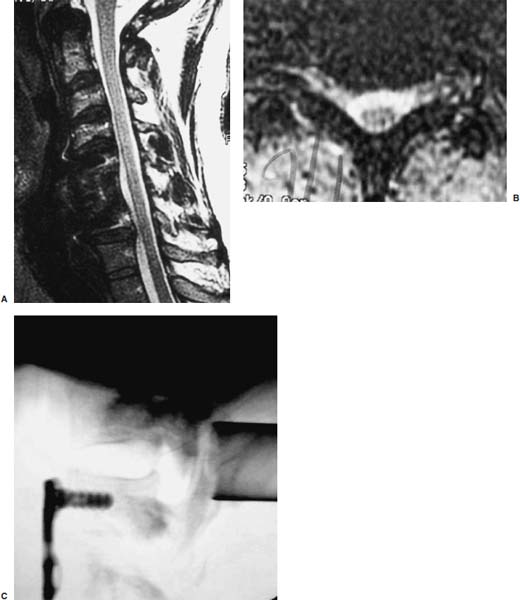
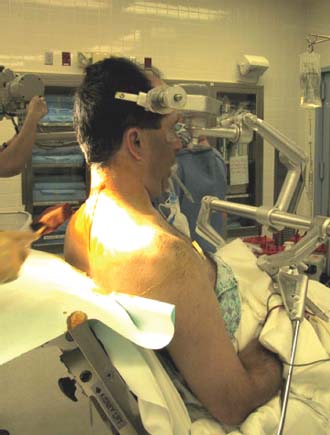
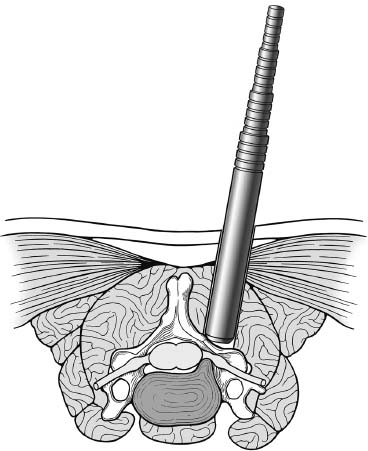
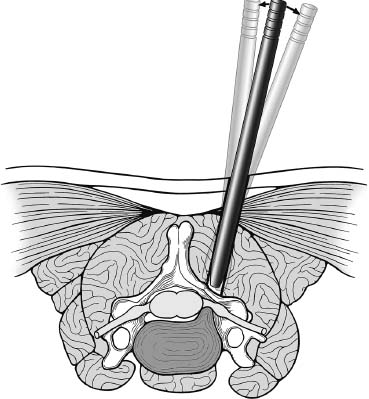
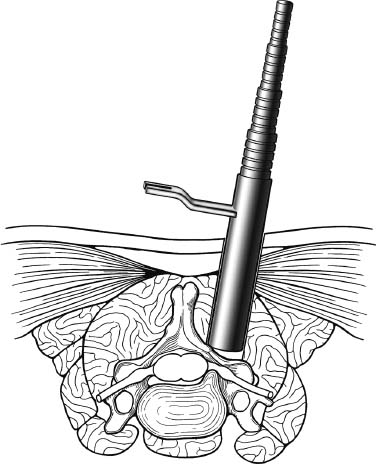
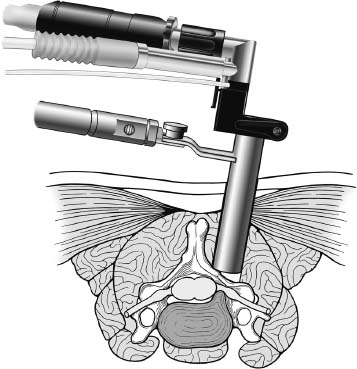
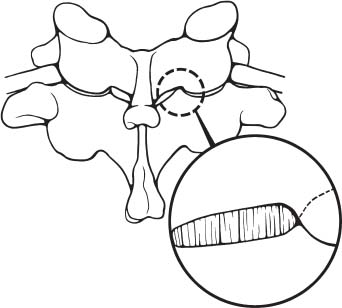
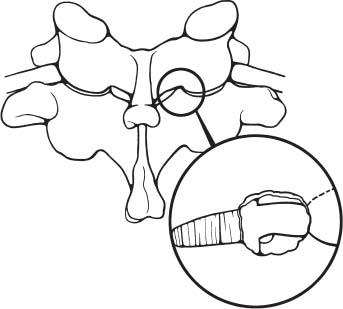
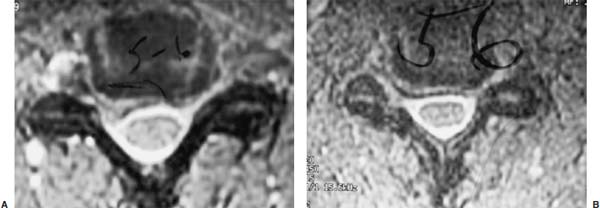
Posterior cervical disk surgery is a well-established and highly effective means of ameliorating disk- and bone-associated compression of cervical nerve roots. It was first proposed by Spurling and Scoville1 in 1944 and remains a procedure of choice for posterolateral disk herniations and focal foraminal stenosis, as well as for patients for whom anterior approaches are contraindicated or associated with increased risk. Despite the effectiveness of the posterior approach, the popularity of the anterior diskectomy procedure has risen steadily. The anterior approach requires an acceptance of the potential risks to the anterior midline structures (e.g., the esophagus and trachea, recurrent laryngeal nerve, and such vascular structures as the carotid artery and its branches).2 In addition, the anterior approach requires near complete disk removal, leading to premature disk space collapse and potential cervical kyphosis. To circumvent this problem, the routine performance of fusion with anterior diskectomy has become standard. The reduction in mobility seen with fusion is associated with an acceleration of degenerative changes at adjacent levels.3 The classic open posterior cervical approach necessitates a moderate-sized incision to develop the relatively deep dissection for visualization of the junction of the lamina with the lateral mass and the underlying neural foramen. Because of the sensitivity of the posterior cervical musculature, the dissection and retraction of ipsilateral and at times bilateral paraspinous musculature can result in significant postoperative pain and muscle spasm. Both anterior and posterior approaches can be performed in the outpatient setting, but posterior approaches can be associated with a longer inpatient postoperative course due to incisional pain. It is therefore very appropriate that initial minimally invasive techniques in spine surgery have attempted to harness the posterior cervical approach, with its relative simplicity and lack of complicated adjacent anatomy, to see if there is a benefit to reducing the extent of the incision and muscle dissection. Reduction in approach-related incisional morbidity has been clearly documented in the laparoscopic experience over the past several decades, and natural progression dictates that this technology be extended to spinal surgical techniques. Minimally invasive surgery is in many ways synonymous with endoscopic techniques that allow visualization of deep structures through small portals in the external body wall. Emphasis is placed on pathology in the case of minimally invasive posterior cervical disk surgery, which results in unilateral radiculopathy, as compared with those that result in central canal stenosis presenting with myelopathy. Classic open treatment of cervical spondylosis has employed both anterior and posterior procedures. Anterior procedures include diskectomy with or without fusion, corpectomy with reconstruction, and, more recently, foraminotomy.4 Smith and Robinson5 initially described the anterior diskectomy procedure in 1955. This approach allows for direct decompression of osteophytes and disk herniations regardless of the location relative to the dorsally located spinal cord and nerve root. The anterior approach relies on naturally occurring dissection planes in the anterior strap muscles, which minimizes potential approach-related morbidity, and thus negates many of the advantages of endoscopic treatment. Anterior percutaneous techniques have had some proponents, but they increase the potential risk to the carotid artery, jugular vein, and esophagus as compared with open surgery. A variation of the anterior approach (i.e., the anterior foraminotomy procedure) allows for preservation of a majority of the disk, thus obviating the need for fusion, but requires dissection immediately adjacent to the ipsilateral vertebral artery, raising some concerns about injury to vascular structures as well as the sympathetic chain. Posterior procedures include laminectomy, laminoplasty, and foraminotomy. These posterior procedures generally allow for indirect decompression, with the exception of foraminal disk herniations amenable to direct removal. Approach morbidity from posterior procedures can be significant, and weakening of the posterior elements can lead to instability. Spurling and Scoville1 initially proposed the posterior approach to cervical disk disease in 1944. Several studies indicated postoperative axial neck pain ranging from 18 to 40%,6,7 and one study failed to include this factor in the outcome.8 The frequently quoted prospective comparison of anterior versus posterior approaches by Herkowitz et al9 had 90% and 75% good to excellent results for both approaches, respectively. It is interesting to note that if the patients were evaluated for resolution of radicular symptoms alone, the results for posterior approaches were good to excellent in 85 to 90% of the cases. This indicated that the major difference in the two approaches was incisional and dissectional morbidity from the open surgical technique. A major innovation allowing for minimally invasive procedures is the development of small-diameter glass rod endoscopes with high resolution. These devices allow for improved visualization of the deep structures through small portals, thereby minimizing soft tissue trauma. The associated development of various endoscopic delivery systems and instrumentation has provided the means for accomplishing such minimally invasive surgery in the cervical spine as the microendoscopic diskectomy (MED) procedure. These innovative procedures are not expected to replace open procedures entirely, but rather to provide surgeons with additional options for treating certain specific problems in the cervical spine. The decisional algorithm to offer anterior versus posterior procedures, single versus multiple levels, and fusion versus decompression alone is often complicated and is beyond the scope of this chapter. Indications Due to the limited exposure of endoscopic techniques, the pathology should ideally be unilateral and restricted to a single level or to two contiguous nerve root levels. Cervical MED is effective for removal of foraminal soft disk herniations, as well as foraminal stenosis from osteophytic spurs or facet arthropathy, including synovial cysts. Endoscopic foraminotomy has been shown to produce an equivalent or slightly larger decompression as compared with standard open surgery.10 Additional indications include multilevel foraminal stenosis without central stenosis, persistent foraminal stenosis after an anterior diskectomy with fusion, and root compression in situations in which anterior approaches may be relatively contraindicated. These anterior issues include the presence of a tracheostomy, history of prior cervical radiation therapy, and disk herniations at the cervicothoracic junction (C7–T1 or T1–T2), as shown in Figure 7–1. Cervical radiculopathy may be associated with significant axial neck pain, but it should not be confused with patients suffering from axial neck pain without nerve root or spinal cord symptoms. Cervical discogenic pain resulting in axial neck pain with referred, nonradicular arm pain is controversial and may require diskography for evaluation; however it is ultimately not amenable to posterior endoscopic treatment. The presence of a brachial plexopathy is frequently ignored in favor of cervical disk disease or distal peripheral nerve entrapment (e.g., the ulnar or median nerve). Shoulder pain with radiation into multiple dermatomal levels, weakness of the hand intrinsics, aggravation with arm elevation, and the absence of significant cervical disk disease should trigger the inclusion of thoracic outlet syndrome into the differential diagnosis. Positioning Surgery may be performed in the prone or sitting position. After general endotracheal anesthesia is induced, the patient is positioned, and narcotics are avoided to minimize postoperative nausea. The authors’ series were initially performed prone with transverse rolls utilized rather than a frame to minimize kyphosis over the cervicothoracic junction. The sitting position (Fig. 7–2) is currently preferred based on a reduction in blood loss and improved visibility, resulting in reduction in operative time. The sitting position also improves the ability to assess the cervicothoracic junction with the fluoroscope (see Fig. 7–1C). Because of the limited size of the exposure and short duration of surgery, the risk of air embolus is negligible, and a central line or precordial Doppler is therefore not needed. A Foley catheter is placed for cases involving multiple levels or bilateral procedures. A prophylactic single dose of intravenous antibiotic (cefazolin or vancomycin) is given at the start of the procedure. FIGURE 7–1 A 38-year-old patient with junctional spondylosis below a prior C5–C7 fusion. (A) Axial magnetic resonance imaging at C7-T1 showing a foraminal disk herniation involving the right C8 foramen. (B) Intraoperative fluoroscopic image showing the position of the tubular retractor over the C7-T1 facet joint at the time of surgery. (C) Fluoroscopic image showing the cervicothoracic junction. Surgical Equipment and Setup Lateral fluoroscopy is used after placement of an 18-gauge spinal needle to confirm the correct level. The fluoroscope can be placed under the drapes because rotation into the anteroposterior (AP) plane is not needed or feasible. The video monitors are placed contralateral to the side of the disk pathology, with the scrub technician on the ipsilateral side. Anesthesia personnel are positioned toward the patient’s feet behind the fluoroscope. Available endoscopic systems include the Flexposure system from Endius (Plainville, MA) and the METRx system by Sofamor Danek (Memphis, TN). The METRx system was chosen for cervical cases because of the availability of downsized instrumentation needed for cervical cases. Both systems use a tubular retractor to deliver an endoscope for visualization and provide a corridor to manipulate surgical instruments. The equipment includes a guidewire that is inserted under fluoroscopic guidance to the correct level onto the desired facet joint. A series of nested sequential dilators are used to create an access corridor. The METRx system is supplied with 16 and 18 mm tubular retractors, but a custom 14 mm tubular retractor has been used for cervical cases to reduce the muscle dissection and retraction. Both the tubular retractor and the endoscope are then fixed to the operating table through adjustable arms. Typical soft tissue and bone instruments have been lengthened for use through the tubular retractor and anodized to eliminate reflective glare. These include curettes, Kerrison rongeurs, pituitary rongeurs, Penfield retractors, nerve hooks, and bipolar cautery, among others. The METRx endoscope has a 25-degree viewing angle, and that of the Endius endoscope is 30 degrees; both provide an improved viewing angle and eliminate obscuration of the operative field, noted with use of the operating microscope. Both endoscopes use glass rod-lens technology, and the clarity of the images compensates for the lack of a three-dimensional image. FIGURE 7–2 Use of the Mayfield head holder and table adaptor for placement in the sitting position. Surgical Technique Following placement in the sitting position, an 18-gauge needle is placed over the correct foraminal level and confirmed with fluoroscopic images. A 1.5 cm incision is made ~2 cm lateral to the midline. If accessible, the fascia is incised with monopolar cautery to facilitate passage of the dilators to the interlaminar space. A K-wire is then passed to the appropriate facet joint, over which a series of dilators is used to create an operative corridor to the spine (Fig. 7–3). The initial dilator is used as a dissector to detach muscular and ligamentous attachments (Fig. 7–4). A 14 mm tubular retractor is then placed over the last dilator (Fig. 7–5) and secured to the operating table with a modified Greenberg articulated arm. Because of the reduced size of the cervical spine, the larger 16 mm tubular retractor has been avoided to prevent excessive resection of the facet joint. The endoscope is then mounted within the tubular retractor (Fig. 7–6), with visualization of the appropriate interlaminar space and facet joint (Fig. 7–7). The 25-degree viewing angle of the endoscope provides a panoramic view of the epidural space and eliminates the obscuration of the operative field that occurs if the operating microscope is used with bayoneted instruments. FIGURE 7–3 A K-wire is placed over the appropriate facet joint under fluoroscopic guidance. A series of dilators are then used to create the operative corridor to the interlaminar space. FIGURE 7–4 After removal of the K-wire, the tip of the smallest dilator is used to dissect soft tissue from the lamina and the interlaminar space. The inferolateral margin of the superior lamina and medial facet joint of the superior vertebral segment are first cleared of soft tissue using bipolar long-tipped cautery, and the ligamentum flavum is separated from the laminar edge using an endoscopic curette. A hemilaminotomy is then performed with a high-speed drill and Kerrison rongeurs. The superior facet of the lower vertebral body is visualized laterally below the initial laminotomy and requires medial resection for access to the exiting nerve root. The epidural space is entered laterally, with resection of the ligamentum flavum and coagulation of the epidural plexus overlying the exiting nerve root. A careful dissection of the ligamentum flavum and removal of an adequate amount of bone decrease the likelihood of dural injury and tears. Kerrison rongeurs are used to accomplish the foraminotomy, and a 90-degree version should be used medially to reduce the potential for a dural tear. After a foraminotomy is performed, a small nerve hook may be used to palpate the medial aspect of the superior and inferior pedicles to define the boundaries of the foramen and lateral margin of the canal. The nerve root is then mobilized using a small Penfield dissector to allow further removal of pathology. Dissection in the axilla of the nerve root usually uncovers the disk herniation or osteophyte, but a disk herniation can occasionally be found at the shoulder of the nerve due to the shape and angulation of the joint of Luschka. In the case of a soft disk herniation, the decompression is complete once the contained or free fragment of disk is removed, and exploration of the disk space is not necessary. Osteophytic spurs are generally not amenable to resection and are treated with a generous foraminotomy (Fig. 7–8). An arthritic, enlarged facet may also be the culprit, and it may be partially resected either through a high-speed drill or Kerrison rongeurs. Once the nerve root has been decompressed, this is confirmed through placement of the nerve hook or Woodson dissector through the foramen along the nerve root. The tubular retractor is removed after ensuring hemostasis, and the fascia is closed with a single absorbable suture. The skin is closed with subcuticular stitches, and a small volume of 0.25% bipuvicaine with epinephrine is injected into the paravertebral muscles. FIGURE 7–5 A tubular retractor is placed over the last dilator. Retractors are available in sizes ranging from 14 to 18 mm. The patient is then moved to the outpatient recovery area and discharged home, usually in 3 to 4 hours. Discharge mediations include antispasmodics for neck spasm and oral opioids for breakthrough pain. FIGURE 7–6 An articulated arm mounted to the operating table is used to stabilize the tubular retractor. The endoscope is then advanced into the tubular retractor. The depth of endoscope within the retractor determines the degree of magnification. Results In the authors’ initial series, 14 patients (nine males and five females) underwent posterior cervical MED with minimum 1.5 years follow up. Two patients presented with symptomatic synovial cysts at C7-T1, and the remainder had spondylosis with or without an associated soft disk herniation (see Fig. 7–8). Twelve had single-level surgery, and two required two-level procedures. All surgeries were performed on an outpatient basis. Thirteen noted complete resolution of radicular symptoms by the first week, and 12 denied the presence of any significant postoperative neck pain. By the sixth postoperative week, axial neck pain had resolved in the remaining two patients. FIGURE 7–7 Scope view of the interlaminar space showing lamina/lateral mass junction over the nerve root, as well as medial facetectomy required for the laminoforaminotomy. FIGURE 7–8 Following bone and ligament removal, instruments and dissectors can be placed through the tubular retractor for removal of the disk herniation, as demonstrated below the exiting nerve root. Complications included one recurrent disk herniation, blood loss greater than 800 cc on one occasion, and one patient with a contralateral neurogenic thoracic outlet syndrome from operative positioning. There was also one open conversion early in the series due to osteophytic foraminal stenosis, which at the time of open exploration had been adequately decompressed with the MED procedure alone. These complications were all noted in patients who were positioned prone. The Neck Disability Index was used to assess outcomes, with a mean entry score of 41/65. The mean score was 12/65 at last follow-up, indicating a marked improvement in function and ability to perform daily activities. This 29-point difference is striking because a 10-point difference is felt to be significant in some studies. The results of this technique reveal a definite improvement over historical controls. There has been virtual elimination of postoperative axial neck pain and uniform resolution of cervical radiculopathy. There has been one open conversion and one recurrent disk herniation requiring an anterior fusion. There have been no long-term neurologic deficits, and significant improvement was confirmed with the Neck Disability Index. FIGURE 7–9 A 42-year-old patient with clinical symptoms of a right C6 radiculopathy. Magnetic resonance imaging (MRI) scan shows a paramedian disk herniation on the right at C5–C6 (A) Postoperative MRI showing decompression of the disk herniation. (B) Note the lack of paraspinal muscle trauma above the site of the foraminotomy. Adamson11 reported on 100 consecutive posterior cervical microendoscopic diskectomies for unilateral cervical radiculopathy from foraminal stenosis or lateral disk herniation. The author used the sitting position with good-to-excellent outcomes in 97% of patients. Adamson compared the results with open posterior or anterior cervical procedures and surmised that posterior cervical MED procedures were superior because of preservation of cervical motion segment, a much faster return to unrestricted activity, and a much lower incidence of complications. Fessler and Khoo12 reported on 25 patients who underwent posterior cervical MEDs, as compared with 26 patients who underwent open cervical laminoforaminotomy for foraminal stenosis from osteophytes or lateral disk herniation. They noted a marked reduction in blood loss, earlier recovery, shorter hospital stay, and fewer narcotics in patients who underwent MED. They noted similar out-comes when evaluating radiculopathy, neck pain, sensory, and motor status. The most remarkable feature was the relative absence of incisional neck pain, which was the most common cause of prolonged hospital stays for patients undergoing open procedures. Complications and Avoidance Our complications have included one recurrent disk herniation, blood loss greater than 800 ml on one occasion, and one patient with a contralateral neurogenic thoracic outlet syndrome from operative positioning. All these cases were performed with prone positioning. Our shift to the sitting position has resulted in a marked decline in blood loss, averaging 150 ml because of the reduction in epidural venous engorgement. Visualization is further facilitated through flow of blood out of the horizontal tubular retractor rather than pooling in the operative field in the prone position. Fessler and Khoo12 have also documented a reduction in blood loss and operative times by employing the sitting position. Although we have not experienced an intraoperative durotomy, Adamson11 described two such cases from his series of 100 cervical MEDs, and Fessler and Khoo12 reported three such cases in their series of 25 patients, as well as a single case of a superficial wound infection. Adamson did not place lumbar drains for his cases of cerebrospinal fluid (CSF) leak, whereas Fessler and Khoo performed 2 to 3 days of lumbar drainage in all cases. No long-term pseudomeningocele or CSF leak-related symptoms have thus far been reported. Given the small size of the incisional exposure, the potential for development of a symptomatic pseudomeningocele is small, and wound closure alone should be sufficient. Fessler and Khoo12 also reported a single case of superficial wound infection. Although no reports have been seen to date, the guidewire must be passed with fluoroscopic guidance to avoid the risk of inadvertent medial passage through the interlaminar space, with potential damage to the spinal cord or the exiting nerve root. A similar deviation laterally may result in injury to the vertebral artery or the venous plexus, with resultant bleeding and hematoma-related complications or arterial dissection and possible stroke. The lateral mass/facet joint complex is therefore the appropriate place for the guidewire and dilators placement, and the guidewire must be in contact with bone prior to passage of the dilators. Posterior cervical microendoscopic diskectomy and laminoforaminotomy can be performed safely, with results comparable to the open posterior approach. MED avoids the approach-related morbidity encountered in open posterior approaches to the posterior paraspinal muscles. The advantages of the microendoscopic approach have been discussed, but the potential for recurrent disk herniations, the added time for surgery, and the requisite learning curve for endoscopic techniques must be taken into consideration. Endoscopic equipment has evolved to hybrid systems that create a working corridor that facilitates recognition of key anatomic structures. For spinal procedures, the ability to identify normal structures is of paramount importance because an open working space (e.g., in laparoscopic techniques) does not exist in the cervical spine. The ability to affect a surgical cure with less destruction of normal structures is clearly a desirable goal. New technology has miniaturized the operative field and created a need for greater understanding of both normal and especially variant anatomy. Although the acceptance of various minimally invasive techniques may not be universal, the trend remains positive in limiting some of the destructive effects of operative exposure and has favorably affected many open surgical techniques. REFERENCES
7

Posterior Cervical Microendoscopic Diskectomy and Laminoforaminotomy

< div class='tao-gold-member'>
Posterior Cervical Microendoscopic Diskectomy and Laminoforaminotomy
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








