouk et al. 2011) and Candida (Phanthumchinda and Kaoropthum 1991). Syringomyelia is also occasionally seen in association with previous epidural inflammatory pathology (Klekamp et al. 1997). All these examples are much less common than post-tuberculous cases. It should, nevertheless, be part of the routine history taking, in all cases of syringomyelia where the cause is not immediately apparent, to enquire about a past history of meningitis, spinal trauma, head injury and subarachnoid haemorrhage.
11.7 Arachnoid Webs and Cysts
When we consider what might happen as a consequence of obstruction to CSF flow within the spinal canal, we might reasonably predict a build-up of fluid on the outside of the cord rather than the more usual finding of syrinx formation. Indeed, we do sometimes see such external, “hydraulic compression” of the cord, in association with arachnoid webs (Fig. 11.3). Such webs, as well as cysts (or pouches), may be congenital lesions or may arise as the result of earlier haemorrhage (Thines et al. 2005) or arachnoiditis from other causes (Gnanalingham et al. 2006; Gopalakrishnan et al. 2010). The obstruction caused by such webs may also result in syrinx formation (Mallucci et al. 1997). The likely mechanism, once again, is that normal arterial and venous pressure waves are not dissipated normally down the spinal canal, leading to creation of the syrinx cavity (Brodbelt and Stoodley 2003a, b; Holly and Batzdorf 2006). The syrinx is the more obvious abnormality on imaging and a careful search should be made to identify the web. Indeed, there may be occasions when it is only revealed at the time of surgical exploration.
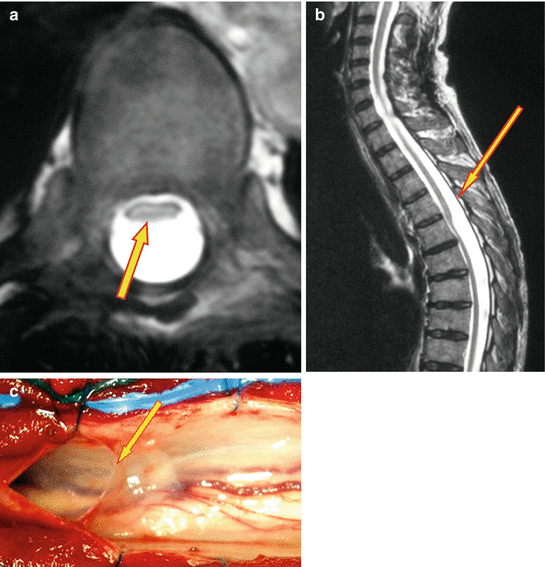

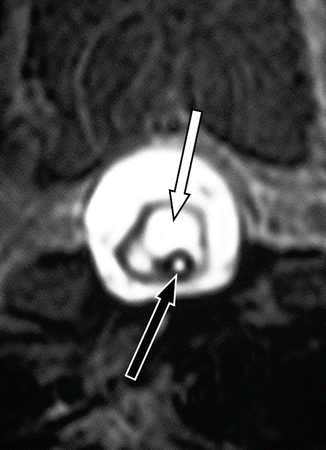
Fig. 11.3
Spinal arachnoid webs. (a) T2-weighted MRI, transverse section: the cord is clearly compressed flat, in the anterior half of the spinal canal (arrow). (b) T2-weighted sagittal MRI: the same feature is evident in the sagittal plane, where the level of the focal point of obstruction to CSF flow is very evident (arrow). (c) Operative appearances of the lesion. With the dura opened, a free edge forms along the dorsal aspect of the web (arrow). Extending anterior to this can be seen the thin membrane, which occupies the full cross-sectional area of the thecal sac
When an operation is planned, the surgeon must realise that the lesion might be an arachnoid cyst rather than a simple web. The myelopathy, be this due to the pressure within the cyst or from a resulting syrinx, might not be relieved unless both the upper and lower limits of the cyst are broached.
11.8 Surgical Management
Compared with operations for hindbrain-related syringomyelia, surgery for cavities caused by spinal arachnoid fibrosis does not yield results that are as good overall. Indeed, this category of syringomyelia is one of the most difficult conditions that neurosurgeons are called upon to treat, at least in terms of gaining consistent results. It is not surprising, therefore, that various techniques have been described over the years (Edgar and Quail 1994). Further, not all syrinx cavities progress relentlessly (Anderson et al. 1986) and it is certainly the author’s experience that many seem to enter a state of “hydrodynamic equilibrium ” (see also Chap. 2). For all these reasons, surgical intervention should be reserved for those cases that show clear evidence of neurological deterioration. Not surprisingly, most published series include significant numbers of patients who do not go on to surgical intervention.
11.8.1 Creation of a CSF Conduit
There are, broadly speaking, three types of surgical manoeuvre that we can offer to a patient with syringomyelia (Table 11.3). Clearly, it makes sense to treat the underlying cause if possible. So, when we can identify a focal point of obstruction to CSF movement, the logical approach is to try and relieve that blockage and create a new conduit for CSF flow. This is, of course, a major operation, involving the removal of several laminae, and it requires microsurgical technique for the intradural part of the procedure. In most cases of post-traumatic syringomyelia, the scar tissue encountered is relatively limited in extent, and breaking it down is not unduly difficult. The offending cicatrix is clearly recognisable by its typical milky-white appearance and can be readily distinguished from normal, translucent arachnoid. Dissection dorsal and dorsolateral to the cord will eventually result in it falling anteriorly, into the spinal canal. At this point, the syrinx has usually collapsed and the cord may begin to pulsate. It is unnecessarily hazardous for the dissection to be carried anteriorly, although opinions vary as to the value of dividing dentate ligaments . The exposure also needs to be of sufficient length to allow free, rhythmic movement of CSF, up and down the spinal canal.
Table 11.3
Treatment options
Creation of a conduit for CSF flow |
Direct drainage of the syrinx cavity |
Lowering the overall CSF pressure |
Conservative management |
The anatomical results from this sort of surgery can be very satisfying (Fig. 11.4). The problem is that blood products and muscle proteins, which are inevitably “spilt” into the laminectomy site, may go on to organise into scar tissue once more, and this leads to recurrent obstruction of the spinal subarachnoid channels (Fig. 11.5). Early postoperative imaging is, therefore, of limited value; it is very likely to show a reduction in the volume of the syrinx, but this by no means guarantees long-term anatomical improvement. If, however, the cavity remains collapsed at 6 months and beyond, then it is likely that a good result will have been achieved in the long term, in anatomical terms at least and probably in terms of function as well.
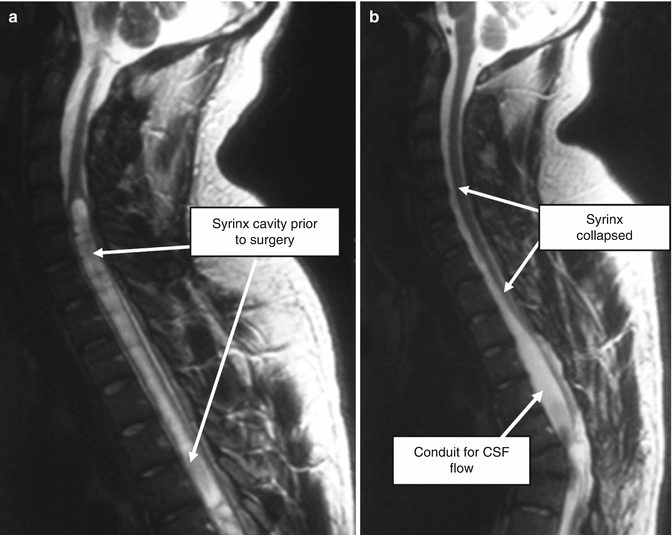
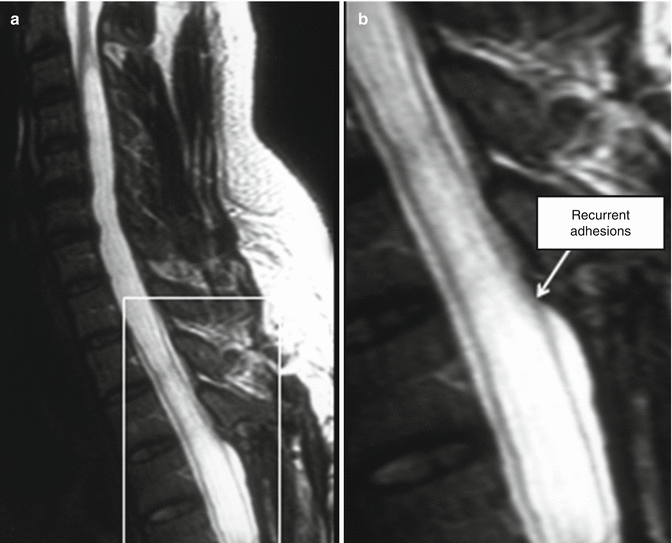

Fig. 11.4
Effective surgery for post-traumatic syringomyelia. Laminectomy, with release of intradural adhesions and creation of an artificial conduit for CSF flow, can produce very rewarding anatomical results, with arrest of clinical deterioration and sometimes recovery of lost function. (a) Prior to surgery. (b) Following laminectomy, release of scar tissue and creation of pseudomeningocoele , to act as an artificial conduit for CSF flow

Fig. 11.5
Recurrence of syringomyelia after laminectomy. (a) Despite the initial operative creation of a good conduit for CSF flow, the syrinx cavity has refilled after an interval. (b) The enlarged inset from “a” reveals that recurrent adhesions at the upper aspect of the laminectomy have once again obstructed normal CSF flow
How the dura is handled is likely to have a major influence on the success or otherwise of this operation. With craniovertebral decompression for hindbrain-related syringomyelia, we have discussions about dural opening – full, partial or not at all – reduction or excision of cerebellar tonsils, use of dural grafts and the role of cranioplasty. We have similar debates about the best methods of exposure and closure, when it comes to operating for post-arachnoiditic syringomyelia. Dural patch grafts might be expected to limit the amount of blood and muscle products entering the spinal theca, from the surrounding tissue planes. This, we might expect, would lessen the amount of postoperative scar tissue formation. Many surgeons therefore feel more comfortable reconstituting the thecal sac, usually augmenting its volume at the same time by suturing in place a patch graft. Autografts are readily obtained from nearby muscle fascia, but synthetic materials are now preferred by most surgeons, mainly because they provoke less in the way of postoperative adhesions. The risk, however, with any form of patch graft, is that CSF may leak around its edges and collect extradurally. This may lead to a build-up of hydrostatic pressure, which pushes the graft onto the cord. This can encourage adhesions to develop between the graft and the cord. This constricting effect becomes more pronounced as healing progresses and defeats the object of opening up CSF channels in the first place. The resultant obstruction to CSF flow may be worse than preoperatively, leading to refilling of the syrinx. Many surgeons therefore make a point of hitching the dural patch graft upwards, to try to prevent such adhesions forming. The author’s preference has been to leave the dura widely open, using lateral retaining sutures. With a good closure of the long paraspinal muscles, a pseudomeningocoele forms between the short muscles and this provides a conduit for CSF flow. It is important to pay close attention to the cranial and caudal aspects of the thecal opening, taking care to suture up the dura under the laminae at the upper and lower aspects of the exposure, to ensure that adhesions do not form at these sites. Leaving the dura open also provides an opportunity for placement of temporary subdural stents or drains (Williams and Page 1987). A soft drainage tube, left in place for no more than 48 h, not only serves as a stent but also allows CSF to “auto-irrigate” the subarachnoid channels and drain blood products away (Fig. 11.6). One study did note, however, a higher syrinx recurrence rate if the dura was left open in this way, although the difference between the two groups did not reach statistical significance (Klekamp et al. 1997).
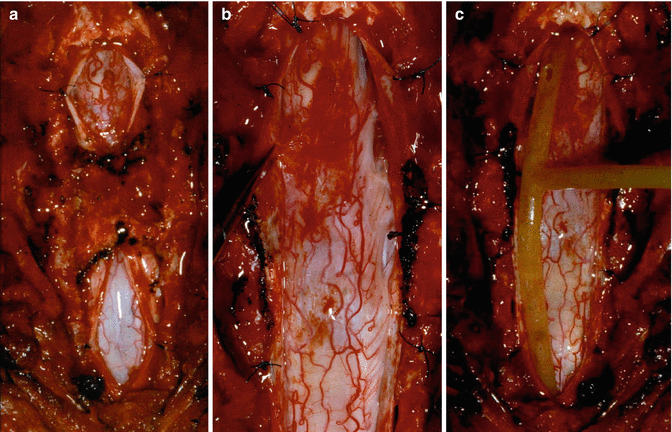

Fig. 11.6
Operative exposure of post-traumatic syringomyelia. (a) Initial dural opening, above and below the level of intradural fibrosis. Note the relative anaemia of the cord in the lower part of the exposure, caused by tension within the syrinx cavity. (b) Following completion of the dural opening and release of arachnoid adhesions. The syrinx has collapsed and the cord is now better perfused. (c) Optional placement of a soft tube, to allow drainage of blood-stained CSF, for 48 h after surgery
11.8.2 Direct Syrinx Drainage
If the attempt to create a conduit fails, or is not suitable for some other reason, then the option of draining the syrinx is available. Indeed, this was a principal means of treatment for some years, and results were considered to be reasonable (Tator et al. 1982; Williams and Page 1987). About half of the patients treated by direct drainage of the syrinx will obtain useful relief (Batzdorf et al. 1998; Sgouros and Williams 1995). The method, however, has a number of drawbacks. In the first place, a myelotomy is required, with an incision through the dorsal columns. Some loss of proprioception is almost inevitable, and this is often more disabling for patients than one might expect, despite the individual retaining good motor power. Further, septa within larger syrinx cavities may obstruct passage of the shunt tubing. The concern then is that the cavity will not drain adequately although, in truth, this is not always a problem. The main difficulty with syrinx drainage tubes is that, in common with all shunt systems, there is a distinct likelihood that they will eventually block. Even if a shunt continues to function and the syrinx remains collapsed, there is a chance that a new cavity may form alongside, simply because the underlying filling mechanism has not been disabled (Fig. 11.7).